The Roles of miRNAs in Predicting Bladder Cancer Recurrence and Resistance to Treatment
Abstract
:1. Introduction
2. Methods
3. EMT
4. Cell Cycle
5. FGFR3
6. Hippo Signaling
7. Wnt Signaling
8. Fatty Acid Metabolism and Synthesis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Matulewicz, R.S.; Steinberg, G.D. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtröder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D.; Fan, C.; Tiganelli, C.J.; Wobker, S.E.; Yeh, J.J.; Milowsky, M.I.; Iyer, G.; Parker, J.S.; et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef] [Green Version]
- Fong, M.H.Y.; Feng, M.; McConkey, D.J.; Choi, W. Update on bladder cancer molecular subtypes. Transl. Androl. Urol. 2020, 9, 2881–2889. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Ward, D.G.; Gordon, N.S.; Boucher, R.H.; Pirrie, S.J.; Baxter, L.; Ott, S.; Silcock, L.; Whalley, C.M.; Stockton, J.D.; Beggs, A.D.; et al. Targeted deep sequencing of urothelial bladder cancers and associated urinary DNA: A 23-gene panel with utility for non-invasive diagnosis and risk stratification. BJU Int. 2019, 124, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Traczyk-Borszynska, M.; Borkowska, E.; Jablonowski, Z.; Jedrzejczyk, A.; Pietrusinski, M.; Kaluzewski, B.; Sosnowski, M.; Borowiec, M. Genetic diversity of urinary bladder cancer and the risk of recurrence based on mutation analysis. Neoplasma 2016, 63, 952–960. [Google Scholar] [CrossRef]
- Yin, X.H.; Jin, Y.H.; Cao, Y.; Wong, Y.; Weng, H.; Sun, C.; Deng, J.H.; Zeng, X.T. Development of a 21-miRNA Signature Associated with the Prognosis of Patients with Bladder Cancer. Front. Oncol. 2019, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019, 110, 408–419. [Google Scholar] [CrossRef]
- Enokida, H.; Yoshino, H.; Matsushita, R.; Nakagawa, M. The role of microRNAs in bladder cancer. Investig. Clin. Urol. 2016, 57 (Suppl. 1), S60–S76. [Google Scholar] [CrossRef] [Green Version]
- Long, J.D.; Sullivan, T.B.; Humphrey, J.; Logvinenko, T.; Summerhayes, K.A.; Kozinn, S.; Harty, N.; Summerhayes, I.C.; Libertino, J.A.; Holway, A.H.; et al. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015, 7, 2500–2509. [Google Scholar] [PubMed]
- Urabe, F.; Matsuzaki, J.; Ito, K.; Takamori, H.; Tsuzuki, S.; Miki, J.; Kimura, T.; Egawa, S.; Nakamura, E.; Matsui, Y.; et al. Serum microRNA as liquid biopsy biomarker for the prediction of oncological outcomes in patients with bladder cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2022, 29, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Xu, W.; Jin, Y.; Wu, J.; Pan, Y.; Zhou, F. MiRNA-217 accelerates the proliferation and migration of bladder cancer via inhibiting KMT2D. Biochem. Biophys. Res. Commun. 2019, 519, 747–753. [Google Scholar] [CrossRef]
- Feng, C.; Sun, P.; Hu, J.; Feng, H.; Li, M.; Liu, G.; Pan, Y.; Feng, Y.; Xu, Y.; Feng, K.; et al. miRNA-556-3p promotes human bladder cancer proliferation, migration and invasion by negatively regulating DAB2IP expression. Int. J. Oncol. 2017, 50, 2101–2112. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Paraskevopoulou, M.D.; Karagkouni, D.; Georgakilas, G.; Vergoulis, T.; Kanellos, I.; Anastasopoulos, I.-L.; Maniou, S.; Karathanou, K.; Kalfakakou, D.; et al. DIANA-TarBase v7.0: Indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015, 43, D153–D159. [Google Scholar] [CrossRef]
- Du, L.; Jiang, X.; Duan, W.; Wang, R.; Wang, L.; Zheng, G.; Yan, K.; Wang, L.; Li, J.; Zhang, X.; et al. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 2017, 8, 40832–40842. [Google Scholar] [CrossRef] [Green Version]
- Juracek, J.; Stanik, M.; Vesela, P.; Radova, L.; Dolezel, J.; Svoboda, M.; Slaby, O. Tumor expression of miR-34a-3p is an inde-pendent predictor of recurrence in non-muscle-invasive bladder cancer and promising additional factor to improve predictive value of EORTC nomogram. Urol. Oncol. 2019, 37, 184.e1–184.e7. [Google Scholar] [CrossRef]
- Andrew, A.S.; Marsit, C.J.; Schned, A.R.; Seigne, J.D.; Kelsey, K.T.; Moore, J.H.; Perreard, L.; Karagas, M.R.; Sempere, L.F. Expression of tumor suppressive microRNA-34a is associated with a reduced risk of bladder cancer recurrence. Int. J. Cancer 2015, 137, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanca, A.; Sanchez-Gonzalez, A.; Requena, M.J.; Carrasco-Valiente, J.; Gomez-Gomez, E.; Cheng, L.; Cimadamore, A.; Mon-tironi, R.; Lopez-Beltran, A. Expression of miR-100 and miR-138 as prognostic biomarkers in non-muscle-invasive bladder cancer. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2019, 127, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, Y.; Wang, Z.; Zhang, X.; Li, D.; Wei, J. A SNP of miR-146a is involved in bladder cancer relapse by affecting the function of bladder cancer stem cells via the miR-146a signallings. J. Cell. Mol. Med. 2020, 24, 8545–8556. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, Z.; Xu, C.; Jiang, X. MicroRNA-148a represents an independent prognostic marker in bladder cancer. Tumor Biol. 2016, 37, 7915–7920. [Google Scholar] [CrossRef]
