Confirmation of ‘Pollen- and Seed-Specific Gene Deletor’ System Efficiency for Transgene Excision from Transgenic Nicotiana tabacum under Field Conditions
Abstract
:1. Introduction
2. Results
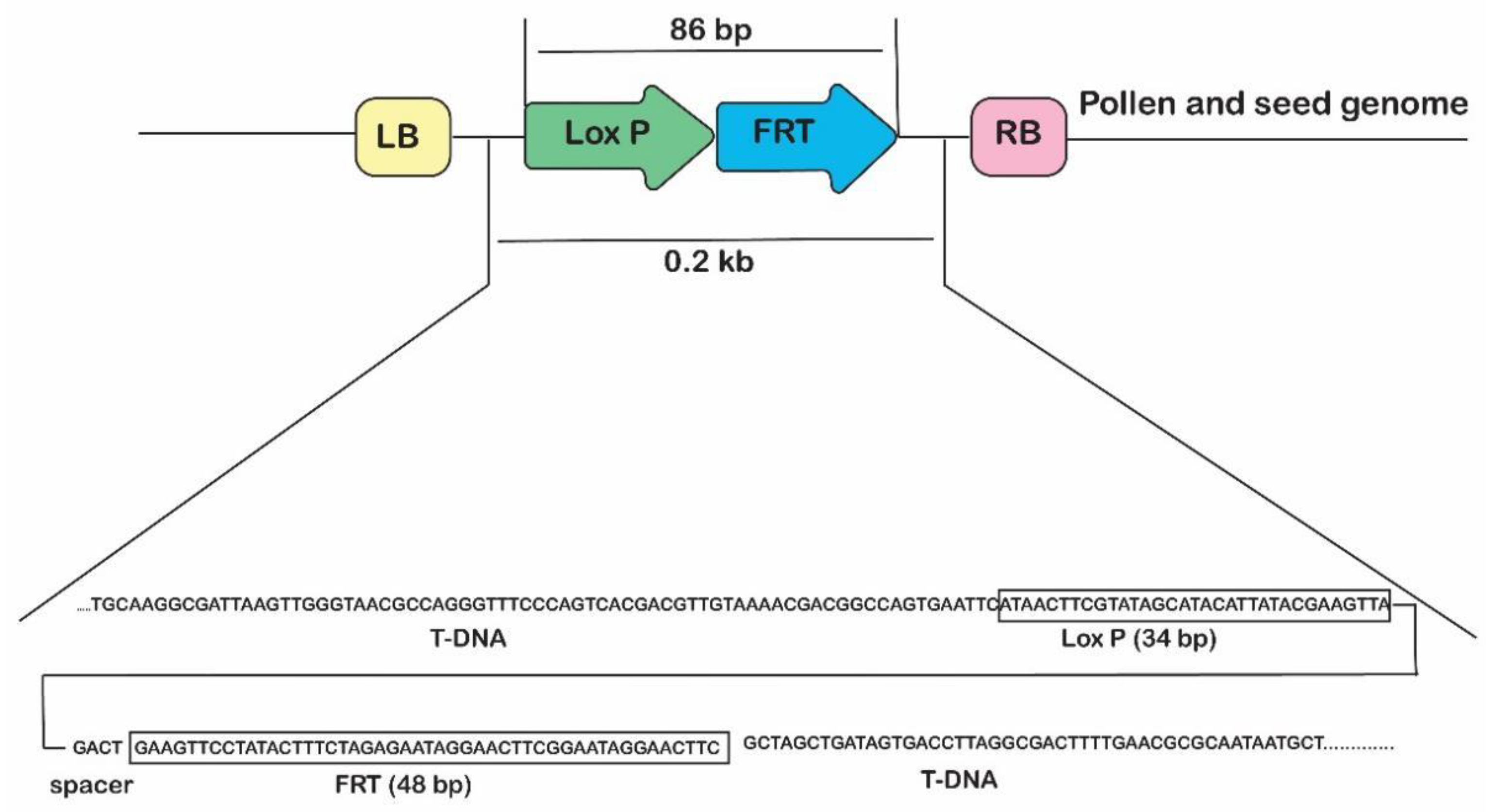
2.1. Gene Deletor Vector Construction
2.2. Production and Confirmation of N. tabacum Transgenic Lines
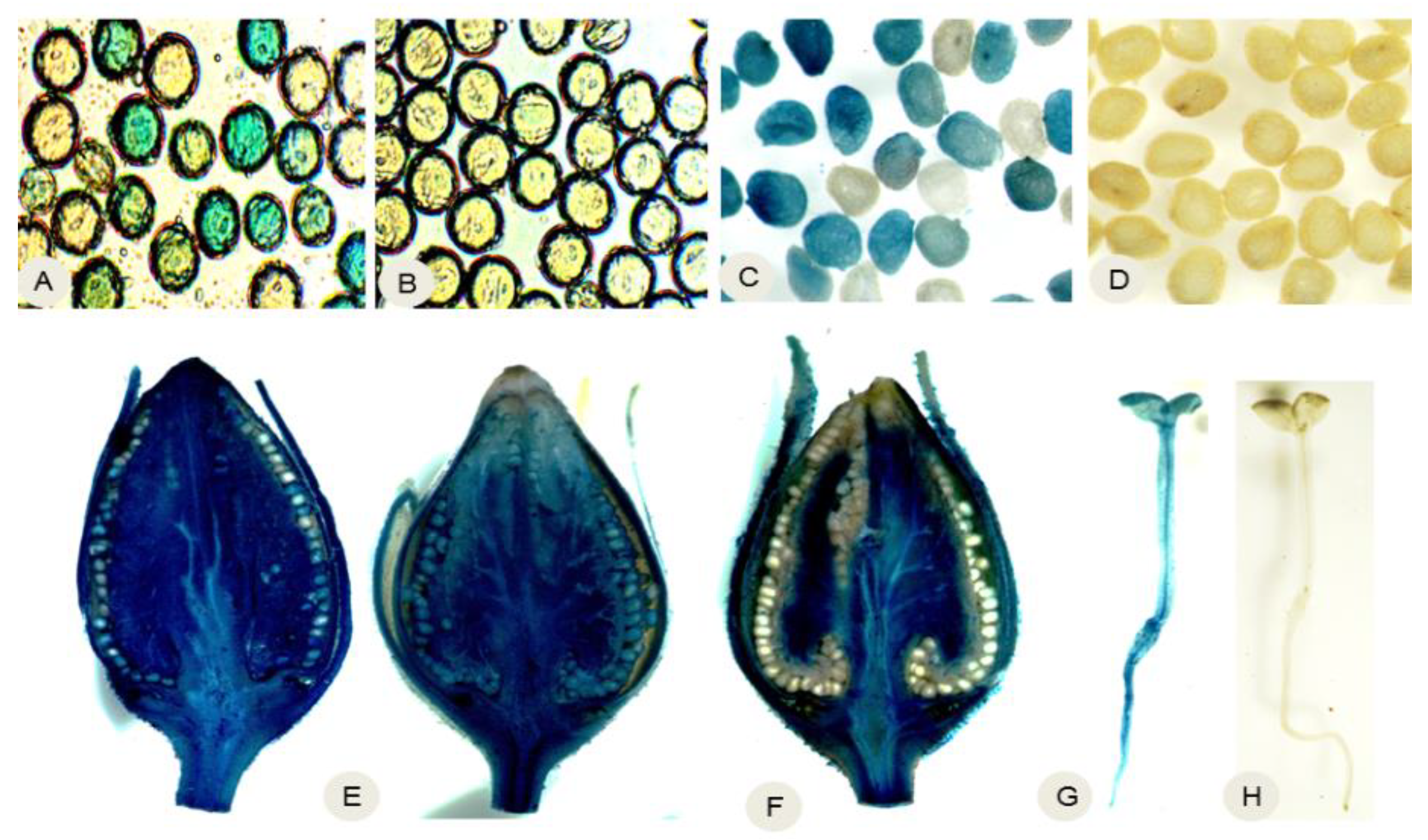
2.3. The ‘Gene Deletor’ System Excised Transgenes from the Pollen and Seeds of Greenhouse-Grown N. Tabacum
2.4. The ‘Gene Deletor’ System Stably and Completely Excised Transgenes from the Pollen and Seeds Produced by Transgenic N. tabacum Grown in the Field
2.5. Molecular Analysis of Transgene Excision from Pollen and Seeds
3. Discussion
4. Materials and Methods
4.1. Nicotiana tabacum L. Transformation Using the ‘Gene Deletor’ Vector
4.2. GUS Histochemical Staining
4.3. PCR Detection of Transgenes
4.4. Detection of Transgene Excision Efficiency in Greenhouse-Grown Transgenic Plants
4.5. Confirmation of Transgene Excision in the T1 Generation of Greenhouse-Grown Transgenic Plants
4.6. Estimation of Transgene Copy Numbers in Transgenic Plants
4.7. Transgene Excision in Field-Grown Transgenic Plants
4.8. Molecular Verification of Transgene Excision in the Transgenic Plants
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaiser, N.; Douches, D.; Dhingra, A.; Glenn, K.C.; Herzig, P.R.; Stowe, E.C.; Swarup, S. The role of conventional plant breeding in ensuring safe levels of naturally occurring toxins in food crops. Trends Food Sci. Technol. 2020, 100, 51–66. [Google Scholar] [CrossRef]
- Li, Y. Gene deletor: A new tool to address gene flow and food safety concerns over transgenic crop plants. Front. Biol. 2012, 7, 557–565. [Google Scholar] [CrossRef]
- Babar, U.; Nawaz, M.A.; Arshad, U.; Azhar, M.T.; Atif, R.M.; Golokhvast, K.S.; Tsatsakis, A.M.; Shcerbakova, K.; Chung, G.; Rana, I.A. Transgenic crops for the agricultural improvement in Pakistan: A perspective of environmental stresses and the current status of genetically modified crops. GM Crops Food 2020, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Fares, N.H.; El-Sayed, A.K. Fine structural changes in the ileum of mice fed on δ-endotoxin-treated potatoes and transgenic potatoes. Nat. Toxins 1998, 6, 219–233. [Google Scholar] [CrossRef]
