Mycobacterium tuberculosis Cell Wall Antigens Induce the Formation of Immune Complexes and the Development of Vasculitis in an Experimental Murine Model
Abstract
:1. Introduction
2. Results
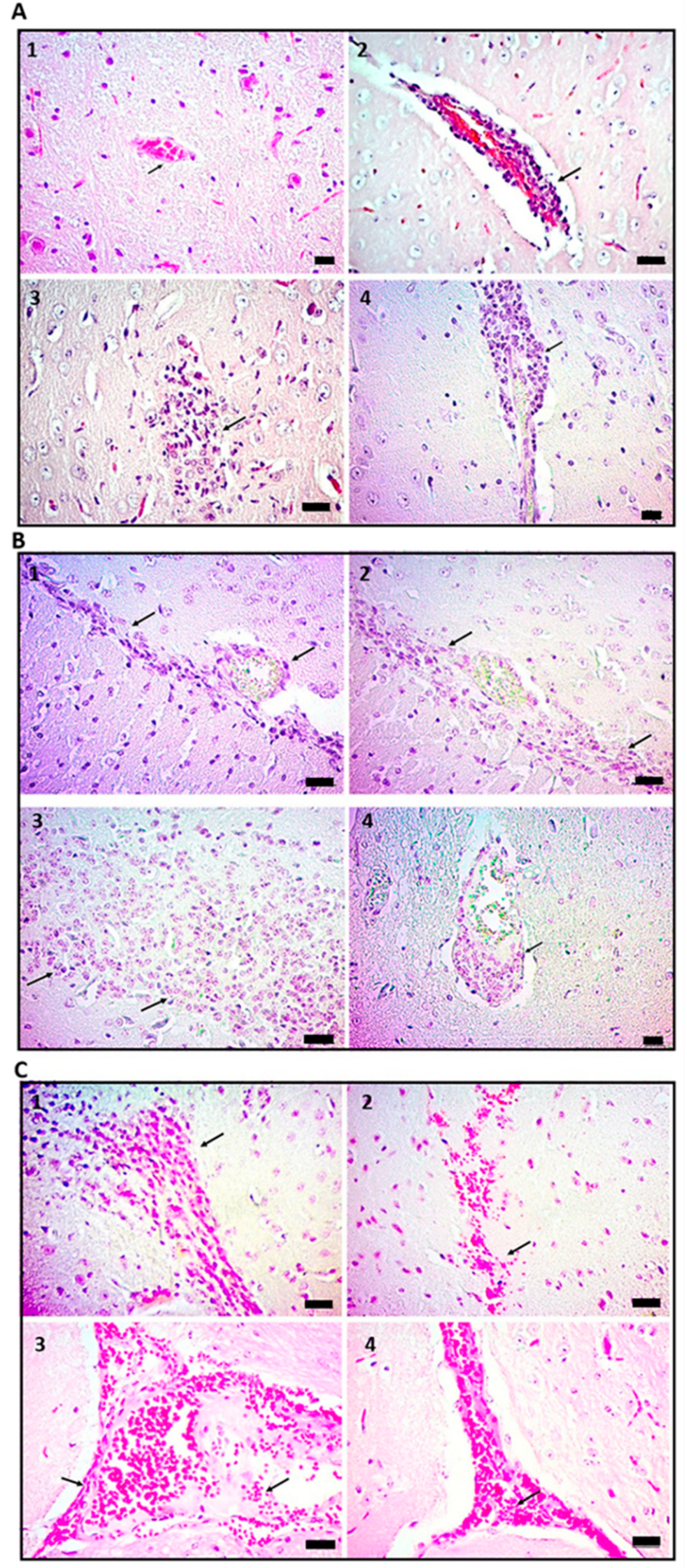
2.1. Administration of Cell Wall Mtb Antigens Induces Cerebral Vasculitis in Mice
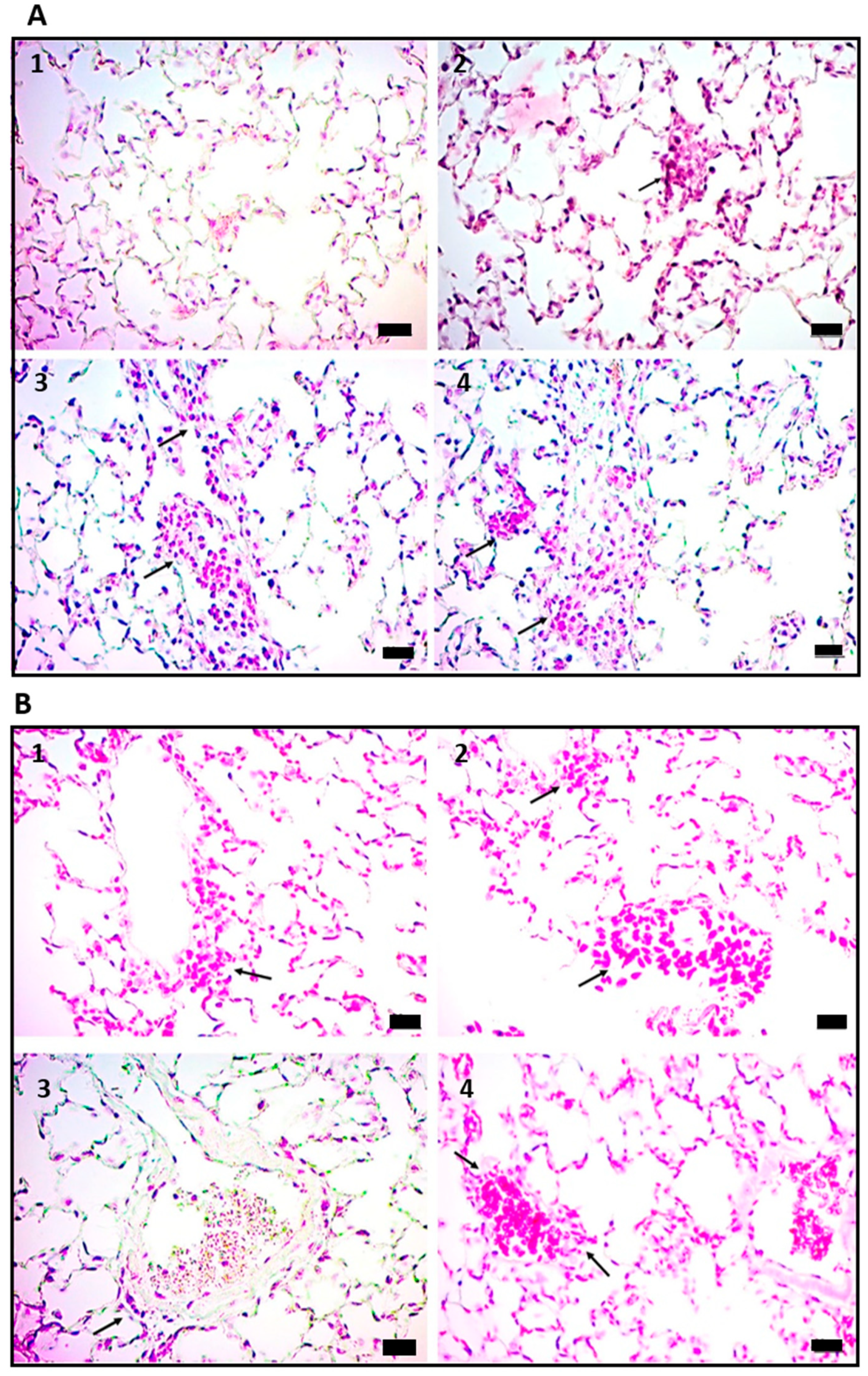
2.2. Vasculitis Induced by Mtb Antigens Is Systemic and Generalizes to Visceral Organs
2.3. Vasculitis Induced by Cell Wall Mtb Antigens Is Mediated by Deposition of ICs
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Mtb Cell Wall Preparations
4.3. Exposure of Mice with the Mtb Cell Wall
4.4. Quantification of Immune Complexes
4.5. Histopathological Analysis
4.6. Evidence of IC Deposition
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Takeuchi, S.Y.; Renjifo, M.E.; Medina, F.J. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics 2019, 39, 2023–2037. [Google Scholar] [CrossRef]
- Moule, M.G.; Cirillo, J.D. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Front. Cell Infect. Microbiol 2020, 10, 65. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sahly, H.M.; Teeter, L.D.; Pan, X.; Musser, J.M.; Graviss, E.A. Mortality associated with central nervous system tuberculosis. J. Infect. 2007, 55, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Rock, R.B.; Olin, M.; Baker, C.A.; Molitor, T.W.; Peterson, P.K. Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin. Microbiol. Rev. 2008, 21, 243–261. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.A.; Wicke, F.; Foerch, C.; Weidauer, S. Central Nervous System Tuberculosis: Etiology, Clinical Manifestations and Neuroradiological Features. Clin. Neuroradiol. 2019, 29, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Kalita, J.; Maurya, P.K. Stroke in tuberculous meningitis. J. Neurol. Sci. 2011, 303, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.G.; Cervantes-Arslanian, A.M. Infectious Etiologies of Stroke. Semin. Neurol. 2019, 39, 482–494. [Google Scholar] [CrossRef]
- Selvaraj, J.U.; Sujalini, B.B.; Rohitson, M.S.; George, A.A.; Arvind, V.H.; Mishra, A.K. Identification of predictors of cerebrovascular infarcts in patients with tuberculous meningitis. Int. J. Mycobacteriol. 2020, 9, 303–308. [Google Scholar]
