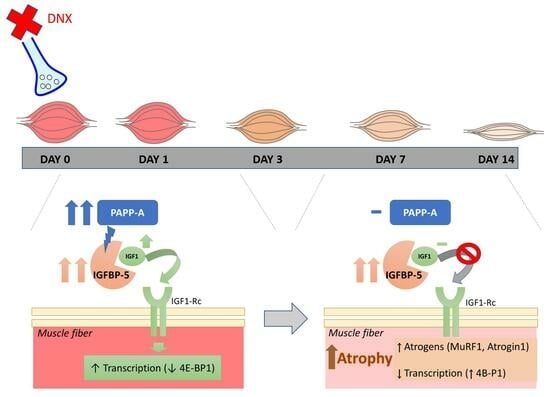
Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation
Abstract
:1. Introduction
2. Results
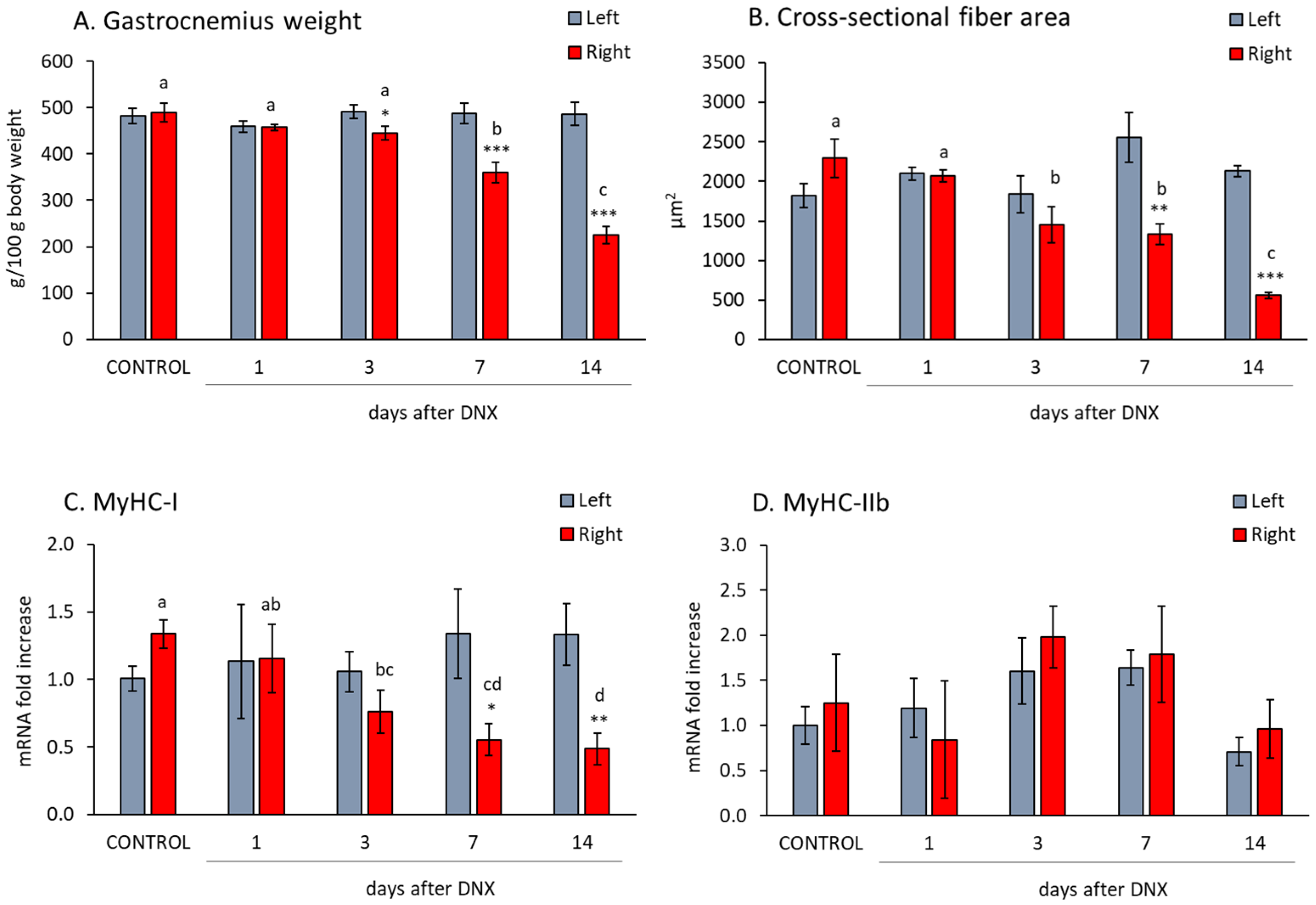
2.1. Denervation Effect on Gastrocnemius Muscle Atrophy and Myofiber Isoform Expression
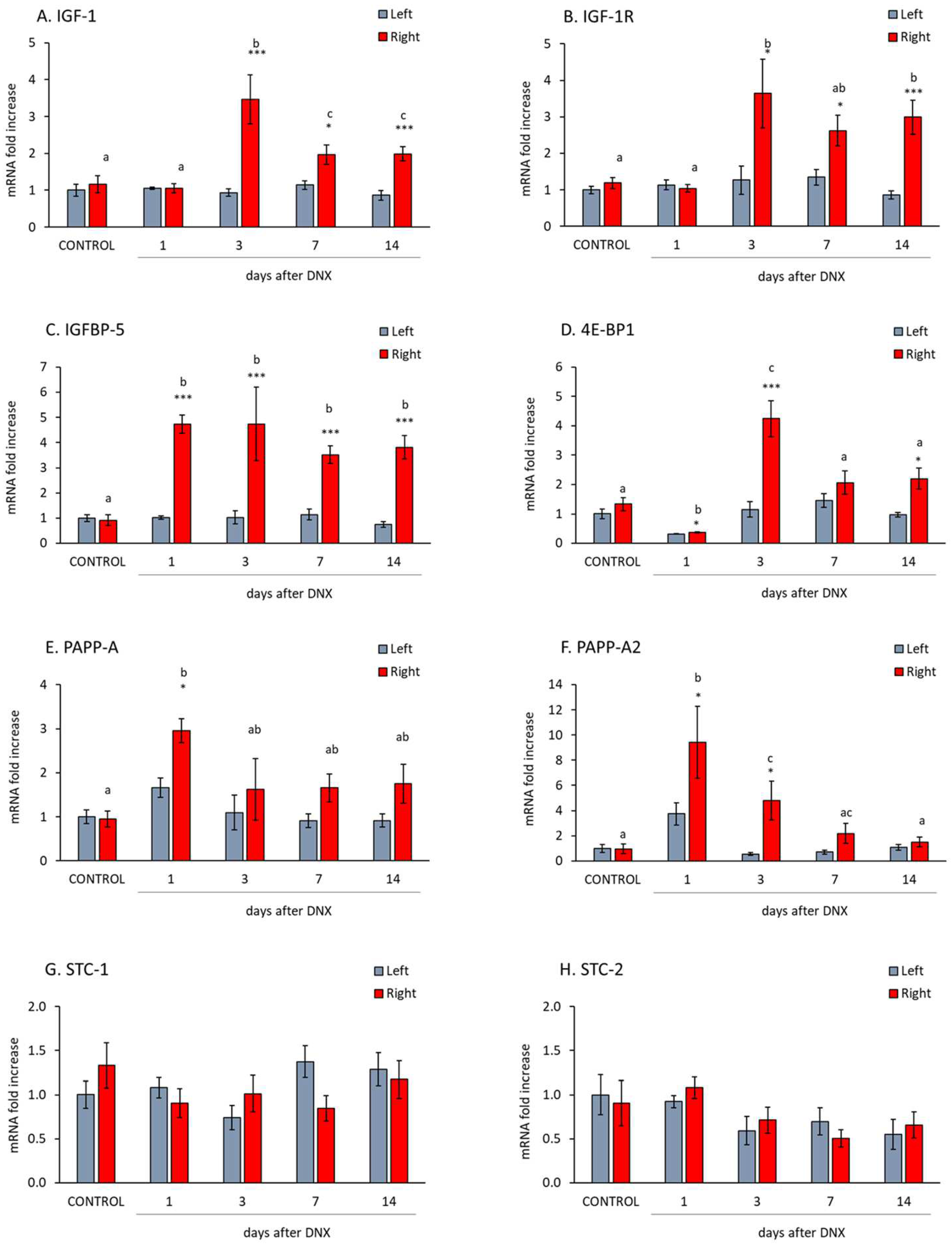
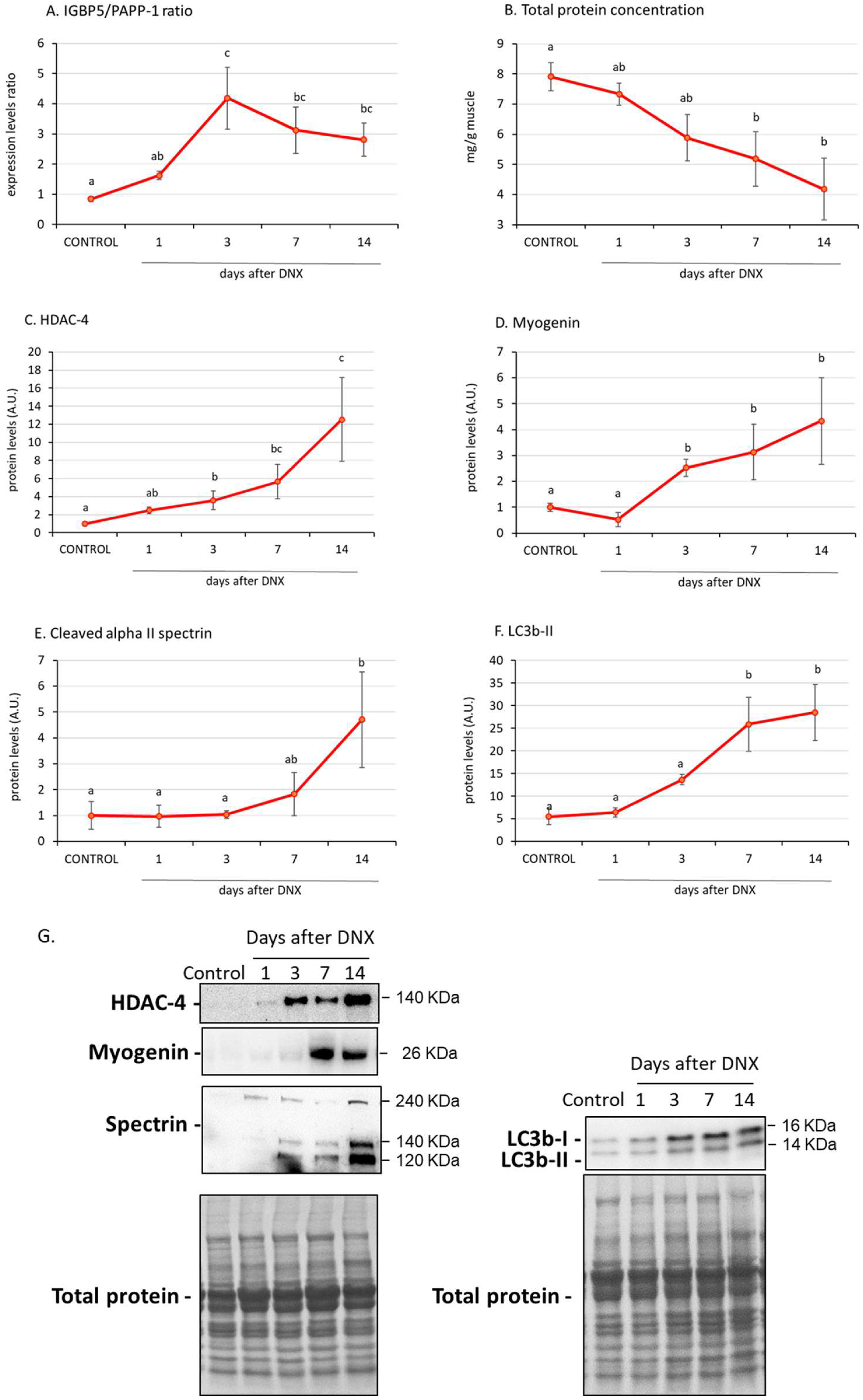
2.2. Denervation Effect on the IGF1-IGFBP5-PAPP System
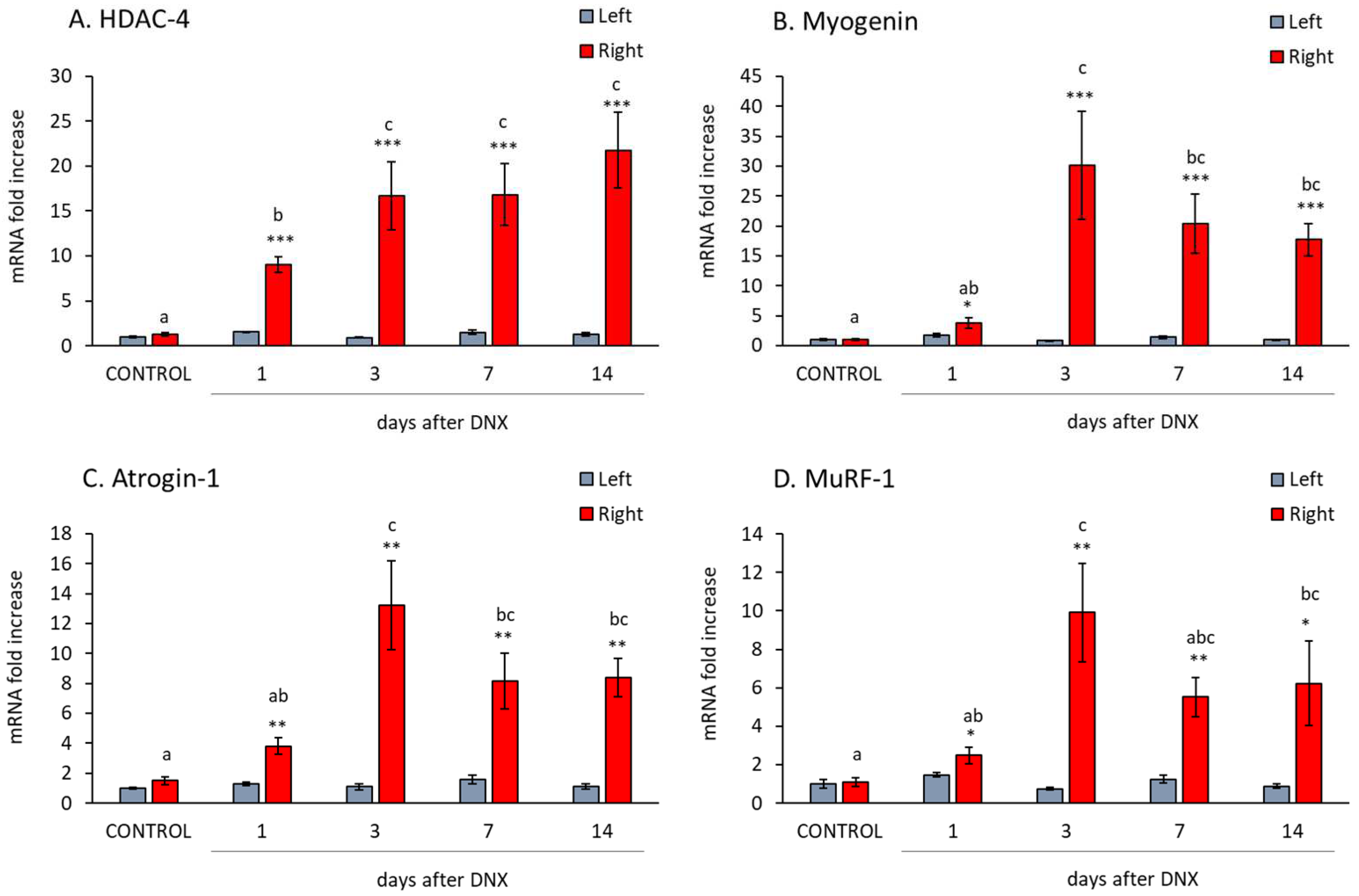
2.3. Denervation Effect on Atrophy Mediators
2.4. IGFBP-5/PAPP-A Correlations with Atrophic Mediators
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Skeletal Muscle Histology
4.3. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.4. Western Blot
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance—A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Kostrominova, T.Y. Skeletal Muscle Denervation: Past, Present and Future. Int. J. Mol. Sci. 2022, 23, 7489. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, R.; Xu, L.; Wan, Q.; Zhu, J.; Gu, J.; Huang, Z.; Ma, W.; Shen, M.; Ding, F.; et al. Microarray Analysis of Gene Expression Provides New Insights Into Denervation-Induced Skeletal Muscle Atrophy. Front. Physiol. 2019, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qiu, J.; Chen, Y.; Yu, M.; Ding, F.; Gu, X. Proteomic and bioinformatic analysis of differentially expressed proteins in denervated skeletal muscle. Int. J. Mol. Med. 2014, 33, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology 2005, 20, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Liu, J.L.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Schulze, P.C.; Gielen, S.; Linke, A.; Mobius-Winkler, S.; Erbs, S.; Kratzsch, J.; Schubert, A.; Adams, V.; Schuler, G. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 401–406. [Google Scholar] [CrossRef]
