Comparative Analysis of Transcriptome and Proteome Revealed the Common Metabolic Pathways Induced by Prevalent ESBL Plasmids in Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Fitness and Persistence of Prevalent MDR Plasmids
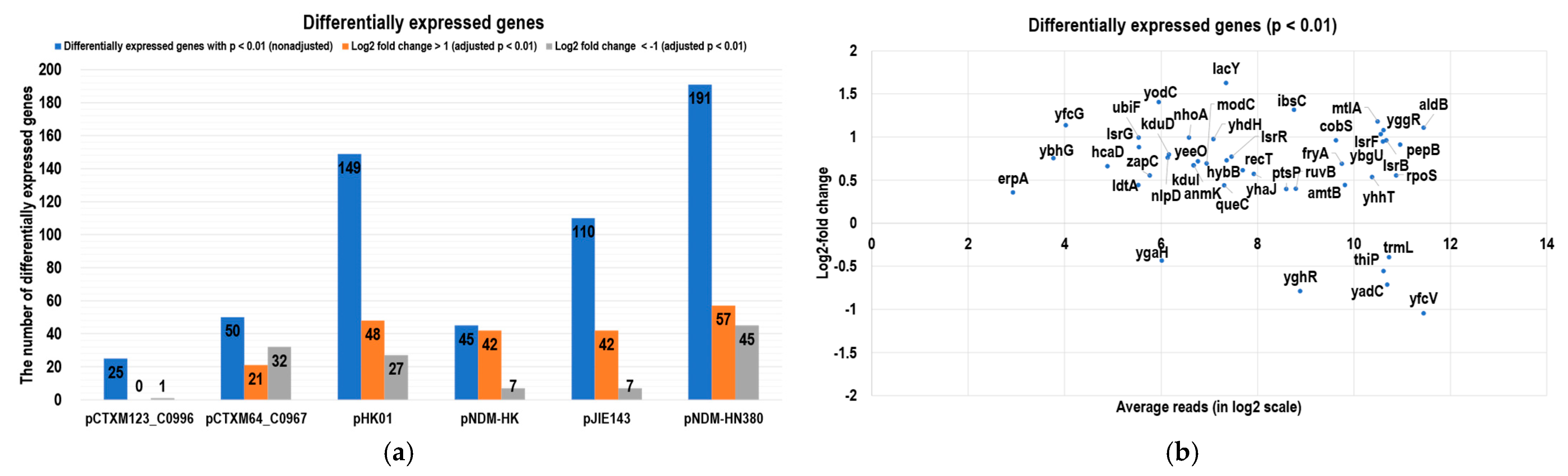
2.2. RNomics Study to Evaluate the Impact of MDR Plasmids on the Host
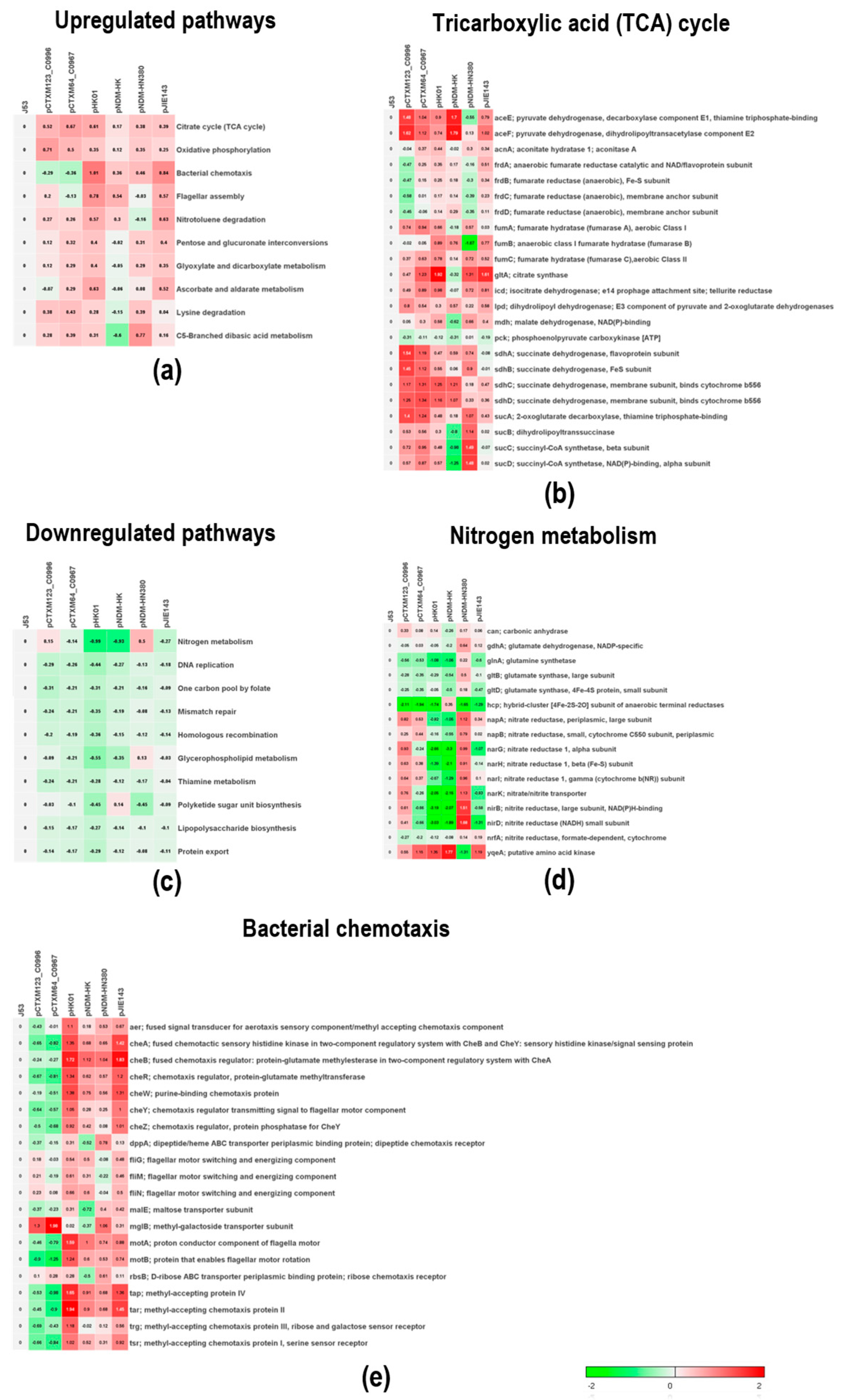
2.3. The Physiological Impacts of Differentially Expressed Genes Using Sample-Level Enrichment Analysis (SLEA)
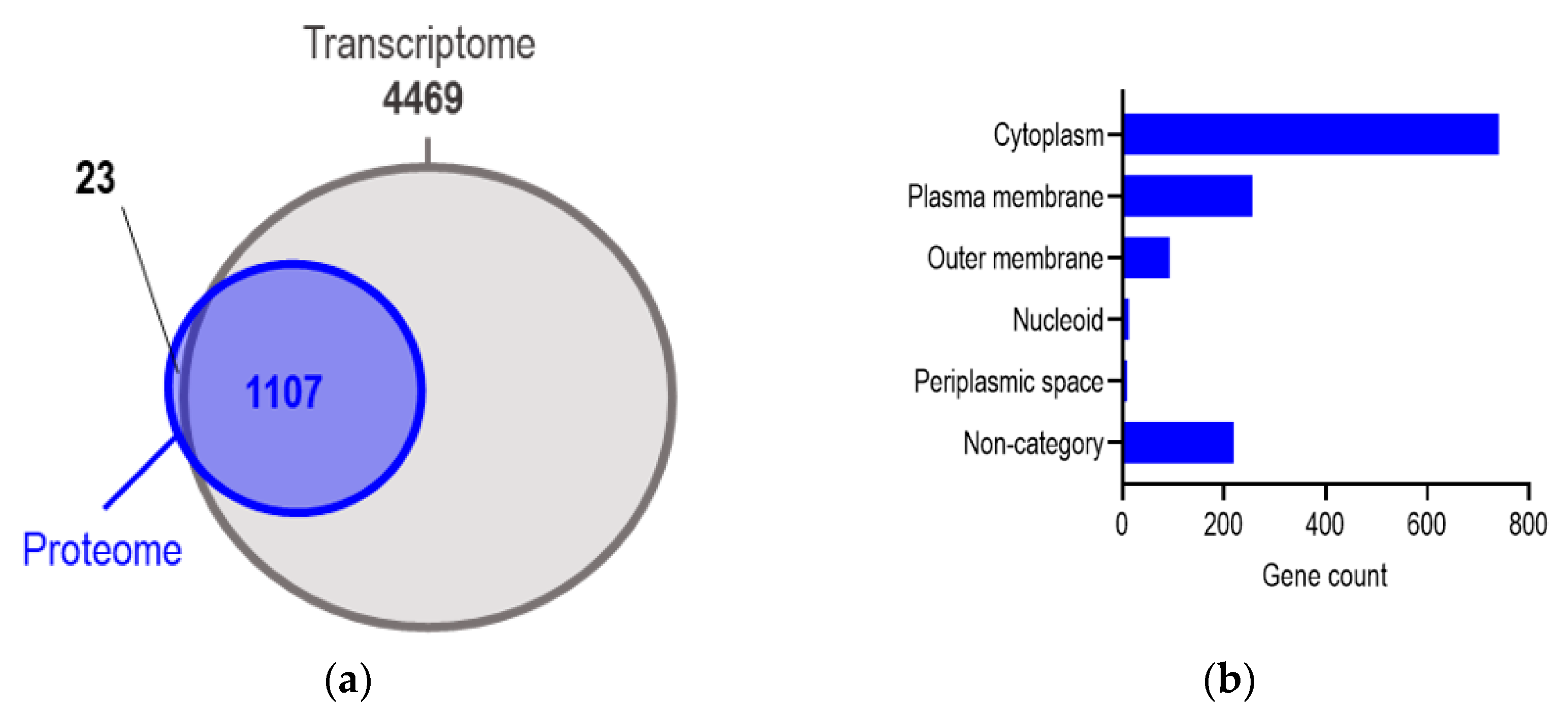
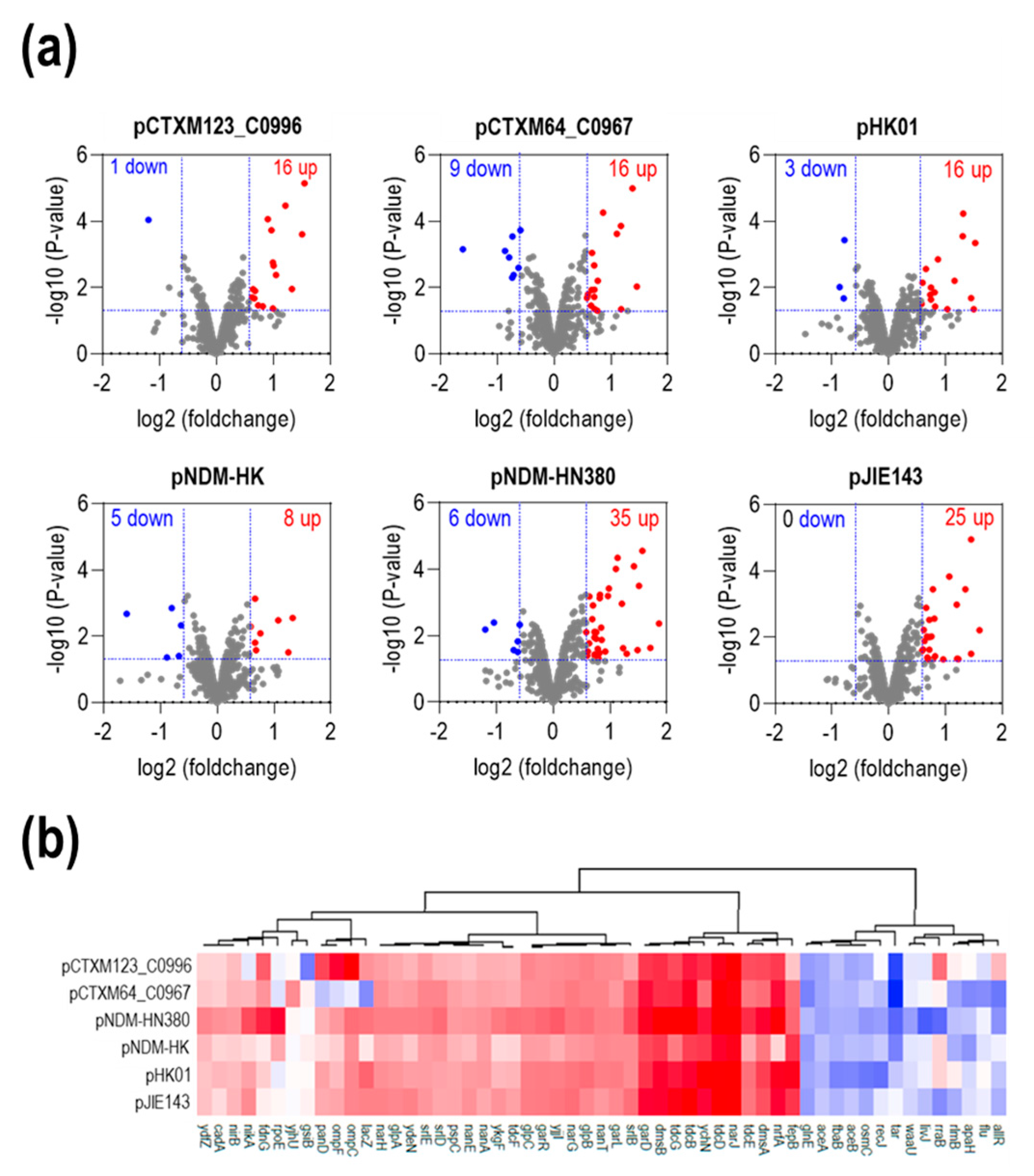
2.4. Mass Spectrometry Revealed the Variation in Proteomes from Bacteria Carrying Different MDR Plasmids
2.5. The Presence of ESBL Conjugative Plasmids Increased the Metabolic Pathway Related to Carbon Utilization at the Translational Level
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids Used in This Study
4.2. Growth and Doubling Time Measurement
4.3. Plasmid Stability Assay
4.4. RNA Isolation
4.5. Sequencing and Bioinformatic Analysis
4.6. Filter-Aided Sample Preparation
4.7. On-Filter Trypsin Digestion
4.8. High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry (HPLC-MS)
4.9. Data Processing
4.10. Protein Quantitation and Analysis
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akter, S.; Chowdhury, A.; Mina, S.A. Antibiotic resistance and plasmid profiling of Escherichia coli isolated from human sewage samples. Microbiol. Insights 2021, 14, 11786361211016808. [Google Scholar] [CrossRef]
- Uma, B.; Prabhakar, K.; Rajendran, S.; Kavitha, K.; Sarayu, Y.L. Antibiotic sensitivity and plasmid profiles of Escherichia coli isolated from pediatric diarrhea. J. Glob. Infect. Dis. 2009, 1, 107–110. [Google Scholar] [CrossRef]