- Jiang, X.; Du, L.; Wang, L.; Li, J.; Liu, Y.; Zheng, G.; Qu, A.; Zhang, X.; Pan, H.; Yang, Y.; et al. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int. J. Cancer 2015, 136, 854–862. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, X.; Fang, A.; Wang, J.; Yang, Y.; Wang, L.; Du, L.; Wang, C. Direct quantitative detection for cell-free miR-155 in urine: A potential role in diagnosis and prognosis for non-muscle invasive bladder cancer. Oncotarget 2016, 7, 3255. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lin, C.; Zhao, L.; Zhou, L.; Pan, X.; Quan, J.; Peng, X.; Li, W.; Li, H.; Xu, J.; et al. Oncogene miR-187-5p is associated with cellular proliferation, migration, invasion, apoptosis and an increased risk of recurrence in bladder cancer. Biomed. Pharmacother. 2018, 105, 461–469. [Google Scholar] [CrossRef]
- Yun, S.J.; Jeong, P.; Kim, W.-T.; Kim, T.H.; Lee, Y.-S.; Song, P.H.; Choi, Y.-H.; Kim, I.Y.; Moon, S.-K.; Kim, W.-J. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int. J. Oncol. 2012, 41, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Qu, A.; Liu, J.; Wang, R.; Liu, Y.; Li, G.; Duan, W.; Fang, Q.; Jiang, X.; Wang, L.; et al. Serum miR-210 Contributes to Tumor Detection, Stage Prediction and Dynamic Surveillance in Patients with Bladder Cancer. PLoS ONE 2015, 10, e0135168. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Wang, L.; Dong, Z.; Du, L.; Yang, Y.; Guo, Y.; Wang, C. Downregulation of urinary cell-free mi-croRNA-214 as a diagnostic and prognostic biomarker in bladder cancer. J. Surg. Oncol. 2015, 111, 992–999. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, H.W.; Kim, W.T.; Kim, Y.J.; Yun, S.J.; Lee, S.C.; Kim, W.J. Cell-Free microRNA-214 From Urine as a Biomarker for Non-Muscle-Invasive Bladder Cancer. Korean J. Urol. 2013, 54, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Tsikrika, F.D.; Avgeris, M.; Levis, P.K.; Tokas, T.; Stravodimos, K.; Scorilas, A. miR-221/222 cluster expression improves clinical stratification of non-muscle invasive bladder cancer (TaT1) patients’ risk for short-term relapse and progression. Genes Chro-Mosomes Cancer 2018, 57, 150–161. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, L.; Lin, C.; Pan, X.; Xie, J.; Zhao, L.; Quan, J.; Xu, J.; Guan, X.; Xu, W.; et al. MiR-302b regulates cell functions and acts as a potential biomarker to predict recurrence in bladder cancer. Life Sci. 2018, 209, 15–23. [Google Scholar] [CrossRef]
- Shee, K.; Seigne, J.D.; Karagas, M.R.; Marsit, C.J.; Hinds, J.W.; Schned, A.R.; Pettus, J.R.; Armstrong, D.A.; Miller, T.W.; Andrew, A.S.; et al. Identification of Let-7f-5p as a novel bi-omarker of recurrence in non-muscle invasive bladder cancer. Cancer Biomark. Sect. Dis. Mark. 2020, 29, 101–110. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Z.; Zhang, Z.; Liu, T.; Zhang, J.; Huang, H.; Li, Y. MiR-7-5p suppresses invasion via downregulation of the autophagy-related gene ATG7 and increases chemoresistance to cisplatin in BCa. Bioengineered 2022, 13, 7328–7339. [Google Scholar] [CrossRef]
- Tao, J.; Lu, Q.; Wu, D.; Li, P.; Xu, B.; Qing, W.; Wang, M.; Zhang, Z.; Zhang, W. microRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol. Rep. 2011, 25, 1721–1729. [Google Scholar]
- Xiao, J.; Niu, S.; Zhu, J.; Lv, L.; Deng, H.; Pan, D.; Shen, D.; Xu, C.; Shen, Z.; Tao, T. miR-22-3p enhances multi-chemoresistance by targeting NET1 in bladder cancer cells. Oncol. Rep. 2018, 39, 2731–2740. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; Efstathiou, J.; Singh, R.; McElroy, J.; Volinia, S.; Cui, R.; Cui, R.; Ibrahim, A.; Johnson, B.; Gupta, N.; et al. MicroRNA Biomarkers for Patients with Mus-cle-Invasive Bladder Cancer Undergoing Selective Bladder-Sparing Trimodality Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 197–206. [Google Scholar] [CrossRef]
- Deng, Y.; Bai, H.; Hu, H. rs11671784 G/A variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem. Biophys. Res. Commun. 2015, 458, 321–327. [Google Scholar] [CrossRef]
- Hwang, T.I.S.; Chen, P.C.; Tsai, T.F.; Lin, J.F.; Chou, K.Y.; Ho, C.Y.; Chen, H.E.; Chang, A.C. Hsa-miR-30a-3p overcomes the acquired protective autophagy of bladder cancer in chemotherapy and suppresses tumor growth and muscle invasion. Cell Death Dis. 2022, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Qin, L.; Zhu, Z.; Wang, X.; Liu, Y.; Fan, Y.; Zhong, S.; Wang, X.; Zhang, X.; Xia, L.; et al. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin α5. Oncotarget 2016, 7, 27445–27457. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Wu, Y.; Fang, Z.; Wu, Q.; Wu, C.; Hao, Y.; Yang, X.; Zhao, J.; Li, J.; et al. MicroRNA-34a Attenuates Metastasis and Chemoresistance of Bladder Cancer Cells by Targeting the TCF1/LEF1 Axis. Cell Physiol. Biochem. 2018, 48, 87–98. [Google Scholar] [CrossRef]
- Vinall, R.L.; Ripoll, A.Z.; Wang, S.; Pan, C.X.; deVere White, R.W. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int. J. Cancer 2012, 130, 2526–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yu, G.; Shi, R.; Lang, B.; Chen, X.; Xia, D.; Xiao, H.; Guo, X.; Guan, W.; Ye, Z.; et al. Cisplatin-induced epigenetic activation of miR-34a sensitizes bladder cancer cells to chemotherapy. Mol. Cancer 2014, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, H.; Wang, Y.; Li, Z.; Pan, Y.; Liu, Q.; Yang, M.; Wang, J. Repression of the miR-93-enhanced sensitivity of bladder carcinoma to chemotherapy involves the regulation of LASS2. OncoTargets Ther. 2016, 9, 1813–1822. [Google Scholar]