- Spradlin, R.A.; Vassilakos, G.; Matheny, M.K.; Jones, N.C.; Goldman, J.L.; Lei, H.; Barton, E.R. Deletion of muscle Igf1 exacerbates disuse atrophy weakness in mice. J. Appl. Physiol. 2021, 131, 881–894. [Google Scholar] [CrossRef]
- Bunn, R.C.; Fowlkes, J.L. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol. Metab. 2003, 14, 176–181. [Google Scholar] [CrossRef]
- Guler, H.P.; Zapf, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol. 1989, 121, 753–758. [Google Scholar] [CrossRef]
- Duan, C.; Ren, H.; Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010, 167, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Gosteli-Peter, M.A.; Winterhalter, K.H.; Schmid, C.; Froesch, E.R.; Zapf, J. Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology 1994, 135, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Allard, J.B. Insulin-like Growth Factor Binding Protein-5 in Physiology and Disease. Front. Endocrinol. 2020, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E.; Abraha, T.; Childs, T.E.; Pattison, J.S.; Booth, F.W. Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E340–E350. [Google Scholar] [CrossRef] [PubMed]
- Awede, B.; Thissen, J.; Gailly, P.; Lebacq, J. Regulation of IGF-I, IGFBP-4 and IGFBP-5 gene expression by loading in mouse skeletal muscle. FEBS Lett. 1999, 461, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1656–E1665. [Google Scholar] [CrossRef] [PubMed]
- González-Hedstrom, D.; Priego, T.; López-Calderón, A.; Amor, S.; de la Fuente-Fernández, M.; Inarejos-García, A.M.; García-Villalón, A.L.; Martín, A.I.; Granado, M. Beneficial Effects of a Mixture of Algae and Extra Virgin Olive Oils on the Age-Induced Alterations of Rodent Skeletal Muscle: Role of HDAC-4. Nutrients 2020, 13, 44. [Google Scholar] [CrossRef]