- Palomino, A.; Gewurz, D.; DeVine, L.; Zajmi, U.; Moralez, J.; Abu-Rumman, F.; Smith, R.P.; Lopatkin, A.J. Metabolic genes on conjugative plasmids are highly prevalent in Escherichia coli and can protect against antibiotic treatment. ISME J. 2023, 17, 151–162. [Google Scholar] [CrossRef]
- Carrilero, L.; Dunn, S.J.; Moran, R.A.; McNally, A.; Brockhurst, M.A. Evolutionary responses to acquiring a multidrug resistance plasmid are dominated by metabolic functions across diverse Escherichia coli lineages. mSystems 2023, 8, e00713–e00722. [Google Scholar] [CrossRef]
- Top, E.M.; Springael, D. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 2003, 14, 262–269. [Google Scholar] [CrossRef]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- Nuti, M.P.; Lepidi, A.A.; Prakash, R.K.; Schilperoort, R.A.; Cannon, F.C. Evidence for nitrogen fixation (nif) genes on indigenous rhizobium plasmids. Nature 1979, 282, 533–535. [Google Scholar] [CrossRef]
- Buchrieser, C.; Glaser, P.; Rusniok, C.; Nedjari, H.; D’Hauteville, H.; Kunst, F.; Sansonetti, P.; Parsot, C. The virulence plasmid pwr100 and the repertoire of proteins secreted by the type iii secretion apparatus of shigella flexneri. Mol. Microbiol. 2000, 38, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Sabater-Munoz, B.; Perez-Brocal, V.; Silva, F.J.; Latorre, A. Plasmids in the aphid endosymbiont buchnera aphidicola with the smallest genomes. A puzzling evolutionary story. Gene 2006, 370, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Holten, K.B.; Onusko, E.M. Appropriate prescribing of oral beta-lactam antibiotics. Am. Fam. Physician 