Loss of MXRA8 Delays Mammary Tumor Development and Impairs Metastasis
Abstract
:1. Introduction
2. Results
2.1. Characterization of MXRA8-Knockout Clones In Vitro
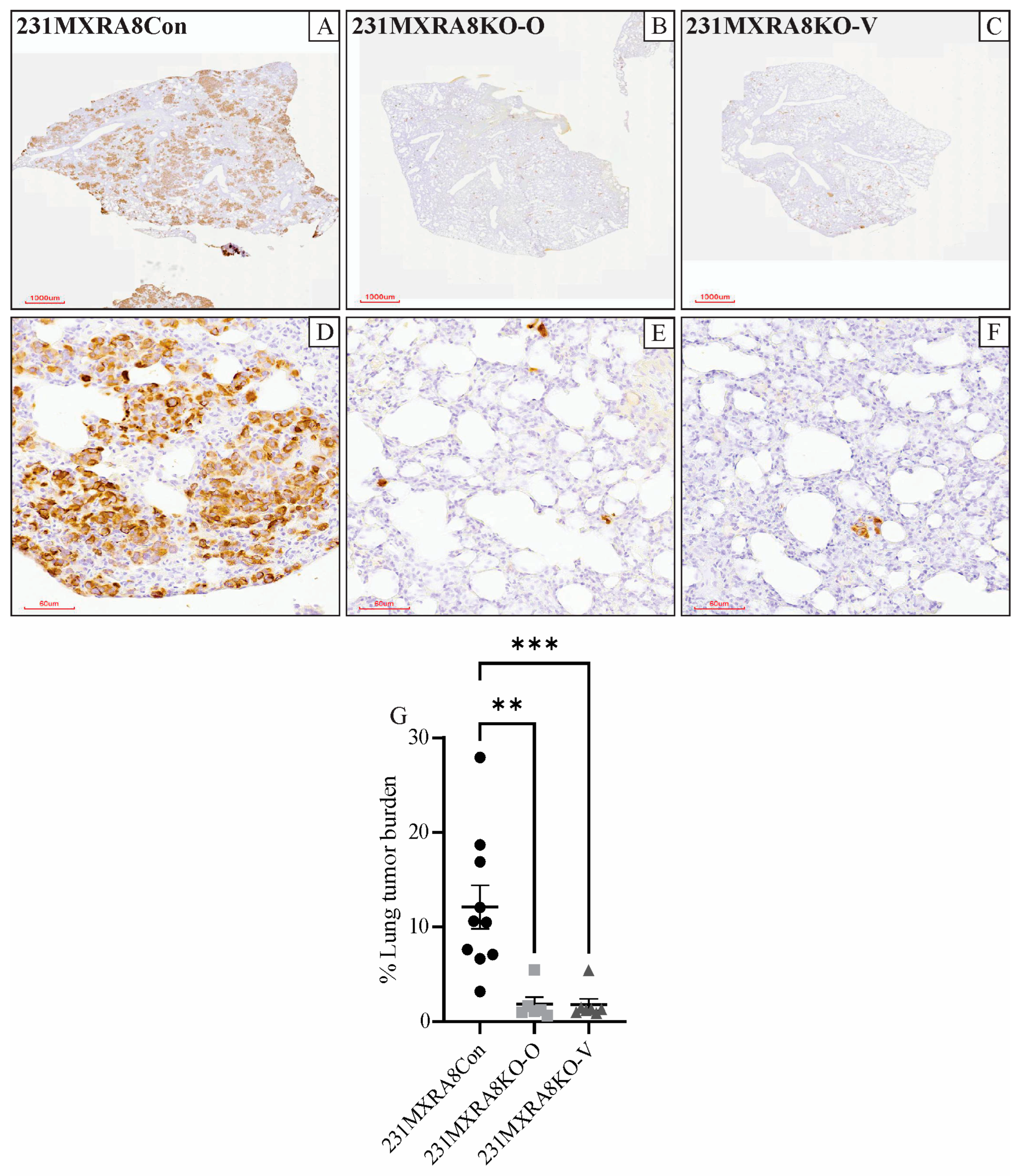
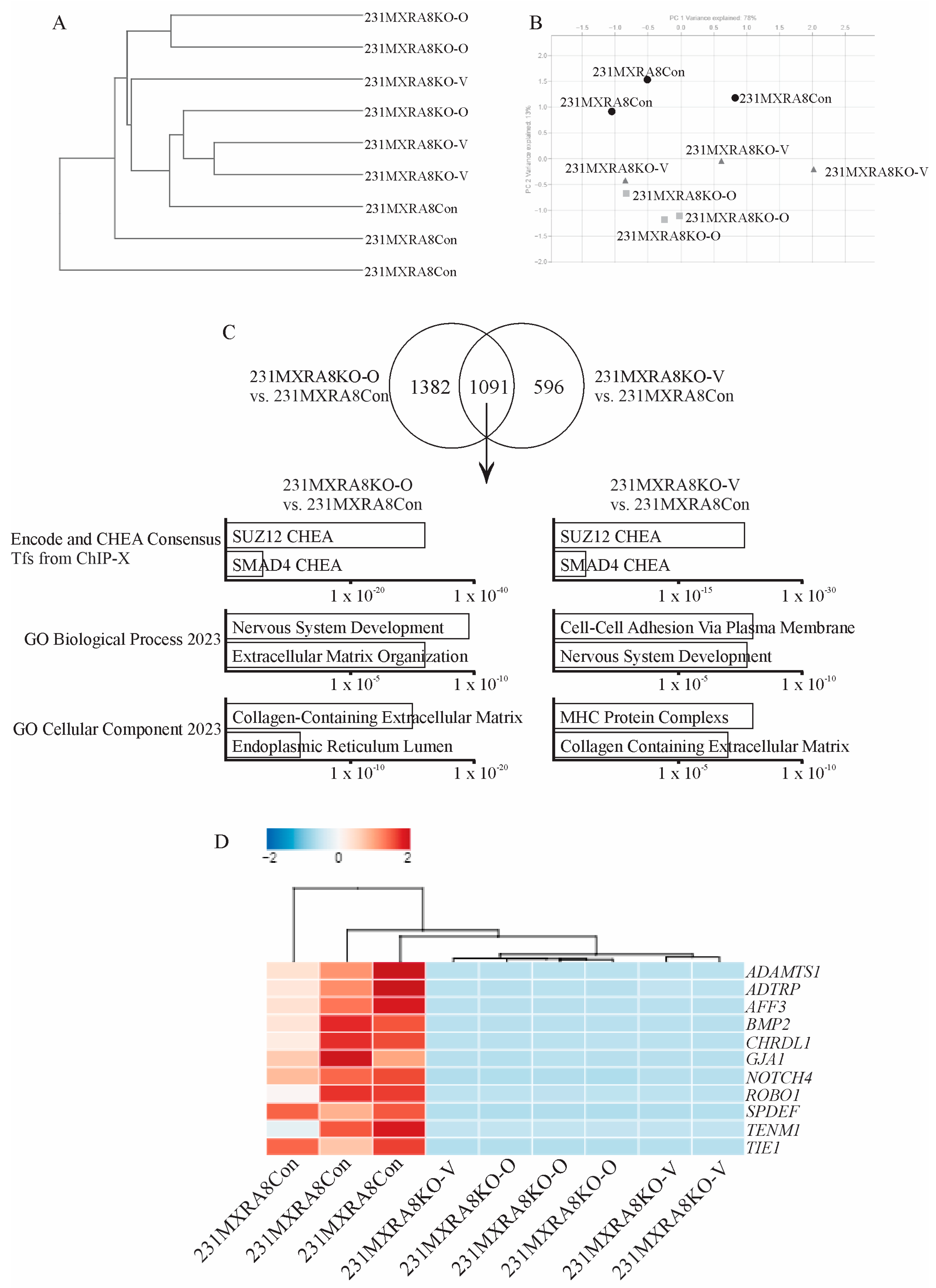
2.2. Characterization of 231MXRA8KO Clones In Vivo
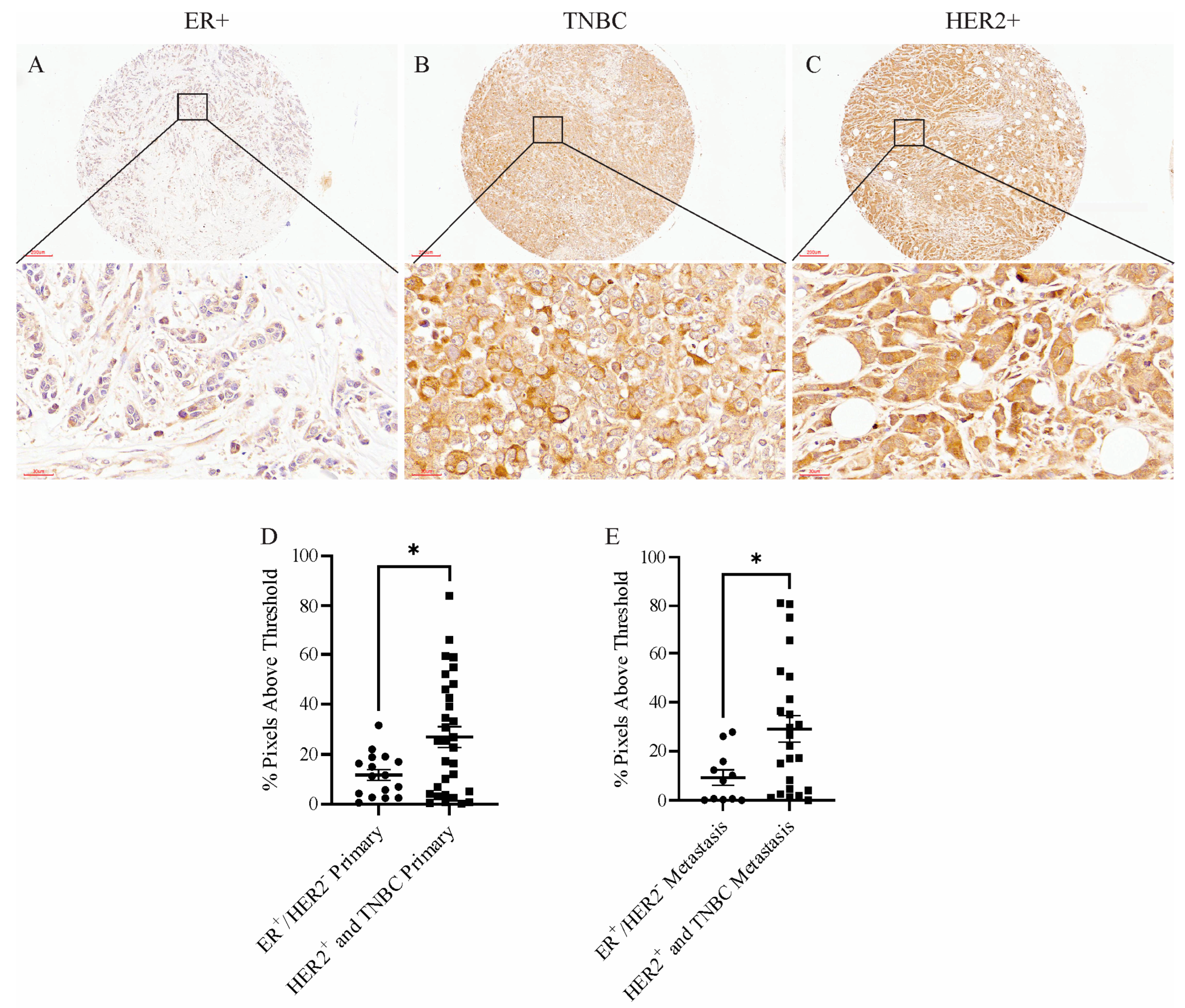
2.3. MXRA8 Protein Levels in Human Breast Cancer
3. Discussion
4. Materials and Methods
4.1. MXRA8 Knockout, Selection, and Cell Culture Conditions
4.2. BrdU and Annexin V Flow Cytometry
4.3. Invasion Chamber Assay
4.4. RNA Extraction and Real-Time PCR
4.5. RNA Sequencing
4.6. Animals and Ethics
4.7. Tumor Specific Growth Rates (SGRs)
4.8. Histology and Immunohistochemistry
4.9. Tissue Microarray
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yonezawa, T.; Ohtsuka, A.; Yoshitaka, T.; Hirano, S.; Nomoto, H.; Yamamoto, K.; Ninomiya, Y. Limitrin, a novel immunoglobulin superfamily protein localized to glia limitans formed by astrocyte endfeet. Glia 2003, 44, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Grasmuck, C.; Ghannam, S.; Bourbonniere, L.; Fournier, A.P.; Lecuyer, M.A.; Tastet, O.; Kebir, H.; Rebillard, R.M.; Hoornaert, C.; et al. DICAM promotes T(H)17 lymphocyte trafficking across the blood-brain barrier during autoimmune neuroinflammation. Sci. Transl. Med. 2022, 14, eabj0473. [Google Scholar] [CrossRef]
- Han, S.W.; Jung, Y.K.; Lee, E.J.; Park, H.R.; Kim, G.W.; Jeong, J.H.; Han, M.S.; Choi, J.Y. DICAM inhibits angiogenesis via suppression of AKT and p38 MAP kinase signalling. Cardiovasc. Res. 2013, 98, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.K.; Han, S.W.; Kim, G.W.; Jeong, J.H.; Kim, H.J.; Choi, J.Y. DICAM inhibits osteoclast differentiation through attenuation of the integrin alphaVbeta3 pathway. J. Bone Miner. Res. 2012, 27, 2024–2034. [Google Scholar] [CrossRef]