- Bayol, S.; Loughna, P.T.; Brownson, C. Phenotypic expression of IGF binding protein transcripts in muscle, in vitro and in vivo. Biochem. Biophys. Res. Commun. 2000, 273, 282–286. [Google Scholar] [CrossRef]
- Lawrence, J.B.; Oxvig, C.; Overgaard, M.T.; Sottrup-Jensen, L.; Gleich, G.J.; Hays, L.G.; Yates, J.R., 3rd; Conover, C.A. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc. Natl. Acad. Sci. USA 1999, 96, 3149–3153. [Google Scholar] [CrossRef]
- Laursen, L.S.; Overgaard, M.T.; Soe, R.; Boldt, H.B.; Sottrup-Jensen, L.; Giudice, L.C.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001, 504, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, M.T.; Boldt, H.B.; Laursen, L.S.; Sottrup-Jensen, L.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J. Biol. Chem. 2001, 276, 21849–21853. [Google Scholar] [CrossRef] [PubMed]
- Argente, J.; Chowen, J.A.; Pérez-Jurado, L.A.; Frystyk, J.; Oxvig, C. One level up: Abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology. EMBO Mol. Med. 2017, 9, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Dauber, A.; Munoz-Calvo, M.T.; Barrios, V.; Domene, H.M.; Kloverpris, S.; Serra-Juhe, C.; Desikan, V.; Pozo, J.; Muzumdar, R.; Martos-Moreno, G.A.; et al. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol. Med. 2016, 8, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mohan, S.; Newton, J.; Rehage, M.; Tran, K.; Baylink, D.J.; Qin, X. Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. J. Biol. Chem. 2005, 280, 37782–37789. [Google Scholar] [CrossRef] [PubMed]
- Rehage, M.; Mohan, S.; Wergedal, J.E.; Bonafede, B.; Tran, K.; Hou, D.; Phang, D.; Kumar, A.; Qin, X. Transgenic overexpression of pregnancy-associated plasma protein-A increases the somatic growth and skeletal muscle mass in mice. Endocrinology 2007, 148, 6176–6185. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Bale, L.K.; Nair, K.S. Comparative gene expression and phenotype analyses of skeletal muscle from aged wild-type and PAPP-A-deficient mice. Exp. Gerontol. 2016, 80, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Reddel, R.R. Identification of a second stanniocalcin cDNA in mouse and human: Stanniocalcin 2. Mol. Cell Endocrinol. 1998, 141, 95–99. [Google Scholar] [CrossRef]
- Jepsen, M.R.; Kloverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Fuchtbauer, E.M.; Laursen, L.S.; Oxvig, C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J. Biol. Chem. 2015, 290, 3430–3439. [Google Scholar] [CrossRef]
- Frystyk, J.; Teran, E.; Gude, M.F.; Bjerre, M.; Hjortebjerg, R. Pregnancy-associated plasma proteins and Stanniocalcin-2—Novel players controlling IGF-I physiology. Growth Horm. IGF Res. 2020, 53–54, 101330. [Google Scholar] [CrossRef]
- Shavlakadze, T.; White, J.D.; Davies, M.; Hoh, J.F.; Grounds, M.D. Insulin-like growth factor I slows the rate of denervation induced skeletal muscle atrophy. Neuromuscul. Disord. 2005, 15, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.K.; Qiao, H.Y.; Fu, M.H.; Li, G.; Li, W.B.; Chen, Z.; Wei, J.; Liang, B.S. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats through Regulation of Satellite Cell Differentiation via TGF-beta1, Smad3, and HDAC4 Signaling. Med. Sci. Monit. 2016, 22, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Priego, T.; Martín, A.I.; González-Hedstrom, D.; Granado, M.; López-Calderón, A. Role of hormones in sarcopenia. Vitam. Horm. 2021, 115, 535–570. [Google Scholar] [CrossRef] [PubMed]