- Ewen, S.W.; Pusztai, A. Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 1999, 354, 1353–1354. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, W.; Momma, K.; Katsube, T.; Ohkawa, Y.; Ishige, T.; Kito, M.; Utsumi, S.; Murata, K. Safety assessment of genetically engineered potatoes with designed soybean glycinin: Compositional analyses of the potato tubers and digestibility of the newly expressed protein in transgenic potatoes. J. Sci. Food Agric. 1999, 79, 1607–1612. [Google Scholar] [CrossRef]
- Momma, K.; Hashimoto, W.; Ozawa, S.; Kawai, S.; Katsube, T.; Takaiwa, F.; Kito, M.; Utsumi, S.; Murata, K. Quality and Safety Evaluation of Genetically Engineered Rice with Soybean Glycinin: Analyses of the Grain Composition and Digestibility of Glycinin in Transgenic Rice. Biosci. Biotechnol. Biochem. 1999, 63, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, T.R.; Andersen, B.; Jørgensen, R.B. The risk of crop transgene spread. Nature 1996, 380, 31. [Google Scholar] [CrossRef]
- Prakash, S.; Bhat, S.; Quiros, C.; Kirti, P.; Chopra, V. 2 Brassica and Its Close Allies: Cytogenetics and Evolution. J. Integr. Agric. 2009, 31, 21–187. [Google Scholar]
- Kwit, C.; Moon, H.S.; Warwick, S.I.; Stewart, C.N., Jr. Transgene introgression in crop relatives: Molecular evidence and mitigation strategies. Trends Biotechnol. 2011, 29, 284–293. [Google Scholar] [CrossRef]
- Piñeyro-Nelson, A.; VAN Heerwaarden, J.; Perales, H.R.; Serratos-Hernández, J.A.; Rangel, A.; Hufford, M.B.; Gepts, P.; Garay-Arroyo, A.; Rivera-Bustamante, R.; Álvarez-Buylla, E.R. Transgenes in Mexican maize: Molecular evidence and methodological considerations for GMO detection in landrace populations. Mol. Ecol. 2009, 18, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Warwick, S.I.; Nair, H.; Séguin-Swartz, G. Gene flow in commercial fields of herbicide-resistant canola (Brassica napus). Ecol. Appl. 2003, 13, 1276–1294. [Google Scholar] [CrossRef]
- Aono, M.; Wakiyama, S.; Nagatsu, M.; Nakajima, N.; Tamaoki, M.; Kubo, A.; Saji, H. Detection of feral transgenic oilseed rape with multiple-herbicide resistance in Japan. Environ. Biosaf. Res. 2006, 5, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichman, J.R.; Watrud, L.S.; Lee, E.H.; Burdick, C.A.; Bollman, M.A.; Storm, M.J.; King, G.A.; Mallory-Smith, C. Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera L.) in nonagronomic habitats. Mol. Ecol. 2006, 15, 4243–4255. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J. Needs for and environmental risks from transgenic crops in the developing world. New Biotechnol. 2010, 27, 522–527. [Google Scholar] [CrossRef]
