Insecticidal Effects of Receptor-Interference Isolated Bioactive Peptides on Fire Ant Colonies
Abstract
:1. Introduction
2. Results
2.1. Fire Ant Worker Ingestion of Bioactive Peptides
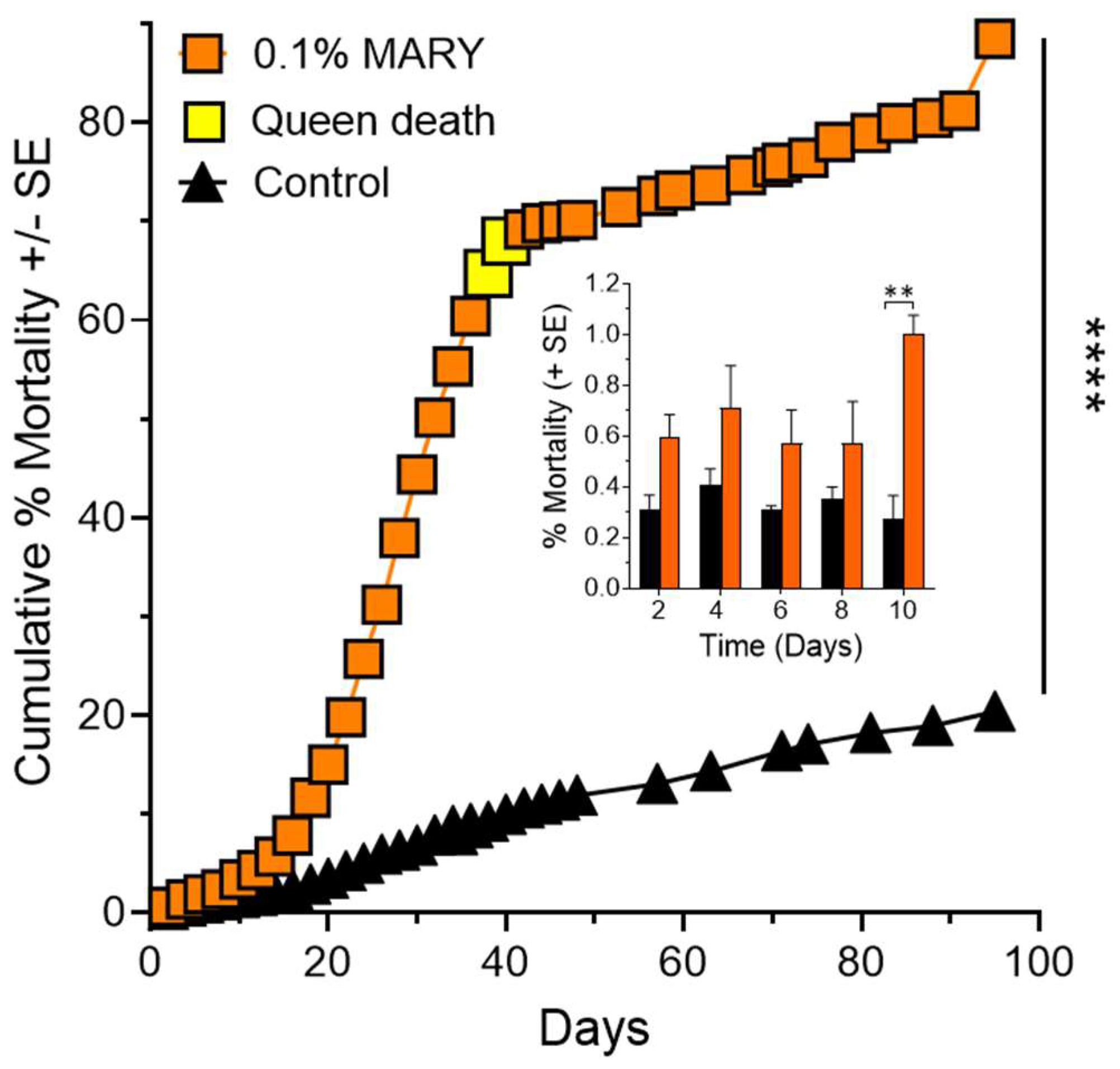
2.2. Effects of Feeding Bioactive Peptide MARY to Queen Right Colonies
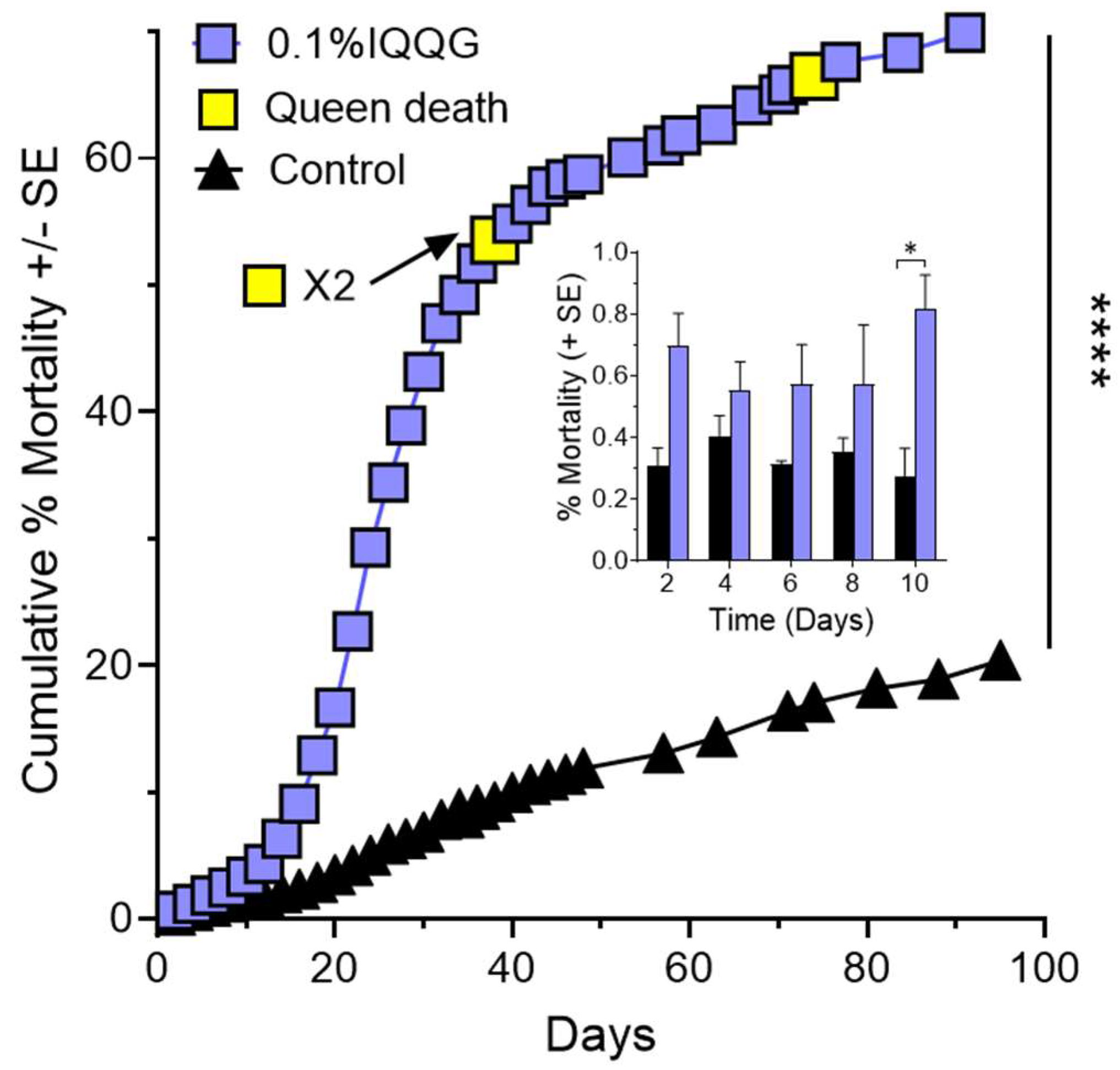
2.3. Effects of Feeding Bioactive Peptide IQQG to Queen Right Colonies
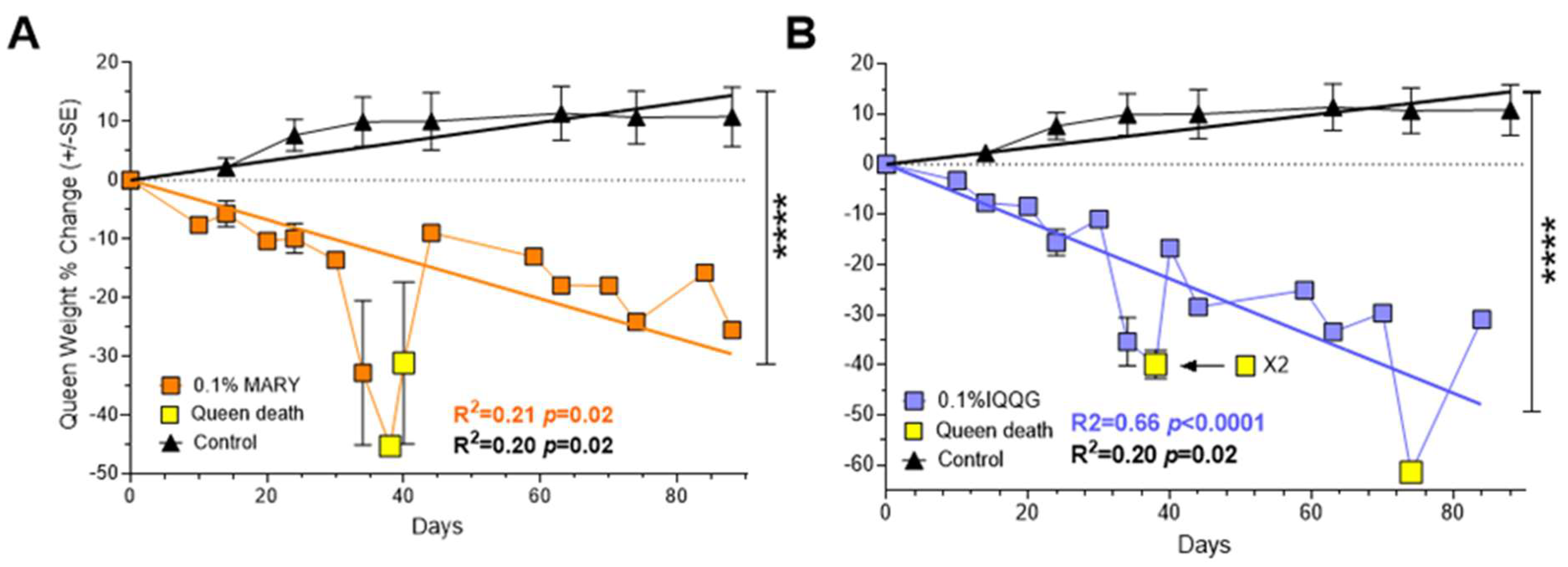
2.4. The Effects of Bioactive Peptides MARY and IQQG on Fire Ant Queens
3. Discussion
3.1. Bioactive Peptides to the Receptor
3.2. Hydrophobicity and Stability of Bioactive Peptides
3.3. The Ant Model Receptor-i
3.4. Effect on the Fire Ant Queens
4. Materials and Methods
4.1. Insects
4.2. Peptides
4.3. Small Scale Feeding Bioassays
4.4. Large Scale Fully Functional Field Colony Collection and Setup
4.5. Peptide Treatments
4.6. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caers, J.; Verlinden, H.; Zels, S.; Vandersmissen, H.P.; Vuerinckx, K.; Schoofs, L. More than two decades of research on insect neuropeptide GPCRs: An overview. Front. Endocrinol. 2012, 3, 151. [Google Scholar] [CrossRef]
- Nassel, D.R.; Zandawala, M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol. 2019, 179, 101607. [Google Scholar] [CrossRef]
- Yeoh, J.G.C.; Pandit, A.A.; Zandawala, M.; Nassel, D.R.; Davies, S.A.; Dow, J.A.T. DINeR: Database for insect neuropeptide research. Insect. Biochem. Mol. Biol. 2017, 86, 9–19. [Google Scholar] [CrossRef]
- Altstein, M.; Nassel, D.R. Neuropeptide signaling in insects. Adv. Exp. Med. Biol. 2010, 692, 155–165. [Google Scholar] [CrossRef]