- Song, D.; Jia, X.; Liu, X.; Hu, L.; Lin, K.; Xiao, T.; Qiao, Y.; Zhang, J.; Dan, J.; Wong, C.; et al. Identification of the receptor of oncolytic virus M1 as a therapeutic predictor for multiple solid tumors. Signal. Transduct. Target Ther. 2022, 7, 100. [Google Scholar] [CrossRef]
- Tan, L.; Fu, D.; Liu, F.; Liu, J.; Zhang, Y.; Li, X.; Gao, J.; Tao, K.; Wang, G.; Wang, L.; et al. MXRA8 is an immune-relative prognostic biomarker associated with metastasis and CD8(+) T cell infiltration in colorectal cancer. Front. Oncol. 2022, 12, 1094612. [Google Scholar] [CrossRef]
- Li, S.; Xu, W. Mining TCGA database for screening and identification of hub genes in kidney renal clear cell carcinoma microenvironment. J. Cell Biochem. 2019, 121, 3952–3960. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Song, L.; Yuan, F.; Yan, Y. Matrix Remodeling-Associated Protein 8 as a Novel Indicator Contributing to Glioma Immune Response by Regulating Ferroptosis. Front. Immunol. 2022, 13, 834595. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, C.; Wei, H.; Qian, X. Identification of the Prognostic Value of Tumor Microenvironment-Related Genes in Esophageal Squamous Cell Carcinoma. Front. Mol. Biosci. 2020, 7, 599475. [Google Scholar] [CrossRef]
- Shen, R.K.; Huang, Z.; Zhu, X.; Lin, J.H. Bioinformatics analysis of differently expressed genes in osteoblastic sarcoma and screening of key genes. Zhonghua Zhong Liu Za Zhi 2022, 44, 147–154. [Google Scholar] [PubMed]
- Wu, L.; Zhou, Y.; Guan, Y.; Xiao, R.; Cai, J.; Chen, W.; Zheng, M.; Sun, K.; Chen, C.; Huang, G.; et al. Seven Genes Associated With Lymphatic Metastasis in Thyroid Cancer That Is Linked to Tumor Immune Cell Infiltration. Front. Oncol. 2021, 11, 756246. [Google Scholar] [CrossRef]