- Stewart, C.N.; Halfhill, M.D.; Warwick, S.I. Transgene introgression from genetically modified crops to their wild relatives. Nat. Rev. Genet. 2003, 4, 806–817. [Google Scholar] [CrossRef]
- Moon, H.S.; Abercrombie, L.L.; Eda, S.; Blanvillain, R.; Thomson, J.G.; Ow, D.W.; Stewart, C. Transgene excision in pollen using a codon optimized serine resolvase CinH-RS2 site-specific recombination system. Plant Mol. Biol. 2011, 75, 621–631. [Google Scholar] [CrossRef]
- Klinger, T.; Ellstrand, N.C. Engineered Genes in Wild Populations: Fitness of Weed-Crop Hybrids of Raphanus Sativus. Ecol. Appl. 1994, 4, 117–120. [Google Scholar] [CrossRef]
- Giddings, G.J.T.; Genetics, A. Modelling the spread of pollen from Lolium perenne. The implications for the release of wind-pollinated transgenics. Theor. Appl. Genet. 2000, 100, 971–974. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Going to “Great Lengths” to Prevent the Escape of Genes That Produce Specialty Chemicals. Plant Physiol. 2003, 132, 1770–1774. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.; Maselko, M. Transgene Biocontainment Strategies for Molecular Farming. Front. Plant Sci. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Halpin, C. Gene stacking in transgenic plants—The challenge for 21st century plant biotechnology. Plant Biotechnol. J. 2005, 3, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.J.; Raybould, A.F. Reducing transgene escape routes. Nature 1998, 392, 653–654. [Google Scholar] [CrossRef]
- Moon, H.S.; Li, Y.; Stewart, C.N., Jr. Keeping the genie in the bottle: Transgene biocontainment by excision in pollen. Trends Biotechnol. 2010, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ow, D.W. Recombinase-directed plant transformation for the post-genome era. Plant Mol. Biol. 2002, 48, 183–200. [Google Scholar] [CrossRef]
- Groth, A.C.; Calos, M.P. Phage integrases: Biology and application. J. Mol. Biol. 2003, 335, 667–678. [Google Scholar] [CrossRef]