- Audsley, N.; Down, R.E. G protein coupled receptors as targets for next generation pesticides. Insect. Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef]
- Birgul Iyison, N.; Shahraki, A.; Kahveci, K.; Duzgun, M.B.; Gun, G. Are insect GPCRs ideal next-generation pesticides: Opportunities and challenges. FEBS J. 2021, 288, 2727–2745. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Wang, Y.; Liu, S. G-Protein Coupled Receptors (GPCRs) in Insects-A Potential Target for New Insecticide Development. Molecules 2021, 26, 2993. [Google Scholar] [CrossRef]
- Coligan, J.E.; Dunn, B.M.; Ploegh, H.L.; Speicher, D.W.; Wingfield, P.T. Current Protocols in Protein Science; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Geary, T.G. Nonpeptide ligands for peptidergic G protein-coupled receptors. Adv. Exp. Med. Biol. 2010, 692, 10–26. [Google Scholar] [CrossRef]
- Grimmelikhuijzen, C.J.; Cazzamali, G.; Williamson, M.; Hauser, F. The promise of insect genomics. Pest. Manag. Sci. 2007, 63, 413–416. [Google Scholar] [CrossRef]
- Meyer, J.M.; Ejendal, K.F.; Avramova, L.V.; Garland-Kuntz, E.E.; Giraldo-Calderon, G.I.; Brust, T.F.; Watts, V.J.; Hill, C.A. A “genome-to-lead” approach for insecticide discovery: Pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors. PLoS Negl. Trop. Dis. 2012, 6, e1478. [Google Scholar] [CrossRef]
- Choi, M.Y. Pheromone Biosynthesis Activating Neuropeptide (PBAN) in Insects. Korean J. Appl. Entomol. 2022, 61, 15–28. [Google Scholar] [CrossRef]
- Jurenka, R. The PRXamide neuropeptide signalling system: Conserved in animals. Adv. Insect Physiol. 2015, 49, 123–170. [Google Scholar] [CrossRef]
- Choi, M.Y.; Vander Meer, R.K. GPCR-Based Bioactive Peptide Screening Using Phage-Displayed Peptides and an Insect Cell System for Insecticide Discovery. Biomolecules 2021, 11, 583. [Google Scholar] [CrossRef]
