Effects of Copper or Zinc Organometallics on Cytotoxicity, DNA Damage and Epigenetic Changes in the HC-04 Human Liver Cell Line
Abstract
:1. Introduction
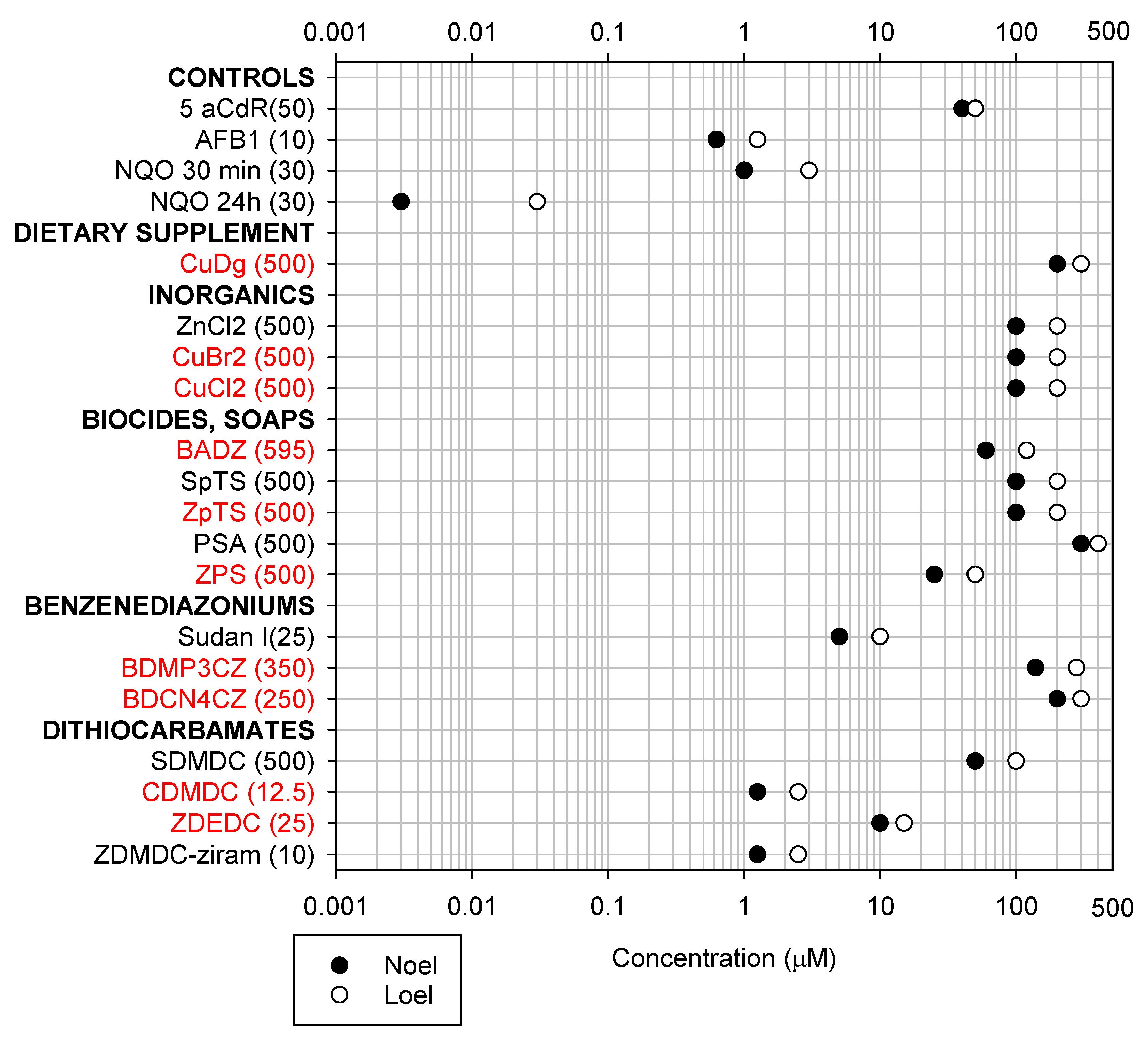
2. Results