- Gidoni, D.; Srivastava, V.; Carmi, N. Site-specific excisional recombination strategies for elimination of undesirable transgenes from crop plants. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 457–467. [Google Scholar] [CrossRef]
- Hu, C.H.; Yang, Q.S.; Shao, X.H.; Dong, T.; Bi, F.C.; Li, C.Y.; Deng, G.M.; Li, Y.; Yi, G.J.; Dou, T.X. The application of the ‘Gene Deletor’ technology in banana. Plant Cell Tissue Organ Cult. 2020, 140, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Luo, K.; Duan, H.; Zhao, D.; Zheng, X.; Deng, W.; Chen, Y.; Stewart, C.N., Jr.; McAvoy, R.; Jiang, X.; Wu, Y.; et al. ‘GM-gene-deletor’: Fused loxP-FRT recognition sequences dramatically improve the efficiency of FLP or CRE recombinase on transgene excision from pollen and seed of tobacco plants. Plant Biotechnol. 2007, 5, 263–274. [Google Scholar] [CrossRef]
- Li, D.; Shi, W.; Deng, X. Agrobacterium-mediated transformation of embryogenic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep. 2002, 21, 153–156. [Google Scholar]
- Morjan, C.L.; Rieseberg, L. How species evolve collectively: Implications of gene flow and selection for the spread of advantageous alleles. Mol. Ecol. 2004, 13, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Deng, H.; Xia, Y.; Zhan, Y.; Tang, N.; Wang, Y.; Cao, M. Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote. Open Life Sci. 2022, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Cho, J.-I.; Ryoo, N.; Qu, S.; Wang, G.-L.; Jeon, J.-S. Development of a simple and efficient system for excising selectable markers in Arabidopsis using a minimal promoter::Cre fusion construct. Mol. Cells 2012, 33, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Chong-Perez, B.; Reyes, M.; Rojas, L.; Ocana, B.; Ramos, A.; Kosky, R.G.; Angenon, G. Excision of a selectable marker gene in transgenic banana using a Cre/lox system controlled by an embryo specifific promoter. Plant Mol. Biol. 2013, 83, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002, 20, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, M. The Ecological Risks of Transgenic Plants; Rivista di Biologia Biology Forum; Anicia SRL: Rome, Italy, 2003; pp. 207–224. [Google Scholar]
- Belostotsky, D.A.; Meagher, R.B. A pollen-, ovule-, and early embryo-specific poly (A) binding protein from Arabidopsis complements essential functions in yeast. Plant Cell 1996, 8, 1261–1275. [Google Scholar]