- Del Brutto, O.H. Infections and stroke. Handb. Clin. Neurol. 2009, 93, 851–872. [Google Scholar]
- Sams, W.M., Jr. Necrotizing vasculitis. J. Am. Acad. Derm. 1980, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, C. Septic vasculitis and vasculopathy in some infectious emergencies: The perspective of the histopathologist. G. Ital. Derm. Venereol. 2015, 150, 73–85. [Google Scholar]
- Chen, Y.H.; Yan, J.J.; Chao, S.C.; Lee, J.Y. Erythema induratum: A clinicopathologic and polymerase chain reaction study. J. Med. Assoc. 2001, 100, 244–249. [Google Scholar]
- Kitamura, H.; Shimizu, K.; Takeda, H.; Tai, H.; Ito, Y.; Fukunaga, M. A case of henoch-schonlein purpura nephritis in pulmonary tuberculosis. Am. J. Med. Sci. 2007, 333, 117–121. [Google Scholar] [CrossRef]
- Chanprapaph, K.; Roongpisuthipong, W.; Thadanipon, K. Annular leukocytoclastic vasculitis associated with anti-tuberculosis medications: A case report. J. Med. Case Rep. 2013, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.; Jung, C.Y. Cutaneous Leukocytoclastic Vasculitis with Gastrointestinal Involvement after Anti-Tuberculosis Treatment. Tuberc. Respir. Dis. 2017, 80, 210–211. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhen, X.; Liu, Y.; Wu, Q. Immunologic Cerebral Vasculitis and Extrapulmonary Tuberculosis: An Uncommon Association. J. Clin. Diagn. Res. 2015, 9, OD03-5. [Google Scholar] [CrossRef]
- Madhavan, H.N.; Therese, K.L.; Doraiswamy, K. Further investigations on the association of Mycobacterium tuberculosis with Eales’ disease. Indian J. Ophthalmol. 2002, 50, 35–39. [Google Scholar]
- Jancar, S.; Sanchez Crespo, M. Immune complex-mediated tissue injury: A multistep paradigm. Trends Immunol. 2005, 26, 48–55. [Google Scholar] [CrossRef]
- Johnson, N.M.; McNicol, M.W.; Burton-Kee, E.J.; Mowbray, J.F. Circulating immune complexes in tuberculosis. Thorax 1981, 36, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Samuel, A.M.; Ashtekar, M.D.; Ganatra, R.D. Significance of circulating immune complexes in pulmonary tuberculosis. Clin. Exp. Immunol. 1984, 58, 317–324. [Google Scholar] [PubMed]