2000, 62, 611–620. [Google Scholar] [PubMed]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Svara, F.; Rankin, D.J. The evolution of plasmid-carried antibiotic resistance. BMC Evol. Biol. 2011, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, C.T.; Feldgarden, M. The ecology and evolution of antibiotic-resistant bacteria. Evol. Health Dis. 2008, 2, 469–476. [Google Scholar]
- Lipsitch, M. The rise and fall of antimicrobial resistance. Trends Microbiol. 2001, 9, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Falkow, S.; Citarella, R.; Wohlhieter, J.; Watanabe, T. The molecular nature of r-factors. J. Mol. Biol. 1966, 17, 102–116. [Google Scholar] [CrossRef]
- Sugino, Y.; Hirota, Y. Conjugal fertility associated with resistance factor r in Escherichia coli. J. Bacteriol. 1962, 84, 902–910. [Google Scholar] [CrossRef]
- Livermore, D.M. Beta-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995, 8, 557–584. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. Rna sequencing and analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Baharoglu, Z.; Bikard, D.; Mazel, D. Conjugative DNA transfer induces the bacterial sos response and promotes antibiotic resistance development through integron activation. PLoS Genet. 2010, 6, e1001165. [Google Scholar] [CrossRef]
- Foster, P.L. Stress-induced mutagenesis in bacteria. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 373–397. [Google Scholar] [CrossRef]
