Reducing Sialylation Enhances Electrotaxis of Corneal Epithelial Cells
Abstract
:1. Introduction
2. Results
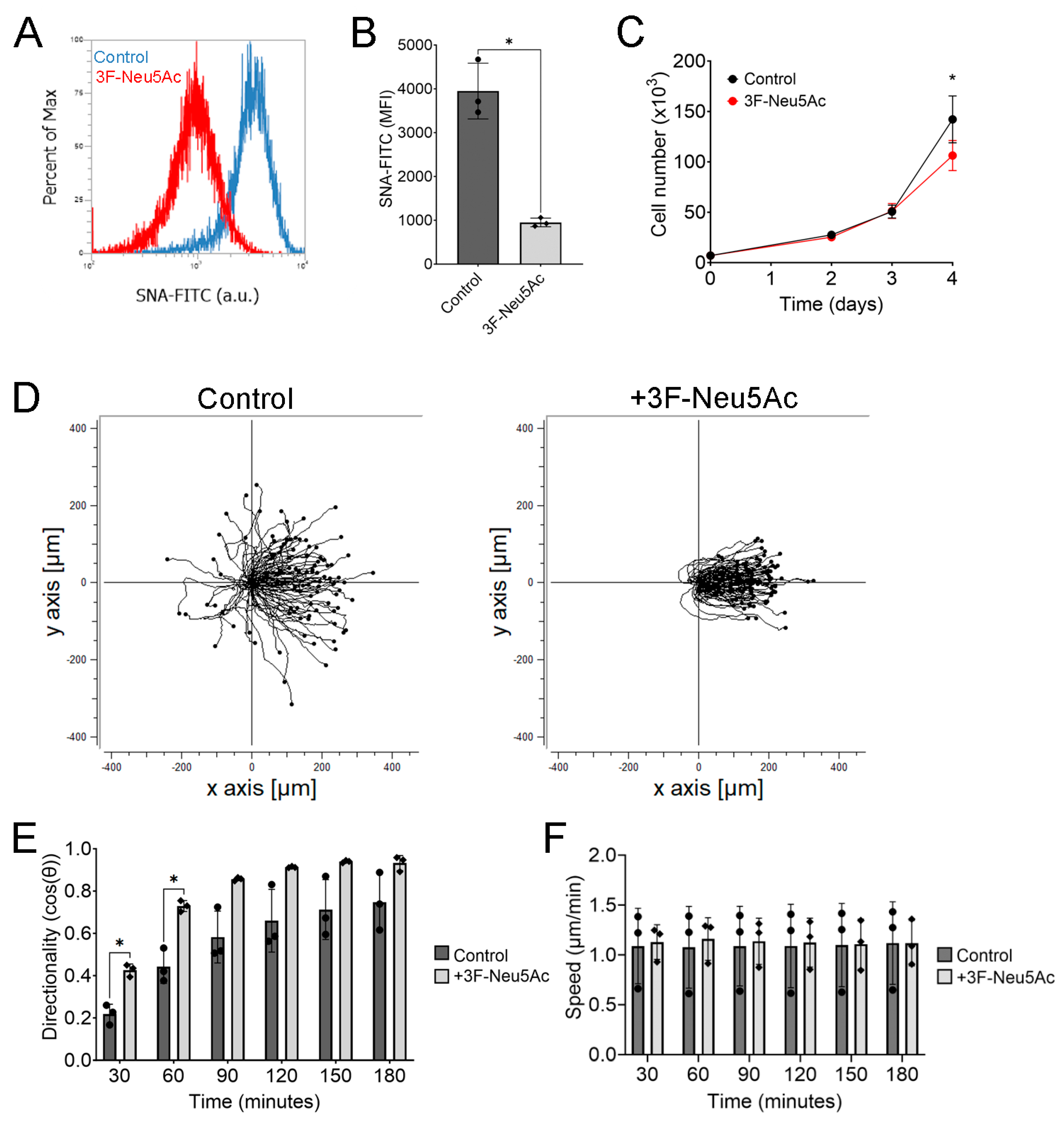
2.1. Reducing Sialylation Enhances Cathodal Migration of hTCEpi Cells
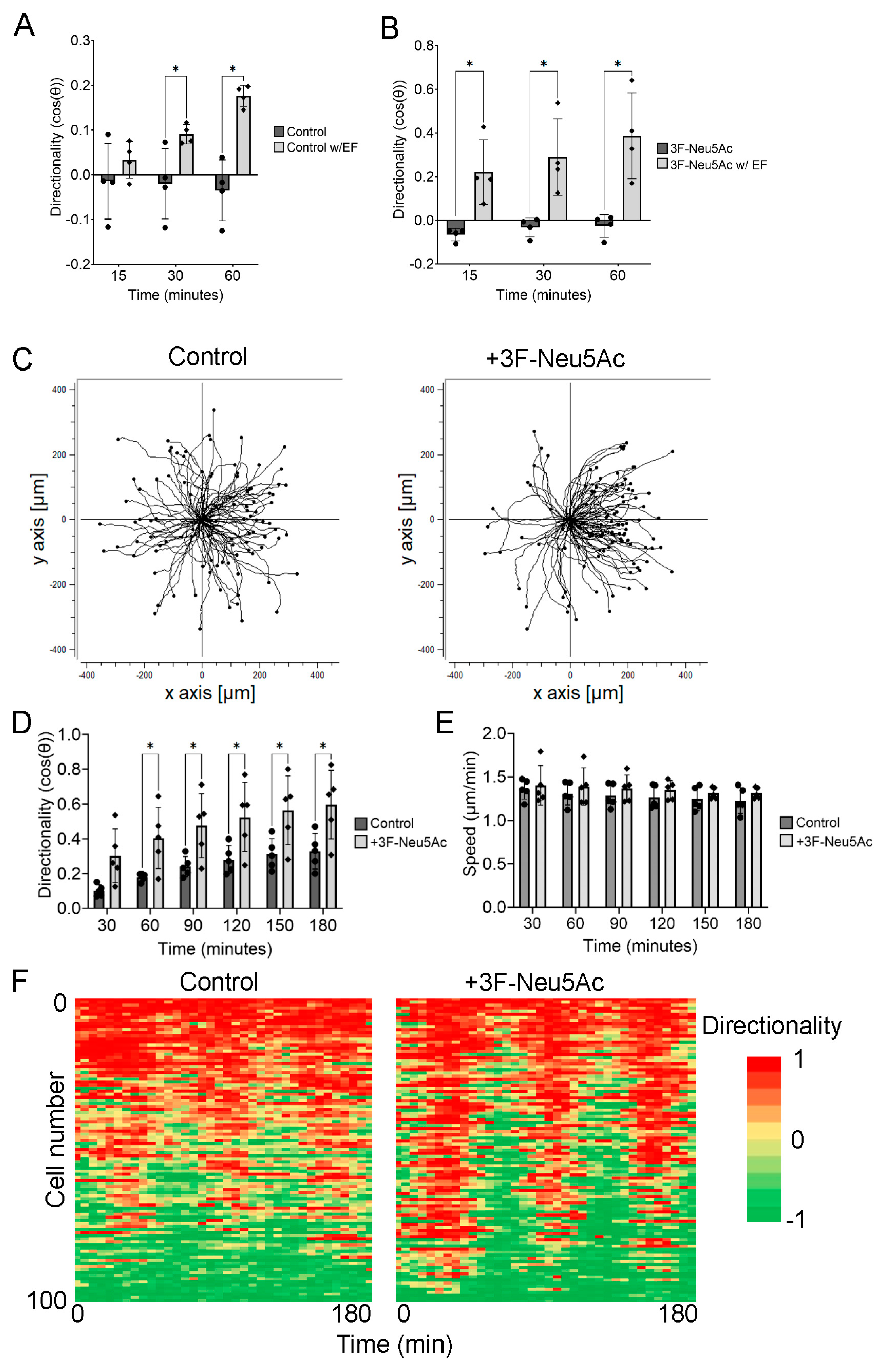
2.2. Reducing Sialylation May Enhance the Sensitivity of hTCEpi Cells to the Electric Field
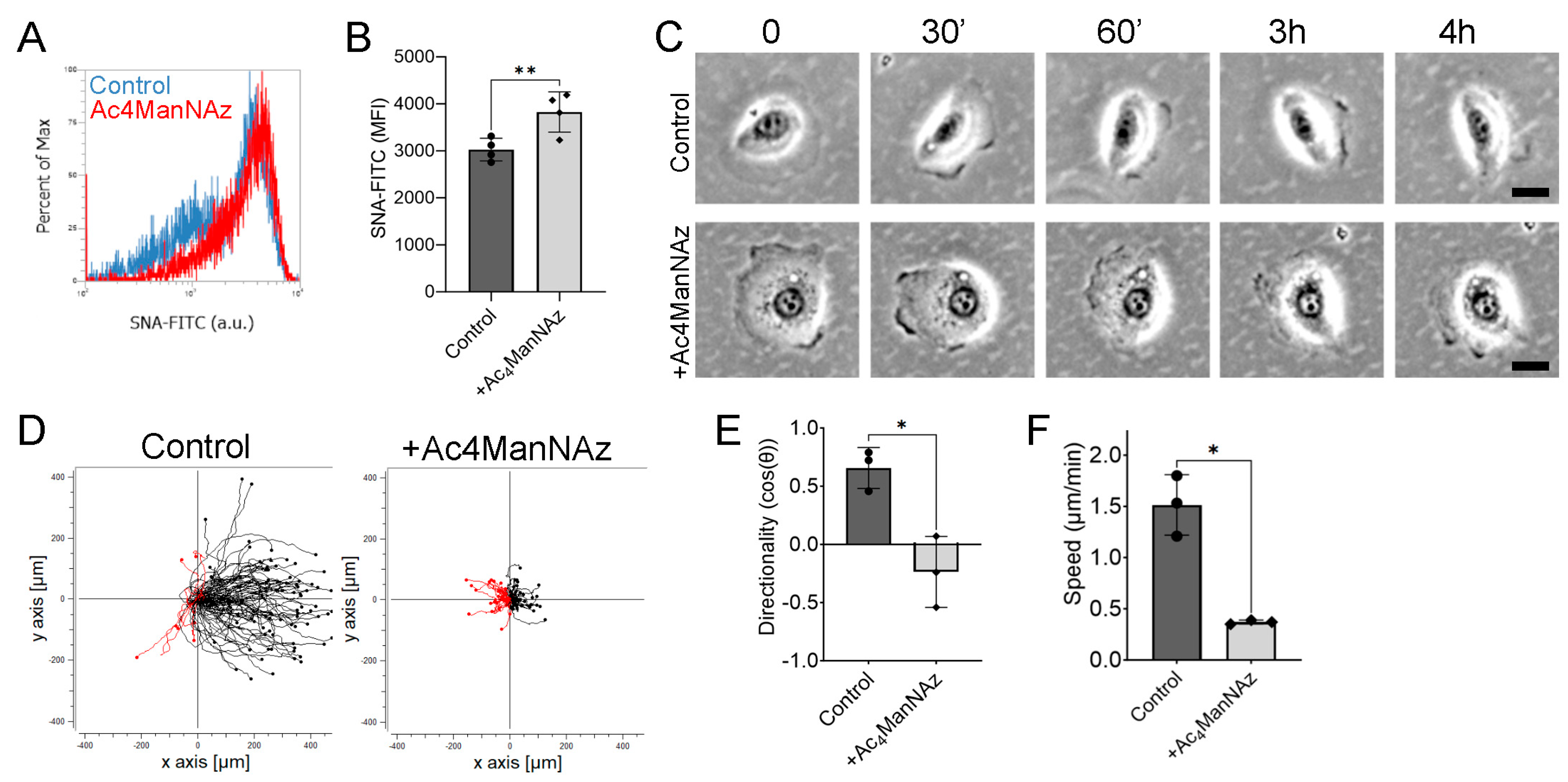
2.3. Increasing Sialylation Impairs Electrotaxis
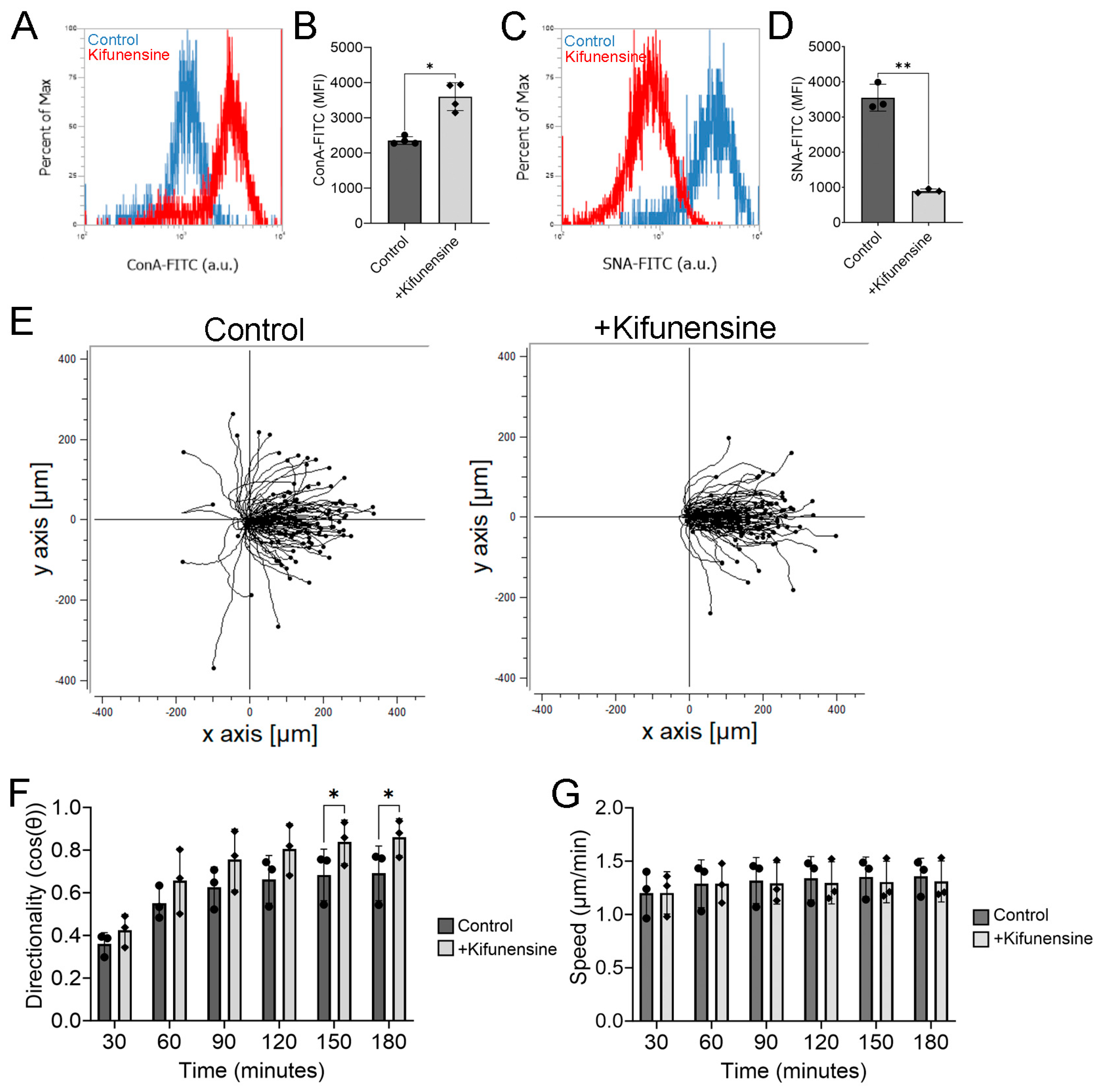
2.4. Pretreating hTCEpi Cells with Kifunensine Enhances Electrotaxis
2.5. Pretreating Enucleated Eyes with 3F-Neu5Ac Promotes Re-Epithelialization in an Ex Vivo Model of Corneal Injury
2.6. hTCEpi Cells Modulate Sialylations in Response to PDGF-BB and Growth Factor Starvation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Flow Cytometry
4.4. Electrotaxis
4.5. Ex Vivo Corneal Wound Healing
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reid, B.; Zhao, M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv. Wound Care 2014, 3, 184–201. [Google Scholar] [CrossRef]
- Liang, Y.; Tian, H.; Liu, J.; Lv, Y.; Wang, Y.; Zhang, J.; Huang, Y. Application of stable continuous external electric field promotes wound healing in pig wound model. Bioelectrochemistry 2020, 135, 107578. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 13577–13582. [Google Scholar] [CrossRef]