- Senbagavalli, P.; Hilda, J.N.; Ramanathan, V.D.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Immune complexes isolated from patients with pulmonary tuberculosis modulate the activation and function of normal granulocytes. Clin. Vaccine Immunol. 2012, 19, 1965–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybylski, G.; Golda, R. Research on the occurrence of Mycobacterium tuberculosis antigens in the circulating immune complexes, isolated from serum of patients with tuberculosis. Med. Sci. Monit. 2014, 20, 6–10. [Google Scholar] [PubMed] [Green Version]
- Patil, S.A.; Gourie-Devi, M.; Anand, A.R.; Vijaya, A.N.; Pratima, N.; Neelam, K.; Chandramuki, A. Significance of mycobacterial immune complexes (IgG) in the diagnosis of tuberculous meningitis. Tuber Lung Dis. 1996, 77, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavon, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar] [PubMed]
- Barbulescu, A.L.; Sandu, R.E.; Vreju, A.F.; Ciurea, P.L.; Criveanu, C.; Firulescu, S.C.; Chisalau, A.B.; Parvanescu, C.D.; Ciobanu, D.A.; Radu, M.; et al. Neuroinflammation in systemic lupus erythematosus—A review. Rom. J. Morphol. Embryol. 2019, 60, 781–786. [Google Scholar]
- Beuker, C.; Schmidt, A.; Strunk, D.; Sporns, P.B.; Wiendl, H.; Meuth, S.G.; Minnerup, J. Primary angiitis of the central nervous system: Diagnosis and treatment. Adv. Neurol. Disord. 2018, 11, 1756286418785071. [Google Scholar] [CrossRef] [Green Version]
- Mandal, J.; Chung, S.A. Primary Angiitis of the Central Nervous System. Rheum. Dis. Clin. N. Am. 2017, 43, 503–518. [Google Scholar] [CrossRef]
- Namer, I.J.; Steibel, J. Antibody directed against mannan of the Mycobacterium tuberculosis cell envelope provokes blood-brain barrier breakdown. J. Neuroimmunol. 2000, 103, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Maker, J.H.; Stroup, C.M.; Huang, V.; James, S.F. Antibiotic Hypersensitivity Mechanisms. Pharmacy 2019, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.K.; Chen, M.L. Crescentic glomerulonephritis associated with miliary tuberculosis. Clin. Nephrol. 