- Vogel, J.; Bartels, V.; Tang, T.H.; Churakov, G.; Slagter-Jager, J.G.; Huttenhofer, A.; Wagner, E.G. Rnomics in Escherichia coli detects new srna species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003, 31, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.-L.; Cheung, Y.-Y.; Lo, W.-U.; Li, Z.; Chow, K.-H.; Lin, C.-H.; Chan, J.F.-W.; Cheng, V.C.-C. Molecular characterization of an atypical incx3 plasmid pkpc-ny79 carrying blakpc-2 in a klebsiella pneumoniae. Curr. Microbiol. 2013, 67, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Fookes, M.; Ivens, A.; Mangan, M.W.; Wain, J.; Dorman, C.J. An h-ns-like stealth protein aids horizontal DNA transmission in bacteria. Science 2007, 315, 251–252. [Google Scholar] [CrossRef]
- Yeh, J.I.; Chinte, U.; Du, S. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 3280. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.B.; Scoffield, J.; Woolnough, J.L.; Silo-Suh, L. Impact of glycerol-3-phosphate dehydrogenase on virulence factor production by pseudomonas aeruginosa. Can. J. Microbiol. 2014, 60, 857–863. [Google Scholar] [CrossRef]
- Alteri, C.J.; Smith, S.N.; Mobley, H.L.T. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the tca cycle. PLOS Pathog. 2009, 5, e1000448. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, Y.Q.; Sadykov, M.R.; Fey, P.D.; Lei, M.G.; Lee, C.Y.; Bayer, A.S.; Somerville, G.A. Tricarboxylic acid cycle-dependent attenuation of staphylococcus aureus in vivo virulence by selective inhibition of amino acid transport. Infect. Immun. 2009, 77, 4256–4264. [Google Scholar] [CrossRef]
- Mou, X.; Spinard, E.J.; Hillman, S.L.; Nelson, D.R. Isocitrate dehydrogenase mutation in vibrio anguillarum results in virulence attenuation and immunoprotection in rainbow trout (oncorhynchus mykiss). BMC Microbiol. 2017, 17, 217. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Wu, Y.; Datta, P. Involvement of fnr and arca in anaerobic expression of the tdc operon of Escherichia coli. J. Bacteriol. 1997, 179, 4868–4873. [Google Scholar] [CrossRef]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar]