- Reid, B.; Song, B.; McCaig, C.D.; Zhao, M. Wound healing in rat cornea: The role of electric currents. FASEB J. 2005, 19, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Xu, J.; Han, D.; Zhang, Q.; Hong, Z. The Applications of Metabolic Glycoengineering. Front. Cell Dev. Biol. 2022, 10, 840831. [Google Scholar] [CrossRef]
- Templeton, K.; Ramos, M.; Rose, J.; Le, B.; Zhou, Q.; Cressman, A.; Ferreyra, S.; Lebrilla, C.B.; Fierro, F.A. Mesenchymal Stromal Cells Regulate Sialylations of N-Glycans, Affecting Cell Migration and Survival. Int. J. Mol. Sci. 2021, 22, 6868. [Google Scholar] [CrossRef]
- Hauser, M.A.; Kindinger, I.; Laufer, J.M.; Spate, A.K.; Bucher, D.; Vanes, S.L.; Krueger, W.A.; Wittmann, V.; Legler, D.F. Distinct CCR7 glycosylation pattern shapes receptor signaling and endocytosis to modulate chemotactic responses. J. Leukoc. Biol. 2016, 99, 993–1007. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Y.; Xu, G.; Lebrilla, C.B. Identification of potential sialic acid binding proteins on cell membranes by proximity chemical labeling. Chem. Sci. 2019, 10, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Wolosin, J.M.; Wang, Y. Alpha-2,3 sialylation differentiate the limbal and corneal epithelial cell phenotypes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2277–2286. [Google Scholar]