- Simpson, K.E.; Watson, K.L.; Moorehead, R.A. Elevated Expression of miR-200c/141 in MDA-MB-231 Cells Suppresses MXRA8 Levels and Impairs Breast Cancer Growth and Metastasis In Vivo. Genes 2022, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Kiflemariam, S.; Ljungstrom, V.; Ponten, F.; Sjoblom, T. Tumor vessel up-regulation of INSR revealed by single-cell expression analysis of the tyrosine kinome and phosphatome in human cancers. Am. J. Pathol. 2015, 185, 1600–1609. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Park, H.R.; Lee, E.J.; Jang, J.A.; Han, M.S.; Kim, G.W.; Jeong, J.H.; Choi, J.Y.; Beier, F.; Jung, Y.K. Dicam promotes proliferation and maturation of chondrocyte through Indian hedgehog signaling in primary cilia. Osteoarthr. Cartil. 2018, 26, 945–953. [Google Scholar] [CrossRef]
- Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N.V.; Monjezi, M.R.; Ilbeigi, S.; Alahari, S.K. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 2021, 40, 1043–1063. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Xu, J.; Zou, J. The role of integrin family in bone metabolism and tumor bone metastasis. Cell Death Discov. 2023, 9, 119. [Google Scholar] [CrossRef]
- Mao, X.G.; Xue, X.Y.; Lv, R.; Ji, A.; Shi, T.Y.; Chen, X.Y.; Jiang, X.F.; Zhang, X. CEBPD is a master transcriptional factor for hypoxia regulated proteins in glioblastoma and augments hypoxia induced invasion through extracellular matrix-integrin mediated EGFR/PI3K pathway. Cell Death Dis. 2023, 14, 269. [Google Scholar] [CrossRef]
- Kovacheva, M.; Zepp, M.; Berger, S.; Berger, M.R. Conditional knockdown of integrin beta-3 reveals its involvement in osteolytic and soft tissue lesions of breast cancer skeletal metastasis. J. Cancer Res. Clin. Oncol. 2021, 147, 361–371. [Google Scholar] [CrossRef]