- Luan, T.; Fu, S.; Huang, L.; Zuo, Y.; Ding, M.; Li, N.; Chen, J.; Wang, H.; Wang, J. MicroRNA-98 promotes drug resistance and regulates mitochondrial dynamics by targeting LASS2 in bladder cancer cells. Exp. Cell Res. 2018, 373, 188–197. [Google Scholar] [CrossRef]
- Bu, Q.; Fang, Y.; Cao, Y.; Chen, Q.; Liu, Y. Enforced expression of miR-101 enhances cisplatin sensitivity in human bladder cancer cells by modulating the cyclooxygenase-2 pathway. Mol. Med. Rep. 2014, 10, 2203–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Xie, D.; Zhang, H. MicroRNA-101-3p advances cisplatin sensitivity in bladder urothelial carcinoma through targeted silencing EZH2. J. Cancer 2019, 10, 2628–2634. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, Q.; Wu, G.; Li, S.; Wang, Q. miR-129-5p inhibits gemcitabine resistance and promotes cell apoptosis of bladder cancer cells by targeting Wnt5a. Int. Urol. Nephrol. 2018, 50, 1811–1819. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H. Knockdown of microRNA-130b improves doxorubicin sensitivity in bladder urothelial carcinoma by nega-tively regulating cylindromatosis expression. Arch. Med. Sci. AMS 2021, 17, 1038–1043. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Q.; Niu, X.; Wang, G.; Zheng, S.; Fu, G.; Wang, Z. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol. Lett. 2017, 13, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. miR-193a-3p regulates the multi-drug resistance of bladder cancer by targeting the LOXL4 gene and the oxidative stress pathway. Mol. Cancer 2014, 13, 234. [Google Scholar] [CrossRef]
- Lv, L.; Li, Y.; Deng, H.; Zhang, C.; Pu, Y.; Qian, L.; Xiao, J.; Zhao, W.; Liu, Q.; Zhang, D.; et al. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015, 357, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. The miR-193a-3p regulated PSEN1 gene suppresses the mul-ti-chemoresistance of bladder cancer. Biochim. Biophys. Acta 2015, 1852, 520–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Deng, H.; Lv, L.; Zhang, C.; Qian, L.; Xiao, J.; Zhao, W.; Liu, Q.; Zhang, D.; Wang, Y.; et al. The miR-193a-3p-regulated ING5 gene activates the DNA damage response pathway and inhibits multi-chemoresistance in bladder cancer. Oncotarget 2015, 6, 10195–10206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Duan, H.; Xie, Y.; Ning, Y.; Zhang, X.; Hui, N.; Wang, C.; Zhang, J.; Zhou, J. MiR-193a-5p Targets the Coding Region of AP-2α mRNA and Induces Cisplatin Resistance in Bladder Cancers. J. Cancer 2016, 7, 1740–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, T.; Niinuma, T.; Nishiyama, N.; Shinkai, N.; Kitajima, H.; Kai, M.; Maruyama, R.; Tokino, T.; Masumori, N.; Suzuki, H. Epigenetic silencing of miR-200b is associated with cisplatin resistance in bladder cancer. Oncotarget 2018, 9, 24457–24469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bi, J.; Li, Z.; Li, Z.; Liu, X.; Kong, C. miR-214 reduces cisplatin resistance by targeting netrin-1 in bladder cancer cells. Int. J. Mol. Med. 2018, 41, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yang, X.; Cheng, Y.; Zhang, X.; Yang, C.; Deng, X.; Li, P.; Tao, J.; Yang, H.; Wei, J.; et al. MicroRNA-218 Increases the Sensitivity of Bladder Cancer to Cisplatin by Targeting Glut1. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2017, 41, 921–932. [Google Scholar] [CrossRef]
- Zeng, L.P.; Hu, Z.M.; Li, K.; Xia, K. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J. Cell Mol. Med. 2016, 20, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Zheng, J.Z.; Huang, X. Suppression of HAX-1 induced by miR-325 resensitizes bladder cancer cells to cisplatin-induced apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9303–9314. [Google Scholar] [PubMed]
- Yu, M.; Ozaki, T.; Sun, D.; Xing, H.; Wei, B.; An, J.; Yang, J.; Gao, Y.; Liu, S.; Kong, C.; et al. HIF-1α-dependent miR-424 induction confers cisplatin resistance on bladder cancer cells through down-regulation of pro-apoptotic UNC5B and SIRT4. J. Exp. Clin. Cancer Res. 2020, 39, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, S.; Wu, Y.; Cui, Y.; Cai, Y.; Lan, L.; Yang, H.; Chen, J.; Chen, W. Reduction of Bladder Cancer Chemosensitivity Induced by the Effect of HOXA-AS3 as a ceRNA for miR-455-5p That Upregulates Notch1. Front. Oncol. 2020, 10, 572672. [Google Scholar] [CrossRef]
- Salimian, J.; Baradaran, B.; Azimzadeh Jamalkandi, S.; Moridikia, A.; Ahmadi, A. MiR-486-5p enhances cisplatin sensitivity of human muscle-invasive bladder cancer cells by induction of apoptosis and down-regulation of metastatic genes. Urol. Oncol. 2020, 38, 738.e9–738.e21. [Google Scholar] [CrossRef]
- Chen, M.K.; Zhou, J.H.; Wang, P.; Ye, Y.L.; Liu, Y.; Zhou, J.W.; Chen, Z.J.; Yang, J.K.; Liao, D.Y.; Liang, Z.J.; et al. BMI1 activates P-glycoprotein via transcription repression of miR-3682-3p and enhances chemoresistance of bladder cancer cell. Aging 2021, 13, 18310–18330. [Google Scholar] [CrossRef]