- Goldberg, R.B. Plants: Novel development processes. Science 1988, 240, 1460–1467. [Google Scholar] [CrossRef]
- Chen, X.; Pascal, J.; Vijayakumar, S.; Wilson, G.M.; Ellenberger, T.; Tomkinson, A.E. Human DNA Ligases I, III, and IV—Purification and New Specific Assays for These Enzymes. Method Enzymol. 2006, 409, 39–52. [Google Scholar]
- Schmidt, G.W.; Delaney, S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genom. 2010, 283, 233–241. [Google Scholar] [CrossRef]

| Transformation | Constructs | No. of Independent Transgenic Lines | Mean Percentage of GUS Negative T1 Seedlings | No. of Lines with 100% Excision Efficiency |
|---|---|---|---|---|
| Experiment No. 1 | Control Cassette | 22 | 19.2% | 0 |
| Gene Deletor | 66 | 37.7% | 2 | |
| Experiment No. 2 | Control Cassette | 19 | 21.4% | 0 |
| Gene Deletor | 81 | 36.1% | 2 | |
| Experiment No. 3 | Control Cassette | 21 | 20.7% | 0 |
| Gene Deletor | 55 | 35.0% | 1 |
| Line | Transgene Copies | Self-Pollinated | WT† as Pollen Recipient | WT as Pollen Donor | Transgene Excision Efficiency (%) | |||
|---|---|---|---|---|---|---|---|---|
| GUS− | GUS+ | GUS− | GUS+ | GUS− | GUS+ | |||
| C1 | 1 | 532 | 1742 | 789 | 834 | 873 | 951 | 0.0 |
| D4 | 1 | 21,862 | 0 | 2654 | 0 | 2476 | 0 | 100.0 |
| D10 | 1 | 41,787 | 0 | 3844 | 0 | 4366 | 0 | 100.0 |
| C14 | 1 | 651 | 1938 | 745 | 817 | 922 | 893 | 0.0 |
| D31 | 1 | 6874 | 0 | 2943 | 0 | 3326 | 0 | 100.0 |
| D56 | 1 | 5796 | 0 | 3219 | 0 | 3543 | 0 | 100.0 |
| C6 | 1 | 717 | 2258 | 1142 | 1271 | 967 | 1055 | 0.0 |
| D43 | 1 | 5864 | 0 | 2227 | 0 | 2581 | 0 | 100.0 |
| Experiment No. | Number of Field Tests | Line | Self-Pollinated | WT as Pollen Recipient | WT as Pollen Donor | Transgene Excision Efficiency (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| GUS− | GUS+ | GUS− | GUS+ | GUS− | GUS+ | ||||
| Experiment 1 | Field Test (1) | C1 | 658 | 2046 | 892 | 973 | 795 | 870 | 0.0 |
| D4 | 49,264 | 0 | 6739 | 0 | 6025 | 0 | 100.0 | ||
| D10 | 53,119 | 0 | 6033 | 0 | 6989 | 0 | 100.0 | ||
| Field Test (2) | C 1 | 506 | 1647 | 768 | 794 | 691 | 715 | 0.0 | |
| D4 | 44,301 | 0 | 5541 | 0 | 5905 | 0 | 100.0 | ||
| D10 | 64,356 | 0 | 7647 | 0 | 8036 | 0 | 100.0 | ||
| Field Test (3) | C1 | 1628 | 5034 | 1773 | 1826 | 1698 | 1735 | 0.0 | |
| D4 | 20,449 | 0 | 4363 | 0 | 4834 | 0 | 100.0 | ||