- Kaymaz, A.Y.; Bal, S.K.; Bora, G.; Talim, B.; Ozon, A.; Alikasifoglu, A.; Topaloglu, H.; Yurter, H.E. Alterations in insulin-like growth factor system in spinal muscular atrophy. Muscle Nerve 2022, 66, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Zeman, R.J.; Zhao, J.; Zhang, Y.; Zhao, W.; Wen, X.; Wu, Y.; Pan, J.; Bauman, W.A.; Cardozo, C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch. 2009, 458, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P.; Schneider, C. Signaling by insulin-like growth factors in paralyzed skeletal muscle: Rapid induction of IGF1 expression in muscle fibers and prevention of interstitial cell proliferation by IGF-BP5 and IGF-BP4. J. Neurosci. 1994, 14, 3378–3388. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Marce, M.; Wee, C.D.; Martinez, T.L.; Lipkes, C.E.; Choe, D.W.; Kong, L.; Van Meerbeke, J.P.; Musaro, A.; Sumner, C.J. Increased IGF-1 in muscle modulates the phenotype of severe SMA mice. Hum. Mol. Genet. 2011, 20, 1844–1853. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Timmer, L.T.; Hoogaars, W.M.H.; Jaspers, R.T. The Role of IGF-1 Signaling in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 109–137. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Argadine, H.M.; Hellyer, N.J.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J. Appl. Physiol. 2009, 107, 438–444. [Google Scholar] [CrossRef]
- Castets, P.; Rion, N.; Theodore, M.; Falcetta, D.; Lin, S.; Reischl, M.; Wild, F.; Guerard, L.; Eickhorst, C.; Brockhoff, M.; et al. mTORC1 and PKB/Akt control the muscle response to denervation by regulating autophagy and HDAC4. Nat. Commun. 2019, 10, 3187. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Sitzmann, J.M.; Dastidar, S.G.; Rodriguez, A.A.; Vu, S.L.; McDonald, C.E.; Academia, E.C.; O’Leary, M.N.; Ashe, T.D.; La Spada, A.R.; et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J. Clin. Investig. 