- Panjwani, N.; Zhao, Z.; Ahmad, S.; Yang, Z.; Jungalwala, F.; Baum, J. Neolactoglycosphingolipids, potential mediators of corneal epithelial cell migration. J. Biol. Chem. 1995, 270, 14015–14023. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, A.J.; Degi, R.M.; Davis, R.M. Corneal wound-associated glycoconjugates analyzed by lectin histochemistry. Curr. Eye Res. 1999, 19, 212–218. [Google Scholar] [CrossRef]
- Saravanan, C.; Cao, Z.; Head, S.R.; Panjwani, N. Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays. Glycobiology 2010, 20, 13–23. [Google Scholar] [CrossRef]
- Sun, Y.H.; Luxardi, G.; Xu, G.; Zhu, K.; Reid, B.; Guo, B.P.; Lebrilla, C.B.; Maverakis, E.; Zhao, M. Surface Glycans Regulate Salmonella Infection-Dependent Directional Switch in Macrophage Galvanotaxis Independent of NanH. Infect. Immun. 2022, 90, e00516-21. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Garcia, V.; Chaboya, C.; Li, Q.; Le, B.; Congleton, T.J.; Florez, J.; Tran, V.; Liu, G.Y.; Yao, W.; Lebrilla, C.B.; et al. High Mannose N-Glycans Promote Migration of Bone-Marrow-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2020, 21, 7194. [Google Scholar] [CrossRef]