- Kuno, K.; Iizasa, H.; Ohno, S.; Matsushima, K. The exon/intron organization and chromosomal mapping of the mouse ADAMTS-1 gene encoding an ADAM family protein with TSP motifs. Genomics 1997, 46, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Boerboom, D.; Pritchard, M.; Lane, M.; Russell, D.L. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 2010, 83, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, M.L.; Carpizo, D.; Luque, A. ADAMTS1: A matrix metalloprotease with angioinhibitory properties. Ann. N. Y. Acad. Sci. 2003, 995, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Carpizo, D.R.; Iruela-Arispe, M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003, 278, 23656–23665. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sone, S.; Takahashi, I.; Mizoguchi, I.; Echigo, S.; Sasano, Y. Expression of versican and ADAMTS1, 4, and 5 during bone development in the rat mandible and hind limb. J. Histochem. Cytochem. 2005, 53, 1553–1562. [Google Scholar] [CrossRef]
- Porter, S.; Scott, S.D.; Sassoon, E.M.; Williams, M.R.; Jones, J.L.; Girling, A.C.; Ball, R.Y.; Edwards, D.R. Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 2004, 10, 2429–2440. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Q.; Hu, G.; Van Poznak, C.; Fleisher, M.; Reiss, M.; Massague, J.; Kang, Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009, 23, 1882–1894. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, Y.; Yu, Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006, 25, 2452–2467. [Google Scholar] [CrossRef]
- Hirano, T.; Hirose, K.; Sakurai, K.; Makishima, M.; Sasaki, K.; Amano, S. Inhibition of tumor growth by antibody to ADAMTS1 in mouse xenografts of breast cancer. Anticancer Res. 2011, 31, 3839–3842. [Google Scholar]
- Ricciardelli, C.; Frewin, K.M.; Tan Ide, A.; Williams, E.D.; Opeskin, K.; Pritchard, M.A.; Ingman, W.V.; Russell, D.L. The ADAMTS1 protease gene is required for mammary tumor growth and metastasis. Am. J. Pathol. 2011, 179, 3075–3085. [Google Scholar] [CrossRef]