2015, 125, 2952–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xue, P.; Liu, Z.; Li, W.; Li, C.; Chen, Z. SESN2 prevents the slow-to-fast myofiber shift in denervated atrophy via AMPK/PGC-1alpha pathway. Cell. Mol. Biol. Lett. 2022, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Martín, A.I.; Priego, T.; López-Calderón, A.; Villanúa, M.A. Tumour necrosis factor blockade did not prevent the increase of muscular muscle RING finger-1 and muscle atrophy F-box in arthritic rats. J. Endocrinol. 2006, 191, 319–326. [Google Scholar] [CrossRef]
- Salih, D.A.; Tripathi, G.; Holding, C.; Szestak, T.A.; González, M.I.; Carter, E.J.; Cobb, L.J.; Eisemann, J.E.; Pell, J.M. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 4314–4319. [Google Scholar] [CrossRef]
- Cobb, L.J.; Salih, D.A.; González, I.; Tripathi, G.; Carter, E.J.; Lovett, F.; Holding, C.; Pell, J.M. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J. Cell Sci. 2004, 117, 1737–1746. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; McCormick, R.; Molloy, A.P.; McArdle, A.; Goljanek-Whysall, K. Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell 2016, 15, 361–369. [Google Scholar] [CrossRef]
- Granado, M.; Priego, T.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E1007–E1014. [Google Scholar] [CrossRef]
- Ren, H.; Yin, P.; Duan, C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J. Cell Biol. 2008, 182, 979–991. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hwa, V.; Dauber, A. Novel Modulators of the Growth Hormone—Insulin-like Growth Factor Axis: Pregnancy-Associated Plasma Protein-A2 and Stanniocalcin-2. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Gagliardi, A.D.; Kuo, E.Y.; Raulic, S.; Wagner, G.F.; DiMattia, G.E. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E92–E105. [Google Scholar] [CrossRef]
- Chang, A.C.; Hook, J.; Lemckert, F.A.; McDonald, M.M.; Nguyen, M.A.; Hardeman, E.C.; Little, D.G.; Gunning, P.W.; Reddel, R.R. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology 2008, 149, 2403–2410. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Atrogin-1 | GAACAGCAAAACCAAAACTCAGTA | GCTCCTTAGTACTCCCTTTGTGAA |