| D10 | 16,841 | 0 | 3961 | 0 | 3589 | 0 | 100.0 | ||
| Experiment 2 | Field Test (4) | C14 | 1275 | 3922 | 1169 | 1352 | 1288 | 1346 | 0.0 |
| D31 | 18,864 | 0 | 4032 | 0 | 3975 | 0 | 100.0 | ||
| D56 | 10,011 | 0 | 2763 | 0 | 2970 | 0 | 100.0 | ||
| Field Test (5) | C14 | 1053 | 3217 | 976 | 1018 | 1127 | 1253 | 0.0 | |
| D31 | 16,797 | 0 | 3524 | 0 | 3276 | 0 | 100.0 | ||
| D56 | 16,231 | 0 | 3226 | 0 | 3537 | 0 | 100.0 | ||
| Experiment 3 | Field Test (6) | C6 | 1136 | 3579 | 1217 | 1188 | 1204 | 1256 | 0.0 |
| D43 | 14,683 | 0 | 2533 | 0 | 2369 | 0 | 100.0 | ||
| Field Test (7) | C6 | 1317 | 4183 | 1479 | 1514 | 1396 | 1447 | 0.0 | |
| D43 | 16,068 | 0 | 2699 | 0 | 2528 | 0 | 100.0 | ||
| Line | PCR Analysis of Self-Pollinated T1 Seedlings | DNA Sequencing Analysis of 0.2 kb Fragments in T1 Seedlings | ||||
|---|---|---|---|---|---|---|
| Seedlings Carrying the 7.6 kb Fragment | Seedlings Carrying the 0.2 kb Fragment | Seedlings Not Producing Any Bands | Total Seedlings Tested for PCR | Seedlings Sequenced | Seedlings Carrying Expected 0.2 kb Sequence | |
| C1 | 21 | 0 | 9 | 30 | 0 | 0 |
| D4 | 0 | 38 | 12 | 50 | 10 | 10 |
| D10 | 0 | 36 | 14 | 50 | 10 | 10 |
| D31 | 0 | 33 | 17 | 50 | 5 | 5 |
| D56 | 0 | 37 | 13 | 50 | 5 | 5 |
| D43 | 0 | 40 | 10 | 50 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; He, M.; Akbar, S.; Zhao, D.; Zhang, M.; Li, Y.; Yao, W. Confirmation of ‘Pollen- and Seed-Specific Gene Deletor’ System Efficiency for Transgene Excision from Transgenic Nicotiana tabacum under Field Conditions. Int. J. Mol. Sci. 2023, 24, 1160. https://doi.org/10.3390/ijms24021160
Duan Z, He M, Akbar S, Zhao D, Zhang M, Li Y, Yao W. Confirmation of ‘Pollen- and Seed-Specific Gene Deletor’ System Efficiency for Transgene Excision from Transgenic Nicotiana tabacum under Field Conditions. International Journal of Molecular Sciences. 2023; 24(2):1160. https://doi.org/10.3390/ijms24021160
Chicago/Turabian StyleDuan, Zhenzhen, Mingyang He, Sehrish Akbar, Degang Zhao, Muqing Zhang, Yi Li, and Wei Yao. 2023. "Confirmation of ‘Pollen- and Seed-Specific Gene Deletor’ System Efficiency for Transgene Excision from Transgenic Nicotiana tabacum under Field Conditions" International Journal of Molecular Sciences 24, no. 2: 1160. https://doi.org/10.3390/ijms24021160