- Du, B.; Js, S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. Available online: https://pubmed.ncbi.nlm.nih.gov/27455225/ (accessed on 28 November 2022). [CrossRef] [Green Version]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Baumgart, E.; Cohen, M.S.; Silva Neto, B.; Jacobs, M.A.; Wotkowicz, C.; Rieger-Christ, K.M.; Biolo, A.; Zeheb, R.; Loda, M.; Libertino, J.A.; et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Kang, Y.; Li, H.; He, H.Q.; Zheng, L.; Wu, S.Q.; Ai, K.; Zhang, L.; Xu, R.; Zhang, X.-Z.; et al. circST6GALNAC6 suppresses bladder cancer metastasis by sponging miR-200a-3p to modulate the STMN1/EMT axis. Cell Death Dis. 2021, 12, 168. [Google Scholar] [CrossRef]
- Li, W.J.; Li, G.; Liu, Z.W.; Chen, Z.Y.; Pu, R. LncRNA LINC00355 promotes EMT and metastasis of bladder cancer cells through the miR-424-5p/HMGA2 axis. Neoplasma 2021, 68, 1225–1235. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, M.; Tan, G.; Liang, Z.; Qin, Y.; Chen, L.; Chen, H.; Liu, J. miR-200c Inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Transl. Med. 2014, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.F.; Zeng, F.; Qi, L.; Zu, X.B.; Wang, J.; Liu, L.F.; Li, Y. Transforming growth factor-β1 induces epithelial-mesenchymal transition and increased expression of matrix metalloproteinase-16 via miR-200b downregulation in bladder cancer cells. Mol. Med. Rep. 2014, 10, 1549–1554. [Google Scholar] [CrossRef]
- Mei, Y.; Zheng, J.; Xiang, P.; Liu, C.; Fan, Y. Prognostic value of the miR-200 family in bladder cancer: A systematic review and meta-analysis. Medicine 2020, 99, e22891. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ho, J.Y.; Yu, C.P.; Cho, C.J.; Wu, C.L.; Huang, C.S.; Gao, H.W.; Yu, D.S. Ellagic Acid Resensitizes Gemcita-bine-Resistant Bladder Cancer Cells by Inhibiting Epithelial-Mesenchymal Transition and Gemcitabine Transporters. Cancers 2021, 13, 2032. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Zang, J.; Wu, G.; Wen, Y.; Liang, Y.; Long, Y.; Guo, W.; Zang, C.; Hu, X.; et al. ADNP prompts the cisplatin-resistance of bladder cancer via TGF-β-mediated epithelial-mesenchymal transition (EMT) pathway. J. Cancer 2021, 12, 5114–5124. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.; Yang, S.; Qiao, R.; Zhu, X.; Zhang, J. CXCL5 promotes mitomycin C resistance in non-muscle invasive bladder cancer by activating EMT and NF-κB pathway. Biochem. Biophys. Res. Commun. 2018, 498, 862–868. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, S.; Lu, M.; Luo, Y.; Wang, G.; Xiao, Y.; Ju, L.; Wang, X. EFEMP2 suppresses epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway in human bladder cancer. Int. J. Biol. Sci. 2019, 15, 2139–2155. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Zhang, X.; Xie, Y.; Ji, Z. LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways. Transl. Androl. Urol. 2020, 9, 2251–2261. [Google Scholar] [CrossRef]
- Yang, N.; Gao, J.; Hou, R.; Xu, X.; Yang, N.; Huang, S. Grape Seed Proanthocyanidins Inhibit Migration and Invasion of Bladder Cancer Cells by Reversing EMT through Suppression of TGF-β Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Fan, L.; Liao, X.; Cui, G.; Hu, H. CRTAC1 (Cartilage acidic protein 1) inhibits cell proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) process in bladder cancer by downregulating Yin Yang 1 (YY1) to inactivate the TGF-β pathway. Bioengineered 2021, 12, 9377–9389. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Zhan, H.; Chen, L.; Deng, Q.; Xiong, T.; Ye, J. lncRNA CASC9 sponges miR-758-3p to promote prolifer-ation and EMT in bladder cancer by upregulating TGF-β2. Oncol. Rep. 2021, 45, 265–277. [Google Scholar] [CrossRef]
- Zhang, Z.; Ao, P.; Han, H.; Zhang, Q.; Chen, Y.; Han, J.; Huang, Q.; Huang, H.; Zhuo, D. LncRNA PLAC2 upregulates miR-663 to downregulate TGF-β1 and suppress bladder cancer cell migration and invasion. BMC Urol. 2020, 20, 94. [Google Scholar] [CrossRef]
- Huang, C.S.; Tsai, C.H.; Yu, C.P.; Wu, Y.S.; Yee, M.F.; Ho, J.Y.; Yu, D.S. Long Noncoding RNA LINC02470 Sponges Mi-croRNA-143-3p and Enhances SMAD3-Mediated Epithelial-to-Mesenchymal Transition to Promote the Aggressive Properties of Bladder Cancer. Cancers 2022, 14, 968. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, N.; Yang, J.; Zhu, Y.; Zhang, Z.; Wang, J.; Xu, X.; Li, Z.; Liu, X.; Li, Z.; et al. Long non-coding RNA ZEB1-AS1 regulates miR-200b/FSCN1 signaling and enhances migration and invasion induced by TGF-β1 in bladder cancer cells. J. Exp. Clin. Cancer Res. CR 2019, 38, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Shen, L.; Yang, L.; Huang, X.; Lu, Q.; Cui, Y.; Zheng, X.; Zhao, X.; Zhang, D.; Huang, R.; et al. TGFβ1 Promotes Gemcitabine Resistance through Regulating the LncRNA-LET/NF90/miR-145 Signaling Axis in Bladder Cancer. Theranostics 2017, 7, 3053–3607. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, L.; Yu, Z.; Liao, G.; Lu, L.; Xu, R.; Zhao, Z.; Chen, G. Increased cyclin-dependent kinase 6 expression in bladder cancer. Oncol. Lett. 2012, 4, 43–46. [Google Scholar] [CrossRef]
- Long, Q.; Ma, A.H.; Zhang, H.; Cao, Z.; Xia, R.; Lin, T.Y.; Sonpavde, G.P.; de Vere White, R.; Guo, J.; Pan, C.-X. Combination of cyclin-dependent kinase and immune check-point inhibitors for the treatment of bladder cancer. Cancer Immunol. Immunother. 2020, 69, 2305–2317. [Google Scholar] [CrossRef]
