Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes
Abstract
:1. Introduction
2. Results
2.1. Ferulic Acid Promotes the Maturation of the Nuclei and Cytoplasms of Bovine Oocytes In Vitro
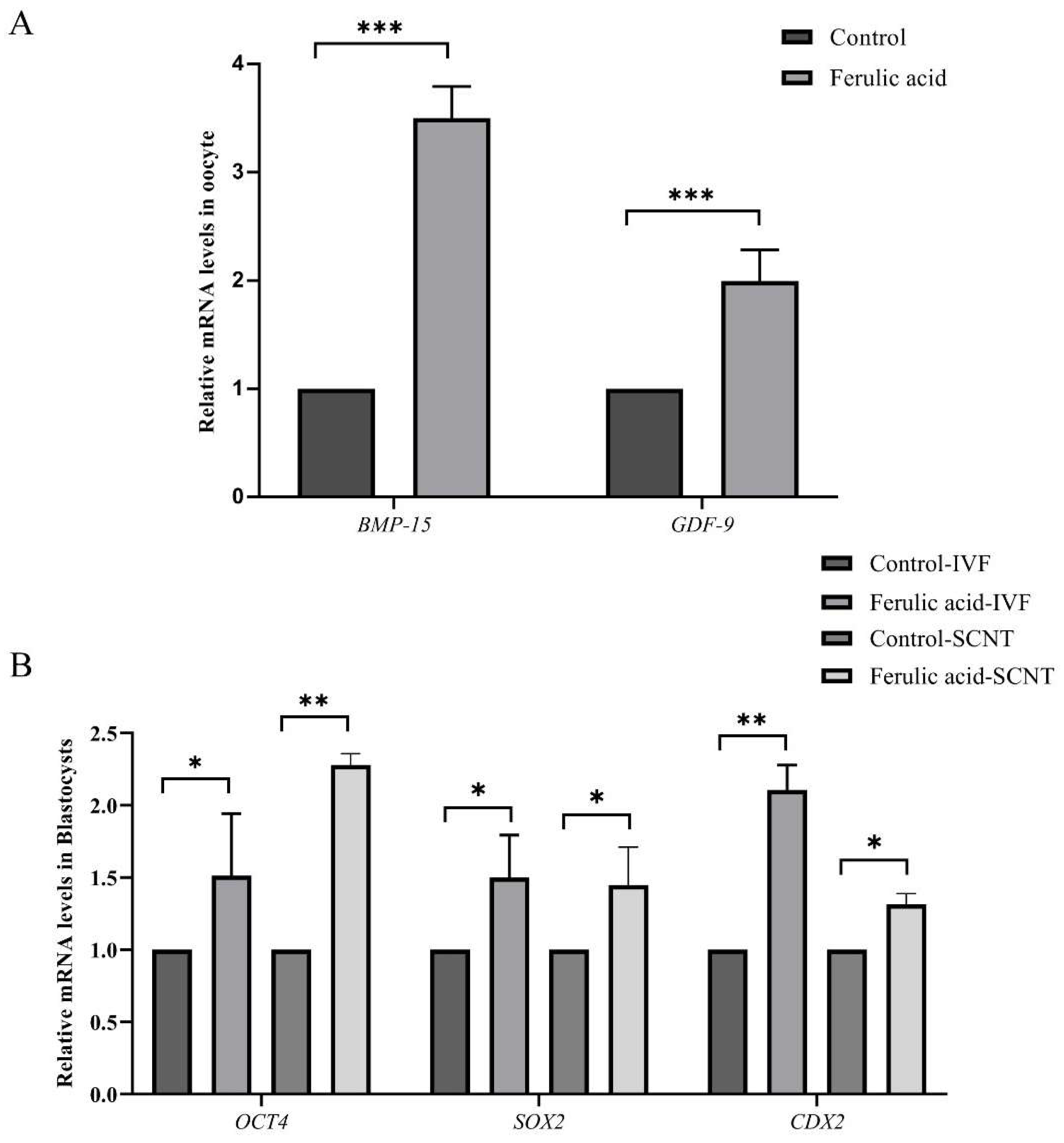
2.2. Ferulic Acid Supplementation Improves the Quality of Oocytes and the Developmental Potential of Subsequent Embryos
2.3. Ferulic Acid Promotes Cumulus Expansion during In Vitro Maturation
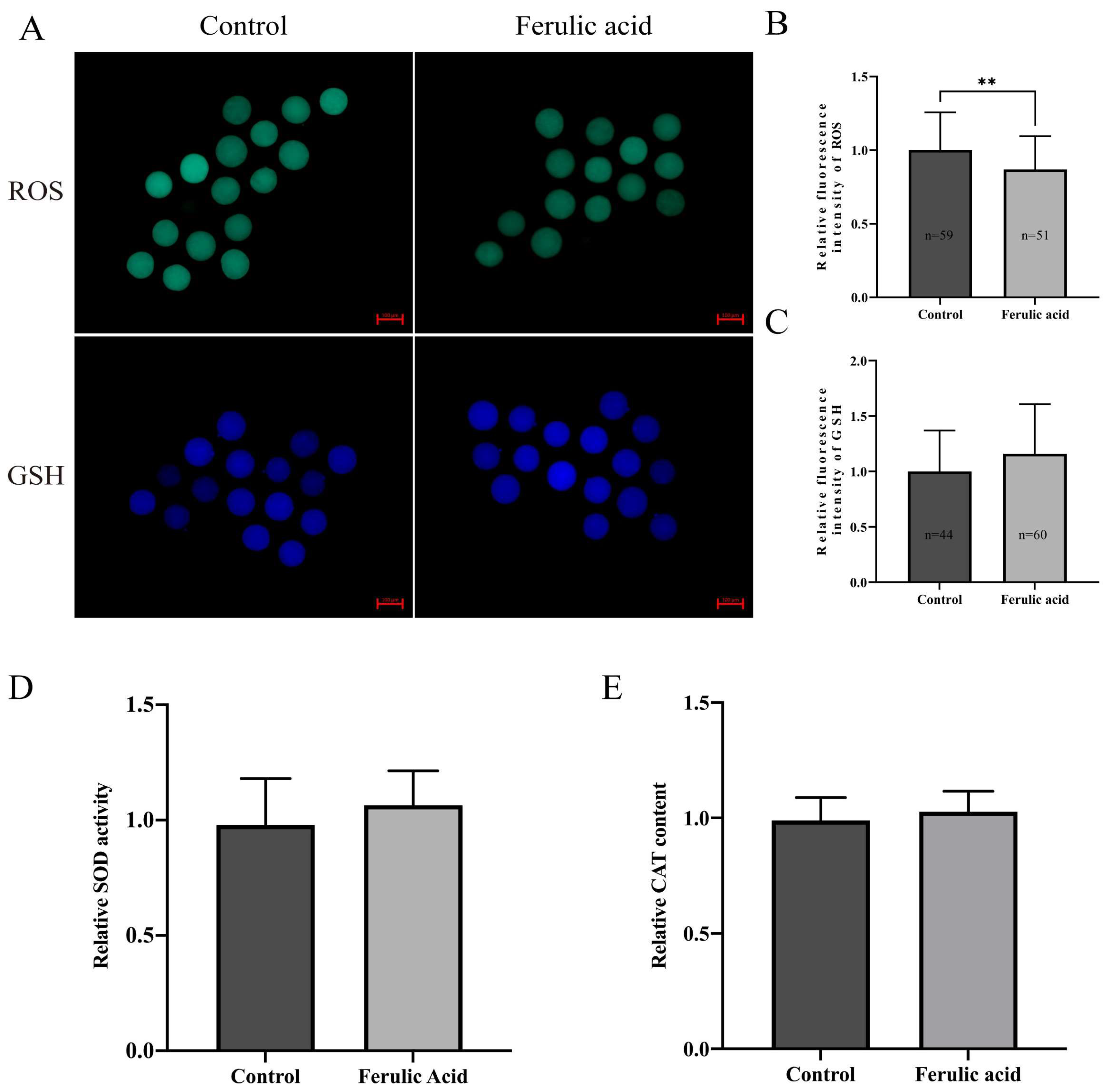
2.4. Ferulic Acid Improves the Antioxidant Capacity of Bovine Oocytes
2.5. Ferulic Acid Improves the Mitochondrial Function of Bovine Oocytes
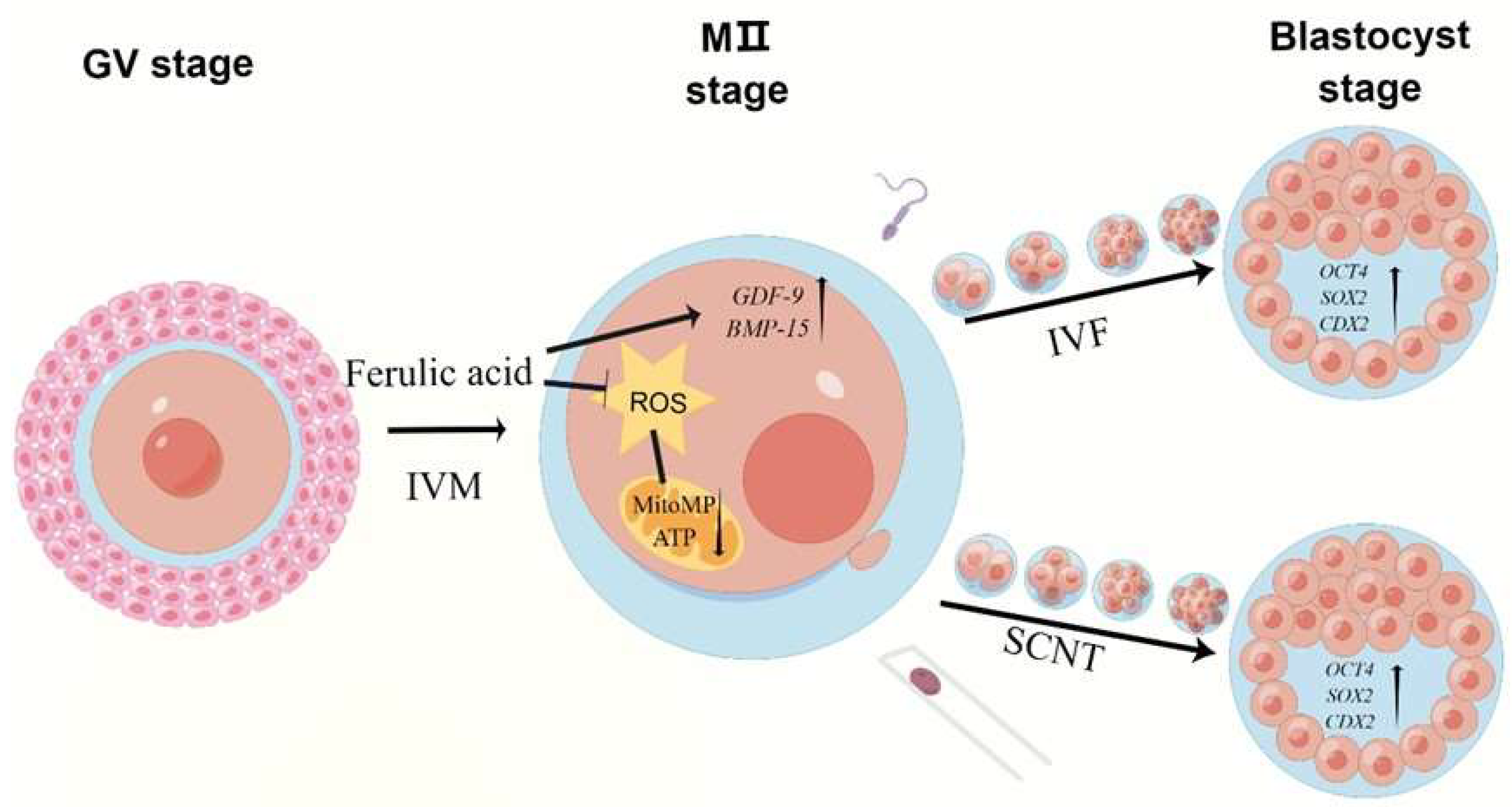
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Chemicals
4.3. Collection and In Vitro Maturation of Oocytes
4.4. Statistics of Cumulus Expansion Area
4.5. In Vitro Fertilization and In Vitro Culture of Embryos
4.6. Somatic Cell Nuclear Transfer and In Vitro Culture of Embryos
4.7. Determination of ROS, GSH and MMP Levels in Bovine Oocytes
4.8. Determination of ATP Levels in Bovine Oocytes
4.9. RNA Extraction, Reverse Transcription and RT–qPCR
4.10. Determination of SOD Activity and CAT Content in Bovine Oocytes
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, J.; Drost, M.; Monson, R.L.; Rutledge, J.J.; Rivera, R.M.; Paula-Lopes, F.F.; Ocon, O.M.; Krininger, C.E., 3rd; Liu, J.; Hansen, P.J. Use of insulin-like growth factor-I during embryo culture and treatment of recipients with gonadotropin-releasing hormone to increase pregnancy rates following the transfer of in vitro-produced embryos to heat-stressed, lactating cows. J. Anim. Sci. 2003, 81, 1590–1602. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Téteau, O.; Liere, P.; Pianos, A.; Desmarchais, A.; Lasserre, O.; Papillier, P.; Vignault, C.; Lebachelier de la Riviere, M.E.; Maillard, V.; Binet, A.; et al. Bisphenol S Alters the Steroidome in the Preovulatory Follicle, Oviduct Fluid and Plasma in Ewes With Contrasted Metabolic Status. Front. Endocrinol. 2022, 13, 892213. [Google Scholar] [CrossRef]
- Fang, X.; Xia, W.; Li, S.; Qi, Y.; Liu, M.; Yu, Y.; Li, H.; Li, M.; Tao, C.; Wang, Z.; et al. SIRT2 Is Critical for Sheep Oocyte Maturation through Regulating Function of Surrounding Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 5013. [Google Scholar] [CrossRef]