- Vestergaard, M.; Nøhr-Meldgaard, K.; Bojer, M.S.; Krogsgård Nielsen, C.; Meyer, R.L.; Slavetinsky, C.; Peschel, A.; Ingmer, H. Inhibition of the atp synthase eliminates the intrinsic resistance of Staphylococcus aureus towards polymyxins. mBio 2017, 8, e01114-17. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Martínez, J.L. Interventions on metabolism: Making antibiotic-susceptible bacteria. mBio 2017, 8, e01950-17. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.S.; Storz, G. Regulatory rnas in bacteria. Cell 2009, 136, 615–628. [Google Scholar]
- Chang, D.E.; Smalley, D.J.; Tucker, D.L.; Leatham, M.P.; Norris, W.E.; Stevenson, S.J.; Anderson, A.B.; Grissom, J.E.; Laux, D.C.; Cohen, P.S.; et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 2004, 101, 7427–7432. [Google Scholar] [CrossRef]
- Faber, F.; Tran, L.; Byndloss, M.X.; Lopez, C.A.; Velazquez, E.M.; Kerrinnes, T.; Nuccio, S.P.; Wangdi, T.; Fiehn, O.; Tsolis, R.M.; et al. ; et al. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 2016, 534, 697–699. [Google Scholar] [CrossRef]
- Kitamoto, S.; Alteri, C.J.; Rodrigues, M.; Nagao-Kitamoto, H.; Sugihara, K.; Himpsl, S.D.; Bazzi, M.; Miyoshi, M.; Nishioka, T.; Hayashi, A.; et al. Dietary l-serine confers a competitive fitness advantage to enterobacteriaceae in the inflamed gut. Nat. Microbiol. 2020, 5, 116–125. [Google Scholar] [CrossRef]