- Wu, S.; Yang, J.; Xu, H.; Wang, X.; Zhang, R.; Lu, W.; Yang, J.; Li, X.; Chen, S.; Zou, Y.; et al. Circular RNA circGLIS3 promotes bladder cancer proliferation via the miR-1273f/SKP1/Cyclin D1 axis. Cell Biol. Toxicol. 2022, 38, 129–146. [Google Scholar] [CrossRef]
- Blanca, A.; Requena, M.J.; Alvarez, J.; Cheng, L.; Montironi, R.; Raspollini, M.R.; Reymundo, C.; Lopez-Beltran, A. FGFR3 and Cyclin D3 as urine biomarkers of bladder cancer recurrence. Biomarkers Med. 2016, 10, 243–253. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karakiewicz, P.I.; Ashfaq, R.; Lerner, S.P.; Palapattu, G.S.; Cote, R.J.; Sagalowsky, A.I.; Lotan, Y. Multiple bi-omarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer 2008, 112, 315–325. [Google Scholar] [CrossRef]
- March-Villalba, J.A.; Ramos-Soler, D.; Soriano-Sarrió, P.; Hervás-Marín, D.; Martínez-García, L.; Martínez-Jabaloyas, J.M. Immunohistochemical expression of Ki-67, Cyclin D1, p16INK4a, and Survivin as a predictive tool for recurrence and pro-gression-free survival in papillary urothelial bladder cancer pTa/pT1 G2 (WHO 1973). Urol. Oncol. 2019, 37, 158–165. [Google Scholar] [CrossRef]
- Jing, W.; Wang, G.; Cui, Z.; Xiong, G.; Jiang, X.; Li, Y.; Li, W.; Han, B.; Chen, S.; Shi, B. FGFR3 Destabilizes PD-L1 via NEDD4 to Control T-cell–Mediated Bladder Cancer Immune Surveillance. Cancer Res 2022, 82, 114–129. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Mertens, L.S.; Mayr, R.; Bostrom, P.J.; Real, F.X.; Zwarthoff, E.C.; Boormans, J.L.; Abas, C.; Geert Jacob Leonard Hubert van Leenders; Götz, S.; et al. FGFR3 Mutation Status and FGFR3 Expression in a Large Bladder Cancer Cohort Treated by Radical Cystectomy: Implications for Anti-FGFR3 Treatment? Eur. Urol. 2020, 78, 682–687. [Google Scholar] [CrossRef]
- Kacew, A.; Sweis, R.F. FGFR3 Alterations in the Era of Immunotherapy for Urothelial Bladder Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Gulìa, C.; Baldassarra, S.; Signore, F.; Rigon, G.; Pizzuti, V.; Gaffi, M.; Briganti, V.; Porrello, A.; Piergentili, R. Role of Non-Coding RNAs in the Etiology of Bladder Cancer. Genes 2017, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Sikic, D.; Taubert, H.; Breyer, J.; Eckstein, M.; Weyerer, V.; Keck, B.; Kubon, J.; Otto, W.; Worst, T.S.; Kriegmair, M.C.; et al. The Prognostic Value of FGFR3 Expression in Patients with T1 Non-Muscle Invasive Bladder Cancer. Cancer Manag. Res. 2021, 13, 6567–6578. [Google Scholar] [CrossRef]
- Beukers, W.; van der Keur, K.A.; Kandimalla, R.; Vergouwe, Y.; Steyerberg, E.W.; Boormans, J.L.; Jensen, J.B.; Lorente, J.A.; Real, F.X.; Segersten, U.; et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J. Urol. 2017, 197, 1410–1418. [Google Scholar] [CrossRef]
- Pal, S.K.; Somford, D.M.; Grivas, P.; Sridhar, S.S.; Gupta, S.; Bellmunt, J.; Sonpavde, G.; Fleming, M.T.; Lerner, S.P.; Loriot, Y.; et al. Targeting FGFR3 alterations with adjuvant infigratinib in invasive urothelial carcinoma: The phase III PROOF 302 trial. Future Oncol. 2022, 18, 2599–2614. [Google Scholar] [CrossRef]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.; Ji, T.; Saleh, M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 522–533. [Google Scholar] [CrossRef]
- Teo, M.Y.; Mota, J.M.; Whiting, K.A.; Li, H.A.; Funt, S.A.; Lee, C.H.; Solit, D.B.; Al-Ahmadie, H.; Milowsky, M.I.; Balar, A.; et al. Fibroblast Growth Factor Receptor 3 Alteration Status is Associated with Differential Sensitivity to Platinum-based Chemotherapy in Locally Advanced and Metastatic Urothelial Carcinoma. Eur. Urol. 2020, 78, 907–915. [Google Scholar] [CrossRef]
- Xie, X.; Lin, J.; Zhong, Y.; Fu, M.; Tang, A. FGFR3S249C mutation promotes chemoresistance by activating Akt signaling in bladder cancer cells. Exp. Ther. Med. 2019, 18, 1226–1234. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, R.; Ge, Y.; Qin, X.; Kang, X.; Wang, Y.; Zhang, X.; Song, C.; Quan, X.; Wang, H.; et al. Somatic FGFR3 Mutations Distinguish a Subgroup of Mus-cle-Invasive Bladder Cancers with Response to Neoadjuvant Chemotherapy. eBioMedicine 2018, 35, 198–203. [Google Scholar] [CrossRef]
- di Martino, E.; Alder, O.; Hurst, C.D.; Knowles, M.A. ETV5 links the FGFR3 and Hippo signalling pathways in bladder cancer. Sci. Rep. 2019, 9, 5740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.-L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, X.; Ma, L.; Guo, Z.; Liu, H.; Du, M.; Chu, H.; Wang, M.; Wang, Z.; Zhang, Z. Genetic variations in Hippo pathway genes influence bladder cancer risk in a Chinese population. Arch. Toxicol. 2020, 94, 785–794. [Google Scholar] [CrossRef]
- Xu, J.; Fang, X.; Long, L.; Wang, S.; Qian, S.; Lyu, J. HMGA2 promotes breast cancer metastasis by modulating Hippo-YAP signaling pathway. Cancer Biol. Ther. 2021, 22, 5–11. [Google Scholar] [CrossRef]
- Xia, J.; Zeng, M.; Zhu, H.; Chen, X.; Weng, Z.; Li, S. Emerging role of Hippo signalling pathway in bladder cancer. J. Cell. Mol. Med. 2018, 22, 4–15. [Google Scholar] [CrossRef]