- Yu, K.; Wang, R.X.; Li, M.H.; Sun, T.C.; Zhou, Y.W.; Li, Y.Y.; Sun, L.H.; Zhang, B.L.; Lian, Z.X.; Xue, S.G.; et al. Melatonin Reduces Androgen Production and Upregulates Heme Oxygenase-1 Expression in Granulosa Cells from PCOS Patients with Hypoestrogenia and Hyperandrogenia. Oxid. Med. Cell. Longev. 2019, 2019, 8218650. [Google Scholar] [CrossRef]
- Liang, Q.X.; Lin, Y.H.; Zhang, C.H.; Sun, H.M.; Zhou, L.; Schatten, H.; Sun, Q.Y.; Qian, W.P. Resveratrol increases resistance of mouse oocytes to postovulatory aging in vivo. Aging 2018, 10, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.Y.; Tu, X.; McAndrews, K.A.; Plotkin, L.I.; Bellido, T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone 2015, 73, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Dong, Q.; Bai, Y.; Yuan, J.; Xu, Q.; Cao, C.; Liu, X. Oxidative stress induces mitotic arrest by inhibiting Aurora A-involved mitotic spindle formation. Free. Radic. Biol. Med. 2017, 103, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020, 30, 101431. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Xiang, W.; Li, Q.; Zhang, H.; Li, Y.; Zhu, G.; Xiong, C.; Jin, L. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 2018, 12, 518–524. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free. Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef]
- Hou, X.; Zhu, S.; Zhang, H.; Li, C.; Qiu, D.; Ge, J.; Guo, X.; Wang, Q. Mitofusin1 in oocyte is essential for female fertility. Redox Biol. 2019, 21, 101110. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; You, S.Y.; Cho, S.; Jeon, H.J.; Lee, S.; Cho, D.H.; Kim, J.S.; Oh, J.S. Eccentric localization of catalase to protect chromosomes from oxidative damages during meiotic maturation in mouse oocytes. Histochem. Cell. Biol. 2016, 146, 281–288. [Google Scholar] [CrossRef]
- Gardiner, C.S.; Reed, D.J. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol. Reprod. 1994, 51, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Curnow, E.C.; Ryan, J.; Saunders, D.; Hayes, E.S. Bovine in vitro oocyte maturation as a model for manipulation of the gamma-glutamyl cycle and intraoocyte glutathione. Reprod. Fertil. Dev. 2008, 20, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free. Radic. Biol. Med. 1999, 26, 463–471. [Google Scholar] [CrossRef]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef]
- Qi, J.J.; Li, X.X.; Zhang, Y.; Diao, Y.F.; Hu, W.Y.; Wang, D.L.; Jiang, H.; Zhang, J.B.; Sun, B.X.; Liang, S. Supplementation with asiatic acid during in vitro maturation improves porcine oocyte developmental competence by regulating oxidative stress. Theriogenology 2021, 172, 169–177. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef]