- Gui, S.H.; Taning, C.N.; De Schutter, K.; Yang, Q.; Chen, P.; Hamshou, M.; Nachman, R.J.; Pandit, A.A.; Dow, J.A.; Davies, S.; et al. Assessment of insecticidal effects and selectivity of CAPA-PK peptide analogues against the peach-potato aphid and four beneficial insects following topical exposure. Pest. Manag. Sci. 2020, 76, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Nachman, R.J.; Gui, S.H.; Piot, N.; Kaczmarek, K.; Zabrocki, J.; Dow, J.A.T.; Davies, S.A.; Smagghe, G. Efficacy and biosafety assessment of neuropeptide CAPA analogues against the peach-potato aphid (Myzus persicae). Insect. Sci. 2022, 29, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Fleites, L.A.; Johnson, R.; Kruse, A.R.; Nachman, R.J.; Hall, D.G.; MacCoss, M.; Heck, M.L. Peptidomics Approaches for the Identification of Bioactive Molecules from Diaphorina citri. J. Proteome Res. 2020, 19, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wulff, J.P.; Nachman, R.J.; Pietrantonio, P.V. Myotropic Activities of Tick Pyrokinin Neuropeptides and Analog in Feeding Tissues of Hard Ticks (Ixodidae). Front. Physiol. 2021, 12, 826399. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Y.; Nachman, R.J.; Pietrantonio, P.V. Tick CAPA propeptide cDNAs and receptor activity of endogenous tick pyrokinins and analogs: Towards discovering pyrokinin function in ticks. Peptides 2021, 146, 170665. [Google Scholar] [CrossRef]
- Ahn, S.J.; Mc Donnell, R.J.; Corcoran, J.A.; Martin, R.C.; Choi, M.Y. Identification and functional characterization of the first molluscan neuromedin U receptor in the slug, Deroceras reticulatum. Sci. Rep. 2020, 10, 22308. [Google Scholar] [CrossRef]