2009, 71, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, J.I.; Kim, J.E.; Choi, G.S.; Park, H.S.; Ye, Y.M.; Yim, H. Cutaneous leukocytoclastic vasculitis due to anti-tuberculosis medications, rifampin and pyrazinamide. Allergy Asthma Immunol. Res. 2010, 2, 55–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, V.; Sibal, A.; Rajgarhia, S. Antituberculosis therapy-associated cutaneous leukocytoclastic vasculitis. J. Trop. Pediatr. 2013, 59, 507–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, A.; Uma Devi, K.R.; Ramalingam, B.; Brennan, P.J. Improved diagnosis of pulmonary tuberculosis by detection of free and immune complex-bound anti-30 kDa antibodies. Diagn. Microbiol. Infect. Dis. 2004, 50, 253–259. [Google Scholar] [CrossRef]
- Ebenezer, R.S.; Gupta, U.D.; Gupta, P.; Shakila, H. Protective effect of antigen excess immune complex in guinea pigs infected with Mycobacterium tuberculosis. Indian J. Med. Res. 2017, 146, 629–635. [Google Scholar]
- Goyal, B.; Sheikh, J.A.; Agarwal, R.; Verma, I. Levels of circulating immune complexes containing Mycobacterium Tuberculosis-specific antigens in pulmonary tuberculosis and sarcoidosis patients. Indian J. Med. Microbiol. 2017, 35, 290–292. [Google Scholar] [CrossRef]
- Sousa, A.O.; Wargnier, A.; Poinsignon, Y.; Simonney, N.; Gerber, F.; Lavergne, F.; Herrmann, J.L.; Lagrange, P.H. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tuber Lung Dis. 2000, 80, 27–33. [Google Scholar] [CrossRef]
- Choreno-Parra, J.A.; Weinstein, L.I.; Yunis, E.J.; Zuniga, J.; Hernandez-Pando, R. Thinking Outside the Box: Innate- and B Cell-Memory Responses as Novel Protective Mechanisms Against Tuberculosis. Front. Immunol. 2020, 11, 226. [Google Scholar] [CrossRef]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Senbagavalli, P.; Geetha, S.T.; Venkatesan, P.; Ramanathan, V.D. Defective solubilization of immune complexes and activation of the complement system in patients with pulmonary tuberculosis. J. Clin. Immunol. 2009, 29, 674–680. [Google Scholar] [CrossRef]
- Shen, C.Y.; Hsieh, S.C.; Yu, C.L.; Wang, J.Y.; Lee, L.N.; Yu, C.J. Autoantibody prevalence in active tuberculosis: Reactive or pathognomonic? BMJ Open 2013, 3, e002665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoenfeld, Y.; Vilner, Y.; Coates, A.R.; Rauch, J.; Lavie, G.; Shaul, D.; Pinkhas, J. Monoclonal anti-tuberculosis antibodies react with DNA, and monoclonal anti-DNA autoantibodies react with Mycobacterium tuberculosis. Clin. Exp. Immunol. 1986, 66, 255–261. [Google Scholar] [PubMed]