- Ghasemi, H.; Mousavibahar, S.H.; Hashemnia, M.; Karimi, J.; Khodadadi, I.; Mirzaei, F.; Tavilani, H. Tissue stiffness contrib-utes to YAP activation in bladder cancer patients undergoing transurethral resection. Ann. N. Y. Acad. Sci. 2020, 1473, 48–61. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Maehama, T.; Nishio, M.; Otani, J.; Hikasa, H.; Mak, T.W.; Sasaki, T.; Honma, T.; Kondoh, Y.; Osada, H.; et al. N-(3,4-dimethoxyphenethyl)-6-methyl-2,3,4,9-tetrahydro-1H-carbazol-1-amine inhibits bladder cancer progression by sup-pressing YAP1/TAZ. Genes Cells Devoted Mol. Cell Mech. 2022, 27, 602–612. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Q.; Xu, S.; Du, C.; Liang, L.; Wu, K.; Wang, K.; Wang, X.; Chang, L.S.; He, D.; et al. Curcumin promotes KLF5 proteasome degradation through down-regulating YAP/TAZ in bladder cancer cells. Int. J. Mol. Sci. 2014, 15, 15173–15187. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, C.; Liu, Y.; Fu, S.; Yuan, C.; Luo, J.; Huang, X.; Yang, W.; Xie, W.; Zhuang, C. Silencing of lncRNA MIR497HG via CRISPR/Cas13d Induces Bladder Cancer Progression Through Promoting the Crosstalk Between Hippo/Yap and TGF-β/Smad Signaling. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, J.; Tang, J.; Zhou, F.; He, Z.; Liu, T.; Liu, T. MINDY1 promotes bladder cancer progression by stabilizing YAP. Cancer Cell Int. 2021, 21, 395. [Google Scholar] [CrossRef]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free. Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef]
- Ciamporcero, E.; Daga, M.; Pizzimenti, S.; Roetto, A.; Dianzani, C.; Compagnone, A.; Palmieri, A.; Ullio, C.; Cangemi, L.; Pili, R.; et al. Crosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancer. Free Radic. Biol. Med. 2018, 115, 447–457. [Google Scholar] [CrossRef]
- Cheng, X.; Lou, K.; Ding, L.; Zou, X.; Huang, R.; Xu, G.; Zou, J.; Zhang, G. Clinical potential of the Hippo-YAP pathway in bladder cancer. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022. [Google Scholar] [CrossRef]
- Du, S.; Sui, Y.; Ren, W.; Zhou, J.; Du, C. PYCR1 promotes bladder cancer by affecting the Akt/Wnt/β-catenin signaling. J. Bioenerg. Biomembr. 2021, 53, 247–258. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Chong, T.; Xue, L.; Luo, Q.; Tang, X.; Zhai, X.; Chen, J.; Zhang, X. TMEM88 exhibits an antiproliferative and anti-invasive effect in bladder cancer by downregulating Wnt/β-catenin signaling. J. Biochem. Mol. Toxicol. 2021, 35, e22835. [Google Scholar] [CrossRef]
- Cai, D.; Zhou, Z.; Wei, G.; Wu, P.; Kong, G. Construction and verification of a novel hypoxia-related lncRNA signature related with survival outcomes and immune microenvironment of bladder urothelial carcinoma by weighted gene co-expression network analysis. Front. Genet. 2022, 13. [Google Scholar] [CrossRef]
- Shan, G.; Zhou, X.; Gu, J.; Zhou, D.; Cheng, W.; Wu, H.; Wang, Y.; Tang, T.; Wang, X. Downregulated exosomal mi-croRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN. Cell Oncol. 2021, 44, 45–59. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, R.; Belmonte-Fernández, A.; Flores, M.L.; González-Moreno, M.; Pérez-Valderrama, B.; Romero, F.; Japón, M.Á.; Sáez, C. Wnt/β-Catenin Signaling Contributes to Paclitaxel Resistance in Bladder Cancer Cells with Cancer Stem Cell-Like Properties. Int. J. Mol. Sci. 2021, 23, 450. [Google Scholar] [CrossRef]
- Jeong, H.; Oh, H.E.; Kim, H.; Lee, J.H.; Lee, E.S.; Kim, Y.S.; Choi, J.W. Upregulation of Fatty Acid Transporters is Associated with Tumor Progression in Non-Muscle-Invasive Bladder Cancer. Pathol. Oncol. Res. 2021, 27, 594705. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Rashed, H.E.; Elkady, E.; Elsebai, E.A.; El-Azony, A.; Matar, I. Fatty acid synthase, Her2/neu, and E2F1 as prognostic markers of progression in non-muscle invasive bladder cancer. Ann. Diagn. Pathol. 2019, 39, 42–52. [Google Scholar] [CrossRef]
- Jiang, B.; Li, E.H.; Lu, Y.Y.; Jiang, Q.; Cui, D.; Jing, Y.F.; Xia, S.J. Inhibition of fatty-acid synthase suppresses P-AKT and induces apoptosis in bladder cancer. Urology 2012, 80, 484.e9–484.e15. [Google Scholar] [CrossRef]
- Okamura, S.; Yoshino, H.; Kuroshima, K.; Tsuruda, M.; Osako, Y.; Sakaguchi, T.; Yonemori, M.; Yamada, Y.; Tatarano, S.; Nakagawa, M.; et al. EHHADH contributes to cisplatin resistance through regulation by tumor-suppressive microRNAs in bladder cancer. BMC Cancer 2021, 21, 48. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Cao, L. LncRNA MST1P2/miR-133b axis affects the chemoresistance of bladder cancer to cisplatin-based therapy via Sirt1/p53 signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22452. [Google Scholar] [CrossRef]
- Dudek, A.M.; van Kampen, J.G.M.; Witjes, J.A.; Kiemeney, L.A.L.M.; Verhaegh, G.W. LINC00857 expression predicts and mediates the response to platinum-based chemotherapy in muscle-invasive bladder cancer. Cancer Med. 2018, 7, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhou, R.; Wang, J.; Han, J.; Yang, X.; Yu, H.; Lu, H.; Zhang, X.; Li, P.; Tao, J.; et al. Circular RNA Cdr1as sensitizes bladder cancer to cisplatin by upregulating APAF1 expression through miR-1270 inhibition. Mol. Oncol. 2019, 13, 1559–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Liu, H.; Cai, Z.; Dong, W.; Jiang, N.; Yang, M.; Huang, J.; Lin, T. Circ-BPTF promotes bladder cancer progression and recurrence through the miR-31-5p/RAB27A axis. Aging 2018, 10, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xie, X.; Li, H.; Li, Z.; Ren, C.; Ming, L. Detection of serum long non-coding RNA UCA1 and circular RNAs for the diagnosis of bladder cancer and prediction of recurrence. Int. J. Clin. Exp. Pathol. 2019, 12, 2951–2958. [Google Scholar] [PubMed]