- Lard, C.F.; Schmidt, J.; Morris, B.; Estes, L.; Ryan, C.; Bergquist, D. An Economic Impact of Imported Fire Ants in the United States of America; Texas A&M University: College Station, TX, USA, 2006; p. 22. [Google Scholar]
- Choi, M.Y.; Fuerst, E.J.; Rafaeli, A.; Jurenka, R. Role of extracellular domains in PBAN/pyrokinin GPCRs from insects using chimera receptors. Insect. Biochem. Mol. Biol. 2007, 37, 296–306. [Google Scholar] [CrossRef]
- Choi, M.Y.; Jurenka, R.A. Site-directed mutagenesis and PBAN activation of the Helicoverpa zea PBAN-receptor. FEBS Lett. 2010, 584, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Vander Meer, R.K. Ant trail pheromone biosynthesis is triggered by a neuropeptide hormone. PLoS ONE 2012, 7, e50400. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehniger Pricinpals of Biochemistry, 3rd ed.; Worth Publishers: Belper, UK, 2000. [Google Scholar]
- Altstein, M. Novel insect control agents based on neuropeptide antagonists: The PK/PBAN family as a case study. J. Mol. Neurosci. 2004, 22, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.; Highsmith, W.E., Jr. Phage display technology: Clinical applications and recent innovations. Clin. Biochem. 2002, 35, 425–445. [Google Scholar] [CrossRef]
- Molek, P.; Strukelj, B.; Bratkovic, T. Peptide phage display as a tool for drug discovery: Targeting membrane receptors. Molecules 2011, 16, 857–887. [Google Scholar] [CrossRef]
- Chen, S.; Rentero Rebollo, I.; Buth, S.A.; Morales-Sanfrutos, J.; Touati, J.; Leiman, P.G.; Heinis, C. Bicyclic peptide ligands pulled out of cysteine-rich peptide libraries. J. Am. Chem. Soc. 2013, 135, 6562–6569. [Google Scholar] [CrossRef]