- Sarmiento-Rubiano, L.A.; Zúñiga, M.; Pérez-Martínez, G.; Yebra, M.J. Dietary supplementation with sorbitol results in selective enrichment of lactobacilli in rat intestine. Res. Microbiol. 2007, 158, 694–701. [Google Scholar] [CrossRef]
- Rhodes, M.; Kator, H. Sorbitol-fermenting bifidobacteria as indicators of diffuse human faecal pollution in estuarine watersheds. J. Appl. Microbiol. 1999, 87, 528–535. [Google Scholar] [CrossRef]
- Chen, C.Y.; Buchmeier, N.A.; Libby, S.; Fang, F.C.; Krause, M.; Guiney, D.G. Central regulatory role for the rpos sigma factor in expression of salmonella dublin plasmid virulence genes. J. Bacteriol. 1995, 177, 5303–5309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, C.; Yu, J.; Ren, J.; Sun, D. Rpos regulates a novel type of plasmid DNA transfer in Escherichia coli. PLoS ONE 2012, 7, e33514. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.; Carrilero, L.; Brockhurst, M.; McNally, A. Limited and strain-specific transcriptional and growth responses to acquisition of a multidrug resistance plasmid in genetically diverse Escherichia coli lineages. mSystems 2021, 6, e00083-21. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Cho, Y.J.; Yong, D.; Chun, J. Genome sequence of Escherichia coli j53, a reference strain for genetic studies. J. Bacteriol. 2012, 194, 3742–3743. [Google Scholar] [CrossRef]
- Godwin, D.; Slater, J.H. The influence of the growth environment on the stability of a drug resistance plasmid in Escherichia coli k12. J. Gen. Microbiol. 1979, 111, 201–210. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Krzywinski, M.I.; Schein, J.E.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]


| Name | pCTXM123_C0996 | pCTXM64_C0967 | pHK01 | pNDM-HK | pNDM-HN380 | pJIE143 |