- Su, F.; He, W.; Chen, C.; Liu, M.; Liu, H.; Xue, F.; Bi, J.; Xu, D.; Zhao, Y.; Huang, J.; et al. The long non-coding RNA FOXD2-AS1 promotes bladder cancer progression and recurrence through a positive feedback loop with Akt and E2F1. Cell Death Dis. 2018, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, M.; Zhang, X.; Zhu, X.; Wang, J. A prognostic index based on a fourteen long non-coding RNA signature to predict the recurrence-free survival for muscle-invasive bladder cancer patients. BMC Med. Inform. Decis. Mak. 2020, 20 (Suppl. 3), 136. [Google Scholar] [CrossRef]
- Charpentier, M.; Gutierrez, C.; Guillaudeux, T.; Verhoest, G.; Pedeux, R. Noninvasive Urine-Based Tests to Diagnose or Detect Recurrence of Bladder Cancer. Cancers 2021, 13, 1650. [Google Scholar] [CrossRef]
- Lodewijk, I.; Dueñas, M.; Rubio, C.; Munera-Maravilla, E.; Segovia, C.; Bernardini, A.; Teijeira, A.; Paramio, J.M.; Suárez-Cabrera, C. Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring. Int. J. Mol. Sci. 2018, 19, 2514. [Google Scholar] [CrossRef] [Green Version]
- Flaig, T.W.; Tangen, C.M.; Daneshmand, S.; Alva, A.; Lerner, S.P.; Lucia, M.S.; McConkey, D.J.; Theodorescu, D.; Goldkorn, A.; Milowsky, M.I.; et al. A Randomized Phase II Study of Coex-pression Extrapolation (COXEN) with Neoadjuvant Chemotherapy for Bladder Cancer (SWOG S1314; NCT02177695). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2435–2441. [Google Scholar] [CrossRef]
- Claps, F.; Mir, M.C.; Zargar, H. Molecular markers of systemic therapy response in urothelial carcinoma. Asian J. Urol. 2021, 8, 376–390. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Porten, S.P.; Willis, D.; Kamat, A.M. Variant histology: Role in management and prognosis of nonmuscle invasive bladder cancer. Curr. Opin. Urol. 2014, 24, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Claps, F.; van de Kamp, M.W.; Mayr, R.; Bostrom, P.J.; Boormans, J.L.; Eckstein, M.; Mertens, L.S.; Boevé, E.R.; Neuzillet, Y.; Burger, M.; et al. Risk factors associated with positive surgical margins’ location at radical cystectomy and their impact on bladder cancer survival. World J. Urol. 2021, 39, 4363–4371. [Google Scholar] [CrossRef]
- Naspro, R.; Finati, M.; Roscigno, M.; Pellucchi, F.; La Croce, G.; Sodano, M.; Manica, M.; Chinaglia, D.; Da Pozzo, L.F. The impact of histological variants on outcomes after open radical cystectomy for muscle-invasive urothelial bladder cancer: Results from a single tertiary referral centre. World J. Urol. 2021, 39, 1917–1926. [Google Scholar] [CrossRef]
- Claps, F.; Mir, M.C.; van Rhijn, B.W.G.; Mazzon, G.; Soria, F.; D’Andrea, D.; Marra, G.; Boltri, M.; Traunero, F.; Massanova, M.; et al. Impact of the controlling nutritional status (CONUT) score on perioperative morbidity and oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol. Oncol. 2023, 41, 49.e13–49.e22. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Rossi de Vermandois, J.A.; Gubbiotti, M.; Giannantoni, A.; Mearini, E.; Maggi, F.; Nabissi, M.; Marinelli, O.; Santoni, M.; et al. Correlation between High PD-L1 and EMT/Invasive Genes Expression and Reduced Recurrence-Free Survival in Blood-Circulating Tumor Cells from Patients with Non-Muscle-Invasive Bladder Cancer. Cancers 2021, 13, 5989. [Google Scholar] [CrossRef]
- Inamoto, T.; Uehara, H.; Akao, Y.; Ibuki, N.; Komura, K.; Takahara, K.; Takai, T.; Uchimoto, T.; Saito, K.; Tanda, N.; et al. A Panel of MicroRNA Signature as a Tool for Predicting Survival of Patients with Urothelial Carcinoma of the Bladder. Dis. Markers 2018, 2018, 5468672. [Google Scholar] [CrossRef]
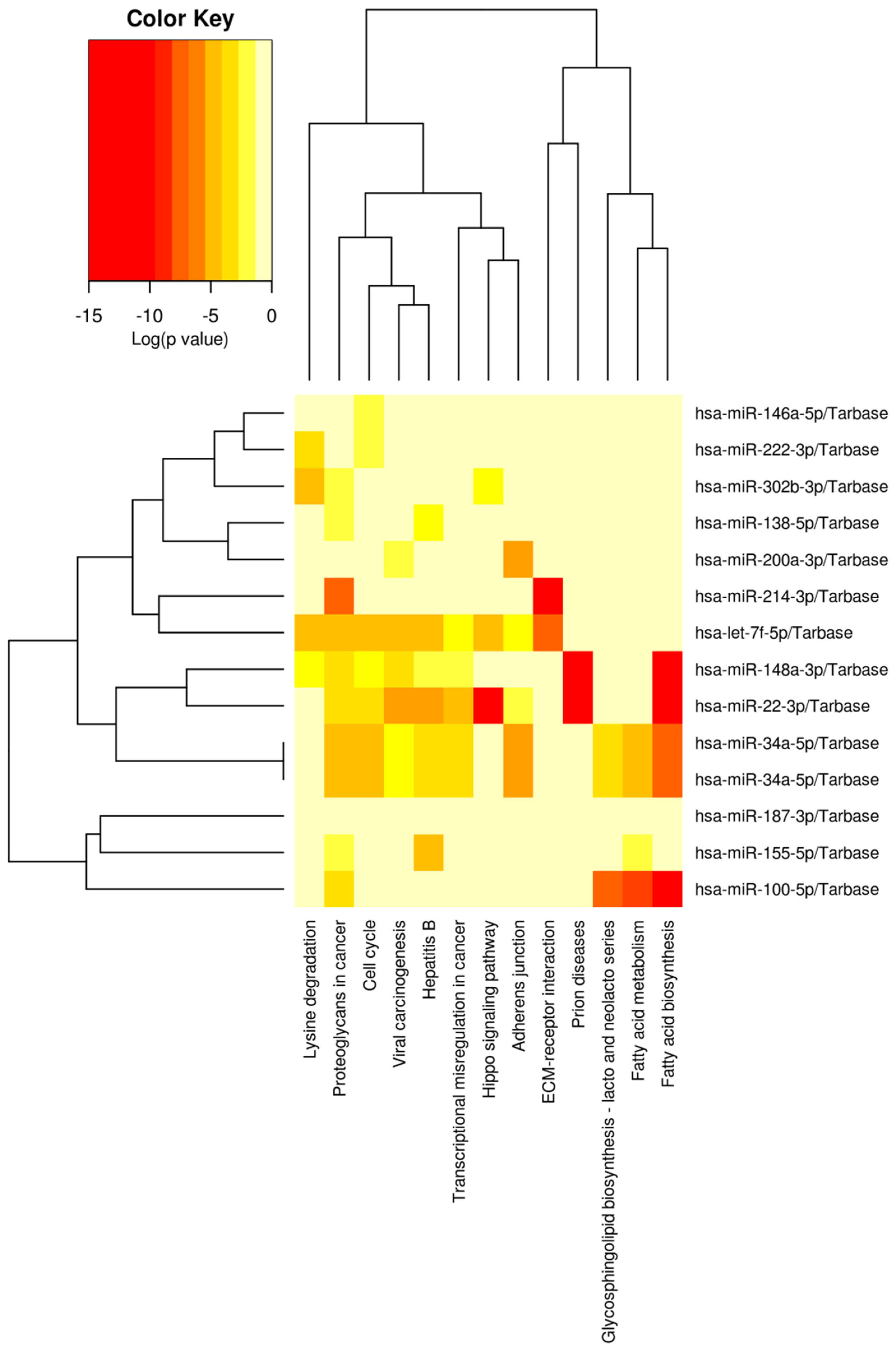

| miRNA | Target/Regulator | Function | Reference |
|---|---|---|---|
| MiR-22-3p | Not identified | Elevated miR-22-3p showed reduced recurrence-free survival (RFS). | [20] |
| MiR-34a | Not identified | Downregulation associated with recurrence and poorer prognosis [21]. Higher expression of miR-34a associated with lower likelihood of recurrence. MiR-34a upregulation showed less invasion and colony formation [22]. | [21,22] |
| MiR-100 | FGFR3 | Reduced miR-100 associated with less recurrence. * | [23] |
| MiR-138 | Cyclin D3 | Downregulation of miR-138 linked to recurrence. | [23] |
| MiR-146a-5p | Two separate pathways involving YAP1 and COX2 | Downregulation associated with recurrence. Subsequent regulation of ALDH1A1 and SOX2. | [24] |
| MiR-148a | Not identified | Downregulation of miR-148a in BC patients linked to recurrence and metastasis. | [25] |
| MiR-152 | Not identified | Higher expression of miR-152 linked to lower RFS in NMIBC. | [26] |
| MiR-155 | Not identified | MiR-155 upregulation associated with recurrence. | [27] |
| MiR-187-5p | Not identified | Oncogene, promotes proliferation and mobility while decreasing apoptosis. | [28] |
| MiR-200a family | Not identified | Reduced miR-200a-3p showed reduced RFS [20]. Lower expression of miR-200a in BCa, and downregulation linked to higher chance of recurrence [29]. | [20,29] |
| MiR-210 | Not identified | Higher expression of miR-210 found in patients with recurrence. | [30] |