| HDAC4 | CACACCTCTTGGAGGGTACAA | AGCCCATCAGCTGTTTTGTC |
| IGF-1 | GCTATGGCTCCAGCATTCG | GGATGAGTGTTGCTTCCGGA |
| IGF-1R | GCCTCCAACTTTGTCTTTGC | TCACTGGGCCAGGAATGT |
| IGFBP5 | GGCGAGCAAACCAAGATAGA | GGTCTCCTCAGCCATCTCG |
| MuRF-1 | TGTCTGGAGGTCGTTTCCG | AAGTGATCATGGACCGGCAT |
| MyHC-I | CCAAGGGCCTGAATGAAGAGT | TGTTTCTGCCTAAGGTGCTGT |
| MyHC-IIb | TCCTATTTTCTGGGGACAA | ACCCTTCTTCTTGCCACCTT |
| Myogenin | CCTTGCTCAGCTCCCTCA | TGGGAGTTGCATTCACTGG |
| PAPP-A | CTCCACACAGAGCCTACTTGG | ATTAGGGCCTCCTTGTCCCA |
| PAPP-A2 | CCCTCCCCCATCTGTACTCA | TGGAGTAGATGAGCCCGGAA |
| STC-1 | CAACAGTGCCCTACAGGTT | TCCCATTGGCGATGCACTTT |
| STC-2 | CACCGACCACCACCTAACAG | CTCACTGCTTCCAGAGGGTC |
| 4E-BP1 | ACTAGCCCTACCAGCGATGA | AGCATCACTGCGTCCTATGG |
| 18S | GGTGCATGGCCGTTCTTA | TCGTTCGTTATCGGAATTAACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, A.I.; Moreno-Rupérez, Á.; Nebot, E.; Granado, M.; Jaque, D.; Nieto-Bona, M.P.; López-Calderón, A.; Priego, T. Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation. Int. J. Mol. Sci. 2023, 24, 14112. https://doi.org/10.3390/ijms241814112
Martín AI, Moreno-Rupérez Á, Nebot E, Granado M, Jaque D, Nieto-Bona MP, López-Calderón A, Priego T. Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation. International Journal of Molecular Sciences. 2023; 24(18):14112. https://doi.org/10.3390/ijms241814112
Chicago/Turabian StyleMartín, Ana Isabel, Álvaro Moreno-Rupérez, Elena Nebot, Miriam Granado, Daniel Jaque, M. Paz Nieto-Bona, Asunción López-Calderón, and Teresa Priego. 2023. "Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation" International Journal of Molecular Sciences 24, no. 18: 14112. https://doi.org/10.3390/ijms241814112