- Bezerra, G.S.N.; Pereira, M.A.V.; Ostrosky, E.A.; Barbosa, E.G.; de Moura, M.d.F.V.; Ferrari, M.; Aragão, C.F.S.; Gomes, A.P.B. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J. Therm. Anal. Calorim. 2017, 127, 1683–1691. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Milenković, D.; Stepanić, V. Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Jin, B.; Zhu, H.; Zhou, P.; Du, L.; Jin, X. Ferulic acid (FA) protects human retinal pigment epithelial cells from H2O2-induced oxidative injuries. J. Cell. Mol. Med. 2020, 24, 13454–13462. [Google Scholar] [CrossRef]
- Zheng, R.-L.; Zhang, H. Effects of Ferulic Acid on Fertile and Asthenozoospermic Infertile Human Sperm Motility, Viability, Lipid Peroxidation, and Cyclic Nucleotides. Free Radic. Biol. Med. 1997, 22, 581–586. [Google Scholar] [CrossRef]
- Tanihara, F.; Hirata, M.; Nhien, N.T.; Hirano, T.; Kunihara, T.; Otoi, T. Effect of ferulic acid supplementation on the developmental competence of porcine embryos during in vitro maturation. J. Vet. Med. Sci. 2018, 80, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Appeltant, R.; Somfai, T.; Nakai, M.; Bodó, S.; Maes, D.; Kikuchi, K.; Van Soom, A. Interactions between oocytes and cumulus cells during in vitro maturation of porcine cumulus-oocyte complexes in a chemically defined medium: Effect of denuded oocytes on cumulus expansion and oocyte maturation. Theriogenology 2015, 83, 567–576. [Google Scholar] [CrossRef]
- Cajas, Y.N.; Cañón-Beltrán, K.; Ladrón de Guevara, M.; Millán de la Blanca, M.G.; Ramos-Ibeas, P.; Gutiérrez-Adán, A.; Rizos, D.; González, E.M. Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality. Int. J. Mol. Sci. 2020, 21, 5340. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, Y.; Zhou, D.; Liu, H.; Zhou, G.; Meng, L.; Hou, Y.; Liu, C.; Li, J.; Fu, X. Leonurine improves bovine oocyte maturation and subsequent embryonic development by reducing oxidative stress and improving mitochondrial function. Theriogenology 2023, 199, 11–18. [Google Scholar] [CrossRef]
- Zhao, Y.; E, Z.; Jiao, A.; Sun, Z.; Zhang, H.; Wang, H.; Fang, N.; Gao, Q.; Jin, Q. Dendrobine enhances bovine oocyte maturation and subsequent embryonic development and quality. Theriogenology 2023, 203, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Estienne, A.; Barbe, A.; Rajska, I.; Soból, K.; Poniedziałek-Kempny, K.; Dupont, J.; Rak, A. Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways. Int. J. Mol. Sci. 2020, 21, 9342. [Google Scholar] [CrossRef]