- Raja, A.; Ranganathan, U.D.; Ramalingam, B. Clinical value of specific detection of immune complex-bound antibodies in pulmonary tuberculosis. Diagn. Microbiol. Infect. Dis. 2006, 56, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.R.; McNeil, M.; Brennan, P.J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J. Bacteriol. 1990, 172, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, U.D.; Bethunaickan, R.; Raja, A. Isolation of Circulating Immune Complexes from TB Patient Serum for Serodiagnosis. Bio-Protocol 2012, 2, e188. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Noriega, F.A.; Salinas-Lara, C.; Sánchez-Garibay, C.; Torres-Ruíz, J.J.; Maravillas-Montero, J.L.; Castañón-Arreola, M.; Hernández-Campos, M.E.; Rodríguez-Balderas, C.; Basurto-López, B.V.; Peñafiel-Salgado, C.; et al. Mycobacterium tuberculosis Cell Wall Antigens Induce the Formation of Immune Complexes and the Development of Vasculitis in an Experimental Murine Model. Int. J. Mol. Sci. 2023, 24, 1242. https://doi.org/10.3390/ijms24021242
Pérez-Noriega FA, Salinas-Lara C, Sánchez-Garibay C, Torres-Ruíz JJ, Maravillas-Montero JL, Castañón-Arreola M, Hernández-Campos ME, Rodríguez-Balderas C, Basurto-López BV, Peñafiel-Salgado C, et al. Mycobacterium tuberculosis Cell Wall Antigens Induce the Formation of Immune Complexes and the Development of Vasculitis in an Experimental Murine Model. International Journal of Molecular Sciences. 2023; 24(2):1242. https://doi.org/10.3390/ijms24021242
Chicago/Turabian StylePérez-Noriega, Flaubert Alexis, Citlaltepetl Salinas-Lara, Carlos Sánchez-Garibay, José Jiram Torres-Ruíz, José Luis Maravillas-Montero, Mauricio Castañón-Arreola, María Elena Hernández-Campos, Cesar Rodríguez-Balderas, Beatriz Victoria Basurto-López, Carlos Peñafiel-Salgado, and et al. 2023. "Mycobacterium tuberculosis Cell Wall Antigens Induce the Formation of Immune Complexes and the Development of Vasculitis in an Experimental Murine Model" International Journal of Molecular Sciences 24, no. 2: 1242. https://doi.org/10.3390/ijms24021242