- Partanen, J.; Puri, M.C.; Schwartz, L.; Fischer, K.D.; Bernstein, A.; Rossant, J. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development 1996, 122, 3013–3021. [Google Scholar] [CrossRef]
- D’Amico, G.; Korhonen, E.A.; Waltari, M.; Saharinen, P.; Laakkonen, P.; Alitalo, K. Loss of endothelial Tie1 receptor impairs lymphatic vessel development-brief report. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 207–209. [Google Scholar] [CrossRef]
- Qu, X.; Tompkins, K.; Batts, L.E.; Puri, M.; Baldwin, H.S. Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development 2010, 137, 1285–1295. [Google Scholar] [CrossRef]
- Saharinen, P.; Jeltsch, M.; Santoyo, M.M.; Leppänen, V.-M.; Alitalo, K. The TIE Receptor Family. In Receptor Tyrosine Kinases: Family and Subfamilies; Wheeler, D.L., Yarden, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 743–775. [Google Scholar]
- Tiainen, L.; Korhonen, E.A.; Leppanen, V.M.; Luukkaala, T.; Hamalainen, M.; Tanner, M.; Lahdenpera, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; et al. High baseline Tie1 level predicts poor survival in metastatic breast cancer. BMC Cancer 2019, 19, 732. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sui, L.; Fang, Z.; Jiang, W.G.; Ye, L. Aberrant expression of bone morphogenetic proteins in the disease progression and metastasis of breast cancer. Front. Oncol. 2023, 13, 1166955. [Google Scholar] [CrossRef] [PubMed]
- Chapellier, M.; Bachelard-Cascales, E.; Schmidt, X.; Clement, F.; Treilleux, I.; Delay, E.; Jammot, A.; Menetrier-Caux, C.; Pochon, G.; Besancon, R.; et al. Disequilibrium of BMP2 levels in the breast stem cell niche launches epithelial transformation by overamplifying BMPR1B cell response. Stem. Cell Rep. 2015, 4, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Clement, F.; Xu, X.; Donini, C.F.; Clement, A.; Omarjee, S.; Delay, E.; Treilleux, I.; Fervers, B.; Le Romancer, M.; Cohen, P.A.; et al. Long-term exposure to bisphenol A or benzo(a)pyrene alters the fate of human mammary epithelial stem cells in response to BMP2 and BMP4, by pre-activating BMP signaling. Cell Death Differ. 2017, 24, 155–166. [Google Scholar] [CrossRef]