- Park, A.; Oh, H.J.; Ji, K.; Choi, E.M.; Kim, D.; Kim, E.; Kim, M.K. Effect of Passage Number of Conditioned Medium Collected from Equine Amniotic Fluid Mesenchymal Stem Cells: Porcine Oocyte Maturation and Embryo Development. Int. J. Mol. Sci. 2022, 23, 6569. [Google Scholar] [CrossRef]
- Artini, P.G.; Scarfò, G.; Marzi, I.; Fusi, J.; Obino, M.E.; Franzoni, F.; Zappelli, E.; Chelucci, E.; Martini, C.; Cela, V.; et al. Oxidative Stress-Related Signaling Pathways Predict Oocytes’ Fertilization In Vitro and Embryo Quality. Int. J. Mol. Sci. 2022, 23, 3442. [Google Scholar] [CrossRef]
- Sun, Y.L.; Tang, S.B.; Shen, W.; Yin, S.; Sun, Q.Y. Roles of Resveratrol in Improving the Quality of Postovulatory Aging Oocytes In Vitro. Cells 2019, 8, 1132. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.S.; Wang, L.J.; Zhang, H.; Li, R.Z.; Cui, C.C.; Li, W.Z.; Zhang, Y.; Jin, Y.P. Vitamin C supplementation enhances compact morulae formation but reduces the hatching blastocyst rate of bovine somatic cell nuclear transfer embryos. Cell. Reprogram. 2014, 16, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, G.; Velasco, I. Activin and TGF-β effects on brain development and neural stem cells. CNS Neurol. Disord. Drug. Targets 2012, 11, 844–855. [Google Scholar] [CrossRef]
- Neely, M.D.; Litt, M.J.; Tidball, A.M.; Li, G.G.; Aboud, A.A.; Hopkins, C.R.; Chamberlin, R.; Hong, C.C.; Ess, K.C.; Bowman, A.B. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: Comparison of PAX6 and SOX1 expression during neural induction. ACS Chem. Neurosci. 2012, 3, 482–491. [Google Scholar] [CrossRef]
- Caixeta, E.S.; Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G.; Price, C.A.; Machado, M.F.; Lima, P.F.; Buratini, J. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction 2013, 146, 27–35. [Google Scholar] [CrossRef]
- Su, Y.Q.; Sugiura, K.; Wigglesworth, K.; O'Brien, M.J.; Affourtit, J.P.; Pangas, S.A.; Matzuk, M.M.; Eppig, J.J. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008, 135, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Min, J.T.; Du, W.H.; Hao, H.S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Krebs, S.; Heininen-Brown, M.; Zakhartchenko, V.; Blum, H.; Wolf, E. Genome activation in bovine embryos: Review of the literature and new insights from RNA sequencing experiments. Anim. Reprod. Sci. 2014, 149, 46–58. [Google Scholar] [CrossRef]