- Schultheiss, P.; Nooten, S.S.; Wang, R.; Wong, M.K.L.; Brassard, F.; Guenard, B. The abundance, biomass, and distribution of ants on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2201550119. [Google Scholar] [CrossRef]
- Choi, M.Y.; Vander Meer, R.K. Identification of a new member of the PBAN family of neuropeptides from the fire ant, Solenopsis invicta. Insect. Mol. Biol. 2009, 18, 161–169. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Morel, L. Oviposition Process of Solenopsis invicta (Hymenoptera: Formicidae) Queens. Ann. Entomol. Soc. Am. 2007, 100, 758–762. [Google Scholar] [CrossRef]
- Banks, W.A.; Lofgren, C.S.; Jouvenaz, D.P.; Stringer, C.E.; Bishop, P.M.; Williams, D.F.; Wojcik, D.P.; Glancey, B.M. Techniques for Collecting, Rearing, and Handling Imported Fire Ants; USDA, SEA, AATS-S-21: Washington, DC, USA, 1981; 9p. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinta, S.; Vander Meer, R.; O’Reilly, E.; Choi, M.-Y. Insecticidal Effects of Receptor-Interference Isolated Bioactive Peptides on Fire Ant Colonies. Int. J. Mol. Sci. 2023, 24, 13978. https://doi.org/10.3390/ijms241813978
Chinta S, Vander Meer R, O’Reilly E, Choi M-Y. Insecticidal Effects of Receptor-Interference Isolated Bioactive Peptides on Fire Ant Colonies. International Journal of Molecular Sciences. 2023; 24(18):13978. https://doi.org/10.3390/ijms241813978
Chicago/Turabian StyleChinta, Satya, Robert Vander Meer, Erin O’Reilly, and Man-Yeon Choi. 2023. "Insecticidal Effects of Receptor-Interference Isolated Bioactive Peptides on Fire Ant Colonies" International Journal of Molecular Sciences 24, no. 18: 13978. https://doi.org/10.3390/ijms241813978