|---|---|---|---|---|---|---|
| Inc Group | I1 | I2 | FII | M2 | X3 | X4 |
| Size | 108 Kb | 62 Kb | 70 Kb | 89 Kb | 54 Kb | 34 Kb |
| Source | Enterobacteriaceae | Enterobacteriaceae | Enterobacteriaceae | Enterobacteriaceae | Enterobacteriaceae | Enterobacteriaceae |
| Resistance Gene | blaCTX-M-123 | blaCTX-M-64 | blaCTX-M-14 | blaTEM-1 blaNDM-1 blaDHA-1 sul1 qacdelta1 | blaNDM-1 blaSHV-12 bleMBL | blaCTX-M-15 |
| CTX Group | CTX-M-1/9 group hybrids | CTX-M-1/9 group hybrids | CTX-M-9 group | - | - | CTX-M-1 group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Pham, H.-Q.; Zhu, L.; Wang, R.; Law, O.-K.; Lin, S.-L.; Nie, Q.-C.; Zhang, L.; Wang, X.; Lau, T.C.-K. Comparative Analysis of Transcriptome and Proteome Revealed the Common Metabolic Pathways Induced by Prevalent ESBL Plasmids in Escherichia coli. Int. J. Mol. Sci. 2023, 24, 14009. https://doi.org/10.3390/ijms241814009
Huang C, Pham H-Q, Zhu L, Wang R, Law O-K, Lin S-L, Nie Q-C, Zhang L, Wang X, Lau TC-K. Comparative Analysis of Transcriptome and Proteome Revealed the Common Metabolic Pathways Induced by Prevalent ESBL Plasmids in Escherichia coli. International Journal of Molecular Sciences. 2023; 24(18):14009. https://doi.org/10.3390/ijms241814009
Chicago/Turabian StyleHuang, Chuan, Hoa-Quynh Pham, Lina Zhu, Rui Wang, Oi-Kwan Law, Shu-Ling Lin, Qi-Chang Nie, Liang Zhang, Xin Wang, and Terrence Chi-Kong Lau. 2023. "Comparative Analysis of Transcriptome and Proteome Revealed the Common Metabolic Pathways Induced by Prevalent ESBL Plasmids in Escherichia coli" International Journal of Molecular Sciences 24, no. 18: 14009. https://doi.org/10.3390/ijms241814009