- Rizzino, A.; Wuebben, E.L. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim. Biophys. Acta 2016, 1859, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Goissis, M.D.; Cibelli, J.B. Functional characterization of SOX2 in bovine preimplantation embryos. Biol. Reprod. 2014, 90, 30. [Google Scholar] [CrossRef]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef]
- Goissis, M.D.; Cibelli, J.B. Functional characterization of CDX2 during bovine preimplantation development in vitro. Mol. Reprod. Dev. 2014, 81, 962–970. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Ynsaurralde-Rivolta, A.E.; Suvá, M.; Luchetti, C.G.; Bevacqua, R.J.; Munilla, S.; Rodriguez-Alvarez, L.; Velasquez, A.; Briski, O.; Lombardo, D.; Salamone, D. DMSO supplementation during in vitro maturation of bovine oocytes improves blastocyst rate and quality. Theriogenology 2020, 148, 140–148. [Google Scholar] [CrossRef]
- Biase, F.H.; Kimble, K.M. Functional signaling and gene regulatory networks between the oocyte and the surrounding cumulus cells. BMC Genomics 2018, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Cillo, F.; Brevini, T.A.; Antonini, S.; Paffoni, A.; Ragni, G.; Gandolfi, F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 2007, 134, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, K.M.; Feil, D.K.; Dunning, K.R.; Lane, M.; Russell, D.L. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil. Steril. 2011, 96, 47–52.e42. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Baltz, J.M. Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus-oocyte complex. Biol. Reprod. 2014, 90, 137. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420–1430. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Li, Y.; Guo, X.; Li, J.; Zhong, R.; Zhang, X. Melatonin-induced demethylation of antioxidant genes increases antioxidant capacity through RORα in cumulus cells of prepubertal lambs. Free Radic. Biol. Med. 2019, 131, 173–183. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Sun, S.; Ruan, Y.; Yan, M.; Xu, K.; Yang, Y.; Shen, T.; Jin, Z. Ferulic Acid Alleviates Oxidative Stress-Induced Cardiomyocyte Injury by the Regulation of miR-499-5p/p21 Signal Cascade. Evid. Based Complement. Altern. Med. 2021, 2021, 1921457. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Wen, D.; Li, R.; Lu, S.; Xu, R.; Tang, Y.; Sun, Y.; Zhao, X.; Pan, M.; et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022, 49, 102215. [Google Scholar] [CrossRef] [PubMed]