- Lomako, J.; Lomako, W.M.; Carothers Carraway, C.A.; Carraway, K.L. Regulation of the membrane mucin Muc4 in corneal epithelial cells by proteosomal degradation and TGF-beta. J. Cell Physiol. 2010, 223, 209–214. [Google Scholar]
- Elbein, A.D.; Tropea, J.E.; Mitchell, M.; Kaushal, G.P. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J. Biol. Chem. 1990, 265, 15599–15605. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef]
- Fujii, A.; Shearer, T.R.; Azuma, M. Galectin-3 enhances extracellular matrix associations and wound healing in monkey corneal epithelium. Exp. Eye Res. 2015, 137, 71–78. [Google Scholar] [CrossRef]
- Holdbrooks, A.T.; Ankenbauer, K.E.; Hwang, J.; Bellis, S.L. Regulation of inflammatory signaling by the ST6Gal-I sialyltransferase. PLoS ONE 2020, 15, e0241850. [Google Scholar] [CrossRef]
- Ednie, A.R.; Bennett, E.S. Modulation of voltage-gated ion channels by sialylation. Compr. Physiol. 2012, 2, 1269–1301. [Google Scholar] [PubMed]
- Cortada, E.; Brugada, R.; Verges, M. N-Glycosylation of the voltage-gated sodium channel beta2 subunit is required for efficient trafficking of Na(V)1.5/beta2 to the plasma membrane. J. Biol. Chem. 2019, 294, 16123–16140. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.I.; Chao, P.H.; Hung, C.T.; Bulinski, J.C. Electric field-induced polarization of charged cell surface proteins does not determine the direction of galvanotaxis. Cell Motil. Cytoskelet. 2007, 64, 833–846. [Google Scholar] [CrossRef]