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, M.; Jiang, H.; Zheng, X. BMP-2 upregulates the AKT/mTOR pathway in breast cancer with microcalcification and indicates a poor prognosis. Clin. Transl. Oncol. 2020, 22, 1263–1271. [Google Scholar] [CrossRef]
- Johnstone, C.N.; Pattison, A.D.; Gorringe, K.L.; Harrison, P.F.; Powell, D.R.; Lock, P.; Baloyan, D.; Ernst, M.; Stewart, A.G.; Beilharz, T.H.; et al. Functional and genomic characterisation of a xenograft model system for the study of metastasis in triple-negative breast cancer. Dis. Model Mech. 2018, 11, dmm032250. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]


| Transcript ID | Transcript Name | 231MXRA8Con | 231MXRA8KO-O | 231MXRA8KO-V |
|---|---|---|---|---|
| ENST00000342753 | MXRA8-202 | 1.8 ± 0.6 | 0.3 ± 0.2 | 0.1 ± 0.1 |
| ENST00000309212 | MXRA8-201 | 21.6 ± 2.1 | 0 ± 0 | 0 ± 0 |
| ENST00000445648 | MXRA8-203 | 3.8 ± 0.7 | 0 ± 0 | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpson, K.E.; Staikos, C.A.; Watson, K.L.; Moorehead, R.A. Loss of MXRA8 Delays Mammary Tumor Development and Impairs Metastasis. Int. J. Mol. Sci. 2023, 24, 13730. https://doi.org/10.3390/ijms241813730
Simpson KE, Staikos CA, Watson KL, Moorehead RA. Loss of MXRA8 Delays Mammary Tumor Development and Impairs Metastasis. International Journal of Molecular Sciences. 2023; 24(18):13730. https://doi.org/10.3390/ijms241813730
Chicago/Turabian StyleSimpson, Kaitlyn E., Christina A. Staikos, Katrina L. Watson, and Roger A. Moorehead. 2023. "Loss of MXRA8 Delays Mammary Tumor Development and Impairs Metastasis" International Journal of Molecular Sciences 24, no. 18: 13730. https://doi.org/10.3390/ijms241813730