- Cetica, P.D.; Pintos, L.N.; Dalvit, G.C.; Beconi, M.T. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 2001, 51, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xuan, B.; Xu, D.; Wang, Q.; Cheng, M.; Jin, Y. Crocin supplementation during oocyte maturation enhances antioxidant defence and subsequent cleavage rate. Reprod. Domest. Anim. 2019, 54, 300–308. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, B.; Hu, W.; Qi, J.; Jiang, H.; Sun, B.; Zhang, J.; Liang, S. Tributyltin Oxide Exposure during In Vitro Maturation Disrupts Oocyte Maturation and Subsequent Embryonic Developmental Competence in Pigs. Front. Cell. Dev. Biol. 2021, 9, 683448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Malik, H.; Saha, A.P.; Dubey, A.; Singhal, D.K.; Boateng, S.; Saugandhika, S.; Kumar, S.; De, S.; Guha, S.K.; et al. Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. J. Assist. Reprod. Genet. 2014, 31, 229–239. [Google Scholar] [CrossRef] [PubMed]
- de Matos, D.G.; Furnus, C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive Effect of Blueberry Extract on Liver Injury Induced by Carbon Tetrachloride in Mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef]
- Farhan, M.; Oves, M.; Chibber, S.; Hadi, S.M.; Ahmad, A. Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy. Int. J. Mol. Sci. 2016, 18, 34. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell'Aquila, M.E.; Bogliolo, L. Protective effect of resveratrol against cadmium-induced toxicity on ovine oocyte in vitro maturation and fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef]
- Rakha, S.I.; Elmetwally, M.A.; El-Sheikh Ali, H.; Balboula, A.Z.; Mahmoud, A.M.; Zaabel, S.M. Lycopene Reduces the In Vitro Aging Phenotypes of Mouse Oocytes by Improving Their Oxidative Status. Vet. Sci. 2022, 9, 336. [Google Scholar] [CrossRef]
- Kwon, K.; Jung, J.; Sahu, A.; Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Release 2022, 344, 160–172. [Google Scholar] [CrossRef]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Reactive oxygen species and oocyte aging: Role of superoxide, hydrogen peroxide, and hypochlorous acid. Free. Radic. Biol. Med. 2008, 44, 1295–1304. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, J.; Majeed, H.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Ferulic acid renders protection to HEK293 cells against oxidative damage and apoptosis induced by hydrogen peroxide. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 722–729. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Xu, X.; Zhao, X.; Han, Z.; Liu, D.; Bo, R.; Li, J.; Liu, Z. Ferulic acid inhibits LPS-induced apoptosis in bovine mammary epithelial cells by regulating the NF-κB and Nrf2 signalling pathways to restore mitochondrial dynamics and ROS generation. Vet. Res. 2021, 52, 104. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology 2006, 228, 249–258. [Google Scholar] [CrossRef]
- Bentov, Y.; Casper, R.F. The aging oocyte—Can mitochondrial function be improved? Fertil. Steril. 2013, 99, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Sun, S.C. Exposure to PBDE47 affects mouse oocyte quality via mitochondria dysfunction-induced oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2020, 198, 110662. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Nie, Z.W.; Zhao, M.; Niu, Y.J.; Shin, K.T.; Cui, X.S. Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation. Sci. Rep. 2017, 7, 17082. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Y.; Wang, D.; Yang, T.; Qi, J.; Zhang, Y.; Jiang, H.; Zhang, J.; Sun, B.; Liang, S. Iron Overload-Induced Ferroptosis Impairs Porcine Oocyte Maturation and Subsequent Embryonic Developmental Competence in vitro. Front. Cell. Dev. Biol. 2021, 9, 673291. [Google Scholar] [CrossRef]
- Liang, S.; Guo, J.; Jin, Y.X.; Yuan, B.; Zhang, J.B.; Kim, N.H. C-Phycocyanin supplementation during in vitro maturation enhances pre-implantation developmental competence of parthenogenetic and cloned embryos in pigs. Theriogenology 2018, 106, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zafeer, M.F.; Firdaus, F.; Anis, E.; Mobarak Hossain, M. Prolong treatment with Trans-ferulic acid mitigates bioenergetics loss and restores mitochondrial dynamics in streptozotocin-induced sporadic dementia of Alzheimer's type. Neurotoxicology 2019, 73, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lai, X.; Yuan, D.; Liu, Y.; Wang, J.; Liang, Y. Effects of ferulic acid, a major component of rice bran, on proliferation, apoptosis, and autophagy of HepG2 cells. Food Res. Int. 2022, 161, 111816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.E.; Yoon, J.W.; Park, H.J.; Oh, S.H.; Lee, D.G.; Pyeon, D.B.; Kim, E.Y.; Park, S.P. Protodioscin protects porcine oocytes against H2O2-induced oxidative stress during in vitro maturation. Anim. Biosci. 2023, 36, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Nie, Z.W.; Guo, J.; Niu, Y.J.; Shin, K.T.; Ock, S.A.; Cui, X.S. Overexpression of MicroRNA-29b Decreases Expression of DNA Methyltransferases and Improves Quality of the Blastocysts Derived from Somatic Cell Nuclear Transfer in Cattle. Microsc. Microanal. 2018, 24, 29–37. [Google Scholar] [CrossRef] [PubMed]