- Ajit, V.; Richard, D.; Cummings, J.D.; Esko, P.S.; Gerald, W.H.; Markus, A.; Debra, M.; Taroh, K.; Nicolle, H.; Packer, J.H.; et al. Essentials of Glycobiology, 4th ed.; Cold Springs Harbor Laboratory Press: Long Island, NY, USA, 2022. [Google Scholar]
- Sun, Y.; Reid, B.; Ferreira, F.; Luxardi, G.; Ma, L.; Lokken, K.L.; Zhu, K.; Xu, G.; Sun, Y.; Ryzhuk, V.; et al. Infection-generated electric field in gut epithelium drives bidirectional migration of macrophages. PLoS Biol. 2019, 17, e3000044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Takada, Y.; Nakajima, K.; Sun, Y.; Jiang, J.; Zhang, Y.; Zeng, Q.; Takada, Y.; Zhao, M. Expression of integrins to control migration direction of electrotaxis. FASEB J. 2019, 33, 9131–9141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Do, K.K.; Wang, F.; Lu, X.; Liu, J.Y.; Li, C.; Ceresa, B.P.; Zhang, L.; Dean, D.C.; Liu, Y. Zeb1 facilitates corneal epithelial wound healing by maintaining corneal epithelial cell viability and mobility. Commun. Biol. 2023, 6, 434. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Song, Y. Role of altered sialylation of the I-like domain of beta1 integrin in the binding of fibronectin to beta1 integrin: Thermodynamics and conformational analyses. Biophys. J. 2010, 99, 208–217. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, L.; Shen, S.; Wu, S.; Burdick, M.M. Effect of alpha 2,6 sialylation on integrin-mediated adhesion of breast cancer cells to fibronectin and collagen IV. Life Sci. 2016, 149, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Gu, Y.; Pu, J.; Reid, B.; Zhao, Z.; Zhao, M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007, 2, 1479–1489. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, B.; Zhu, K.; Brown, C.; Reid, B.; Cressman, A.; Zhao, M.; Fierro, F.A. Reducing Sialylation Enhances Electrotaxis of Corneal Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 14327. https://doi.org/10.3390/ijms241814327
Le B, Zhu K, Brown C, Reid B, Cressman A, Zhao M, Fierro FA. Reducing Sialylation Enhances Electrotaxis of Corneal Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(18):14327. https://doi.org/10.3390/ijms241814327
Chicago/Turabian StyleLe, Bryan, Kan Zhu, Chelsea Brown, Brian Reid, Amin Cressman, Min Zhao, and Fernando A. Fierro. 2023. "Reducing Sialylation Enhances Electrotaxis of Corneal Epithelial Cells" International Journal of Molecular Sciences 24, no. 18: 14327. https://doi.org/10.3390/ijms241814327