| MiR-214 | Not identified | Reduced miR-214 expression in BCa urines pre-op compared to post. Linked to RFS [31]. Mir-214 downregulation linked to recurrence [32]. | [29,30,31,32] |
| MiR-221/222 | Not identified | Downregulated in BCa, but miR-222 is upregulated in high grade/invasive BCas. MiR-222 upregulation (Ta/T1 cancers) linked to recurrence. | [33] |
| MiR-302b | EPS8 (potential) | Tumor suppressor, lessens proliferation, migration, and invasion. Promotes apoptosis. | [34] |
| Let-7f-5p | LIN28 | Tumor suppressor, represses cell viability and migration. | [35] |
| miRNA | Target/Regulator | Function | Reference |
|---|---|---|---|
| MiR-7-5p | ATG7 | Upregulation of miR-7-5p inhibited invasive characteristics. Promotes chemosensitivity. | [36] |
| MiR-21 | PTEN | Promotes chemoresistance to doxorubicin and proliferation in transitional cell carcinoma; inhibits doxorubicin-induced apoptosis. | [37] |
| MiR-22-3p | NET1 | MiR-22-3p promotes chemoresistance. More cell viability, colony formation, and less apoptosis with upregulation of miR-22-3p via mimic. | [38] |
| MiR-23a | SFRP1 protein and Wnt signaling | Linked to chemoradiation response. | [39] |
| MiR-27a | SFRP1 protein and Wnt signaling, RUNX-1 | Linked to chemoradiation response [39]. Rs11671784 SNP (wherein A is replaced with G) results in reduced chemosensitivity [40]. | [39,40] |
| MiR-30a-3p | MMP2, MMP9 | Combination of cisplatin and miR-30a-3p resulted in improved apoptosis and reduced cell viability. Upregulation of miR-30a-3p via mimic lessened migration and invasion. | [41] |
| MiR-31 | ITGA5 | MiR-31 promotes chemosensitivity to mitomycin-C and upregulation inhibits proliferation, migration, and invasion. Downregulation associated with higher risk of recurrence in noninvasive UBC. | [42] |
| MiR-34a | TCF1, LEF1, Cdk6, SRT-1 (sirtuin), CD44 | Downregulated in BCa; promotes chemosensitivity to epirubicin [43] and to cisplatin [44,45]. Higher expression of miR-34a represses metastatic characteristics [43,45]. | [43,44,45] |
| MiR-93 | LASS2 (but no direct binding) | MiR-93 promotes chemoresistance. | [46] |
| MiR-98 | LASS2 | Expressed at higher levels in BCa. Upregulation via mimic resulted in increased proliferation, greater cisplatin and doxorubicin resistance, and repression of apoptosis. | [47] |
| MiR-101 | COX2 | MiR-101 promotes chemosensitivity to cisplatin. | [48] |
| MiR-101-3p | EZH2, affects MRP1 expression | MiR-101-3p promotes chemosensitivity. | [49] |
| MiR-129-5p | Wnt5a | Expression of miR-129-5p promotes response to gemcitabine. | [50] |
| MiR-130b | CYLD | Involved in promoting chemoresistance. | [51] |
| MiR-143 | IGF-1R | MiR-143 promotes chemosensitivity. Upregulation of IGF-1R linked to reduced survival and recurrence. | [52] |
| MiR-193a-3p | LOXL4, HOXC9, PSEN1, ING5 | MiR-193a-3p promotes chemoresistance (oxidative stress pathway) [53,54]. MiR-193a-3p reported to target PSEN1 gene and affect DNA damage response [55]. Interaction with ING5 also occurs through DNA damage response pathway [56]. | [53,54,55,56] |
| MiR-193a-5p | AL-2α | MiR-193a-5p is involved in chemoresistance. Upregulation of miR-193a-5p linked to increased migration and resistance to cisplatin. | [57] |
| MiR-200b | IGFBP3, ICAM1, TNFSD10 | MiR-200b promotes chemosensitivity. More broadly, miR-200 family members (miR-200b, miR-200a, and miR-429) were downregulated in cisplatin-resistant cell lines. | [58] |
| MiR-214 | Netrin-1 | Tumor suppressor activity; miR-214 upregulation resulted in reduced colony formation and invasion. MiR-214 promotes chemosensitivity. | [59] |
| MiR-218 | Glut1 | MiR-218 promotes chemosensitivity to cisplatin. | [60] |
| MiR-222 | PPP2R2A | MiR-222 is implicated in chemoresistance. Acts through AKT/mTOR and autophagy pathways. | [61] |
| MiR-325 | HAX-1 | MiR-325 promotes chemosensitivity. | [62] |
| MiR-424 | UNC5B and SIRT4 | Promotes cisplatin resistance via downregulation of UNC5B and SIRT4. | [63] |
| MiR-455-5p | Regulated by HOXA-As3 | Promotes sensitivity to cisplatin, reduces proliferation, and promotes apoptosis. | [64] |
| MiR-486-5p | Gene expression changes observed in caspase-9, caspase03, P53, SIRT1, OLFM4, SMAD2, Bcl-2, ROCK, CD44, MMP9 | MiR-486-5p functions as tumor suppressor and promotes chemosensitivity. | [65] |
| miR-3682-3p | Regulated by BMI1 and regulates ABCB1 | BMI1 inhibits miR-3682-3p transcription to induce chemoresistance. Elevated BMI1 is also associated with poorer RFS. | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Hayden, J.; Sullivan, T.; Rieger-Christ, K. The Roles of miRNAs in Predicting Bladder Cancer Recurrence and Resistance to Treatment. Int. J. Mol. Sci. 2023, 24, 964. https://doi.org/10.3390/ijms24020964
Das S, Hayden J, Sullivan T, Rieger-Christ K. The Roles of miRNAs in Predicting Bladder Cancer Recurrence and Resistance to Treatment. International Journal of Molecular Sciences. 2023; 24(2):964. https://doi.org/10.3390/ijms24020964
Chicago/Turabian StyleDas, Sanjna, Joshua Hayden, Travis Sullivan, and Kimberly Rieger-Christ. 2023. "The Roles of miRNAs in Predicting Bladder Cancer Recurrence and Resistance to Treatment" International Journal of Molecular Sciences 24, no. 2: 964. https://doi.org/10.3390/ijms24020964