| Parameters Evaluated | Total Number of Oocytes n | First Polar Body Extrusion n (%) |
|---|---|---|
| Control | 122 | 69 (56.55 ± 4.116) |
| 2.5 μM Ferulic acid | 102 | 58 (56.86 ± 2.318) |
| 5 μM Ferulic acid | 125 | 89 (71.2 ± 3.838) * |
| 10 μM Ferulic acid | 97 | 57 (58.76 ± 6.052) |
| 20 μM Ferulic acid | 145 | 78 (53.79 ± 2.775) |
| Gene Symbol | Primer | Primer Sequences (5′–3′) | Accession Number (NCBI) |
|---|---|---|---|
| GAPDH | F-primer | TTCAACGGCACAGTCAAGG | NM_001034034 |
| R-primer | ACATACTCAGCACCAGCATCAC | ||
| PTX3 | F-primer | CACAGGTCATGTTGTTCCTGAG | NM_001076259.2 |
| R-primer | CAGATATTGAAGCCTGTGAGTCTG | ||
| HAS2 | F-primer | TCTCTAGAAACCCCCATTAAGTTG | NM_174079.3 |
| R-primer | ATCTTCCGAGTTTCCATCTATGAC | ||
| CX43 | F-primer | CTTTCGTTGTAACACTCAACAACC | NM_174068.2 |
| R-primer | GTAGAACACATGAGCCAGGTACAG | ||
| CX37 | F-primer | AGCCCGTGTTTGTGTGCCAG | NM_001083738.1 |
| R-primer | ACCAGGGAGATGAGTCCGACCA | ||
| OCT4 | F-primer | GGAGAGCATGTTCCTGCAGTGC | NM_174580 |
| R-primer | ACACTCGGACCACGTCCTTCTC | ||
| CDX2 | F-primer | CCTGTGCGAGTGGATGCGGAAG | XM_871005 |
| R-primer | CCTTTGCTCTGCGGTTCT | ||
| SOX2 | F-primer | CGAGTGGAAACTTTTGTCCG | NM_001105463 |
| R-primer | GGTATTTATAATCCGGGTGTT | ||
| GDF-9 | F-primer | TCCAGAACCTTGTCAATGAG | NM_001031752.1 |
| R-primer | GGGCAATCATACCCTCATAC | ||
| BMP-15 | F-primer | GGACCCCTAAATCCAACAGA | NM_174681.2 |
| R-primer | ACAGTAACACGATCCAGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qi, J.-J.; Yin, Y.-J.; Jiang, H.; Zhang, J.-B.; Liang, S.; Yuan, B. Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes. Int. J. Mol. Sci. 2023, 24, 14804. https://doi.org/10.3390/ijms241914804
Wang Y, Qi J-J, Yin Y-J, Jiang H, Zhang J-B, Liang S, Yuan B. Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes. International Journal of Molecular Sciences. 2023; 24(19):14804. https://doi.org/10.3390/ijms241914804
Chicago/Turabian StyleWang, Yu, Jia-Jia Qi, Yi-Jing Yin, Hao Jiang, Jia-Bao Zhang, Shuang Liang, and Bao Yuan. 2023. "Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes" International Journal of Molecular Sciences 24, no. 19: 14804. https://doi.org/10.3390/ijms241914804