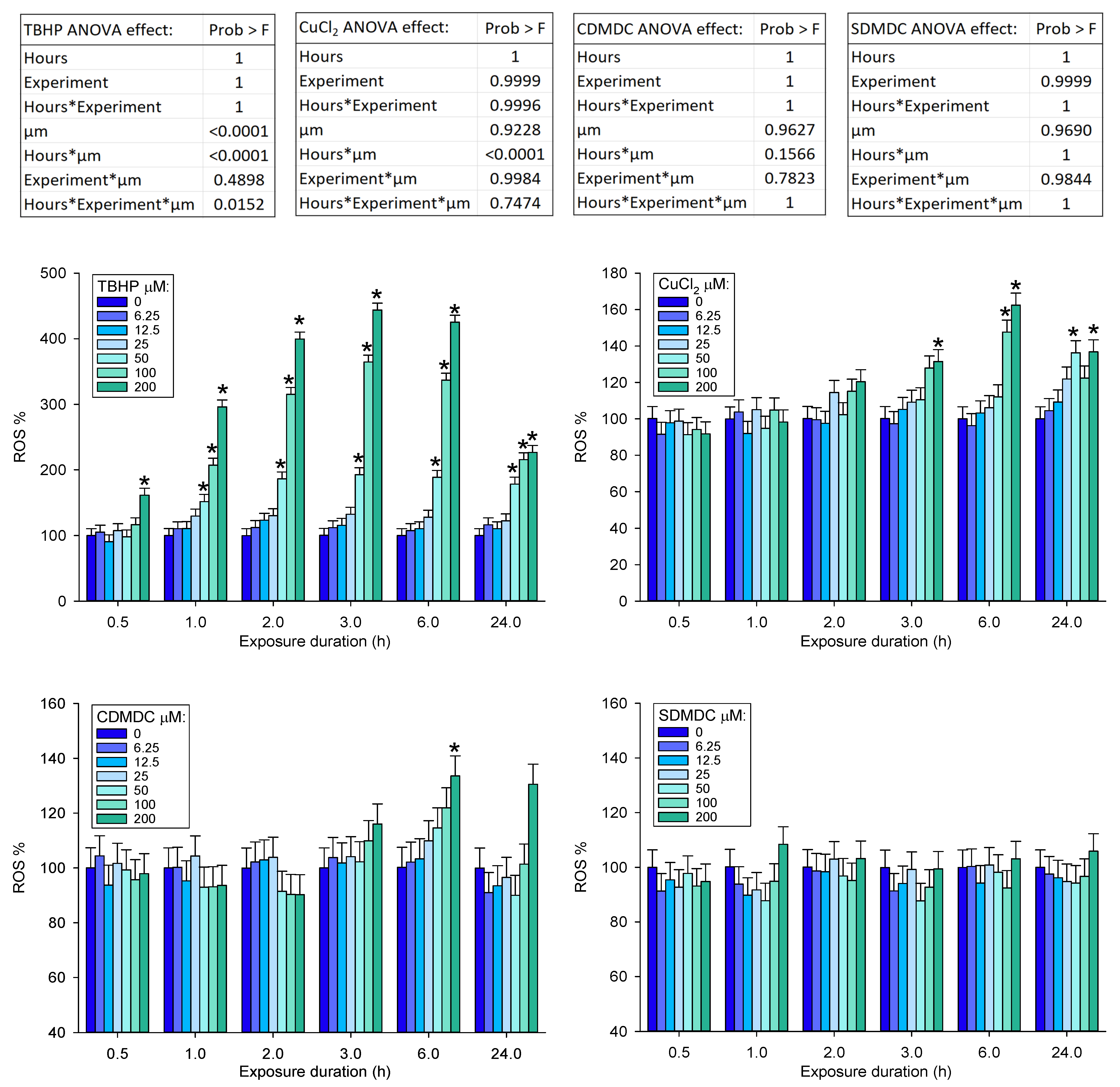
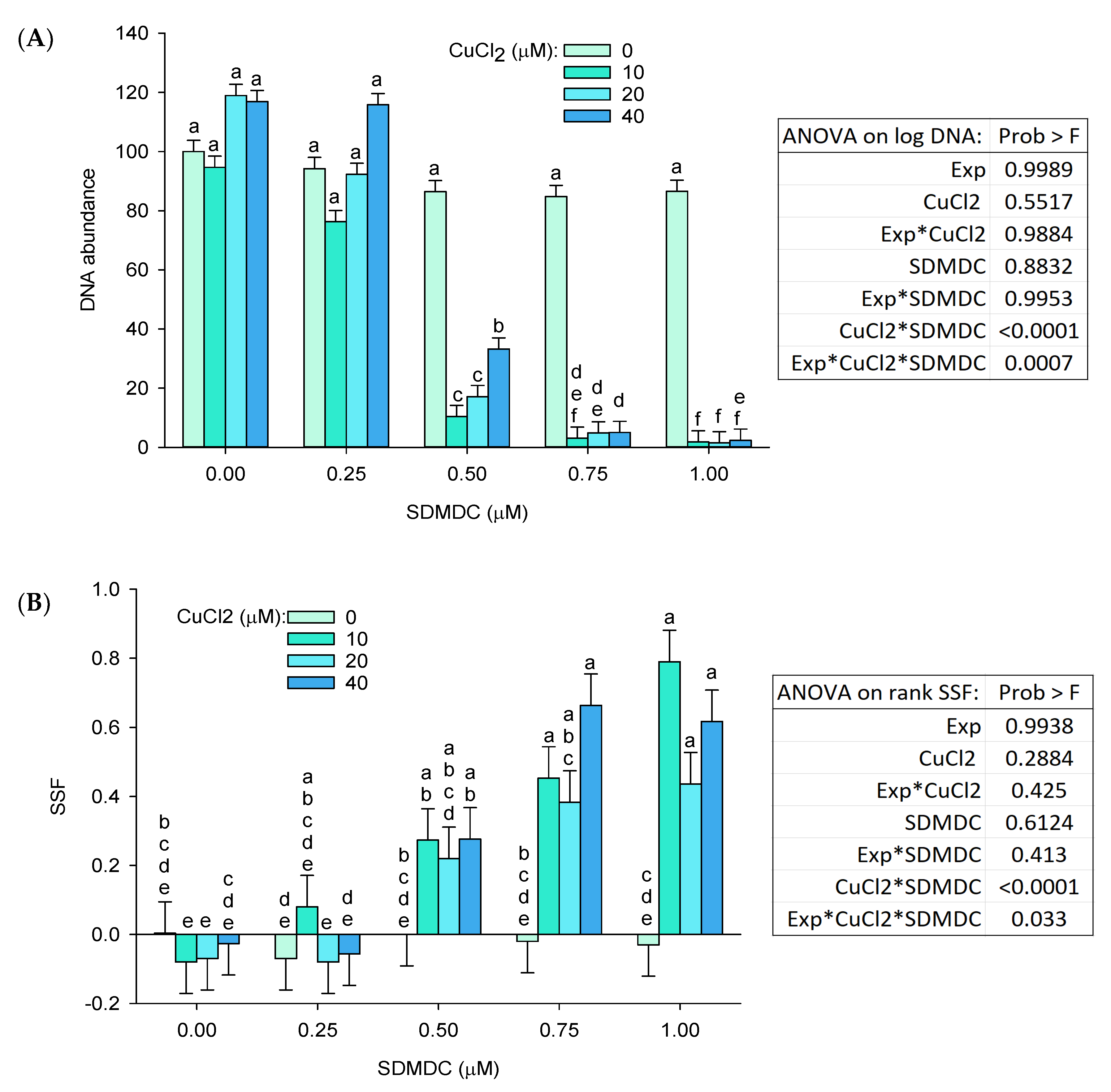
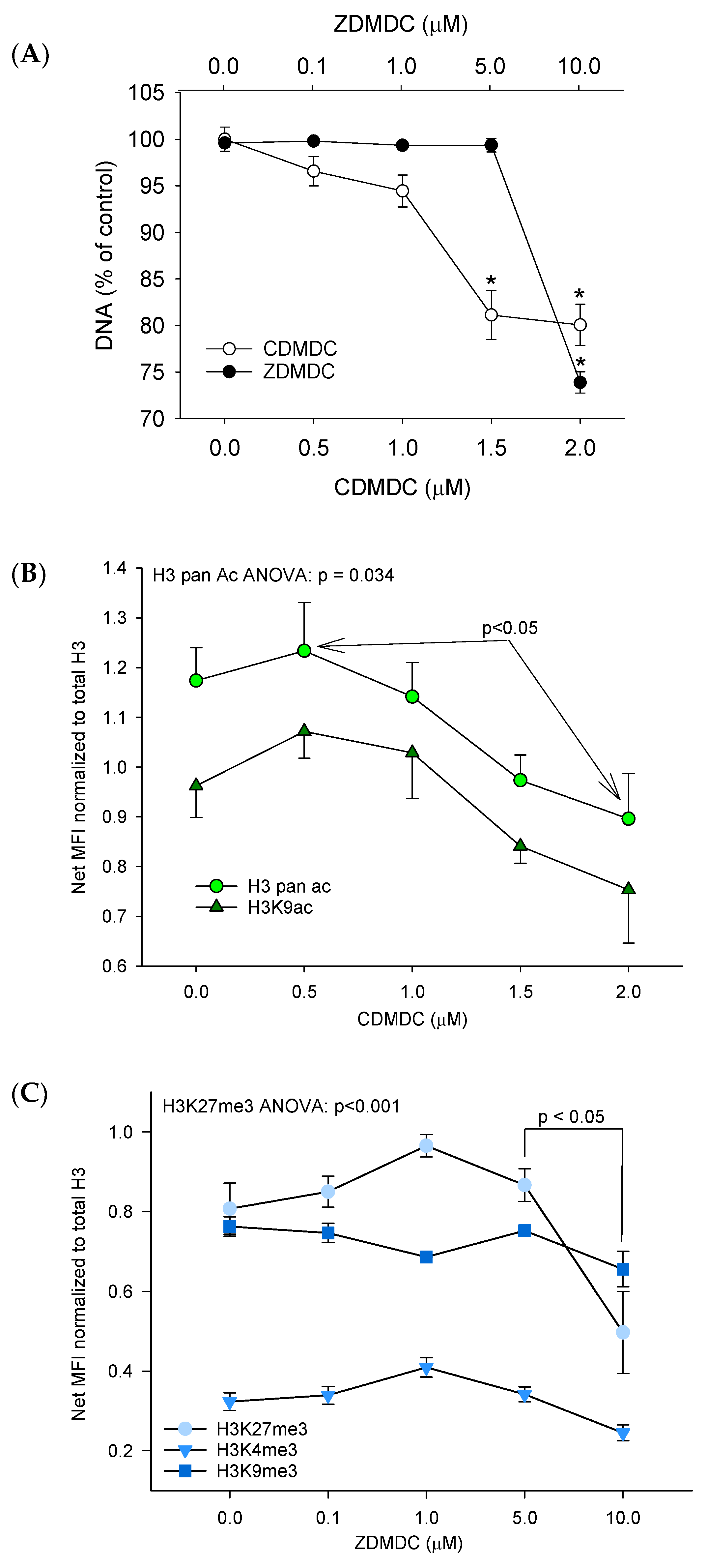
2.1. Cytotoxicity and DNA Damage Induction
2.2. Epigenetic Effects
2.2.1. Global Genome Histone Modifications
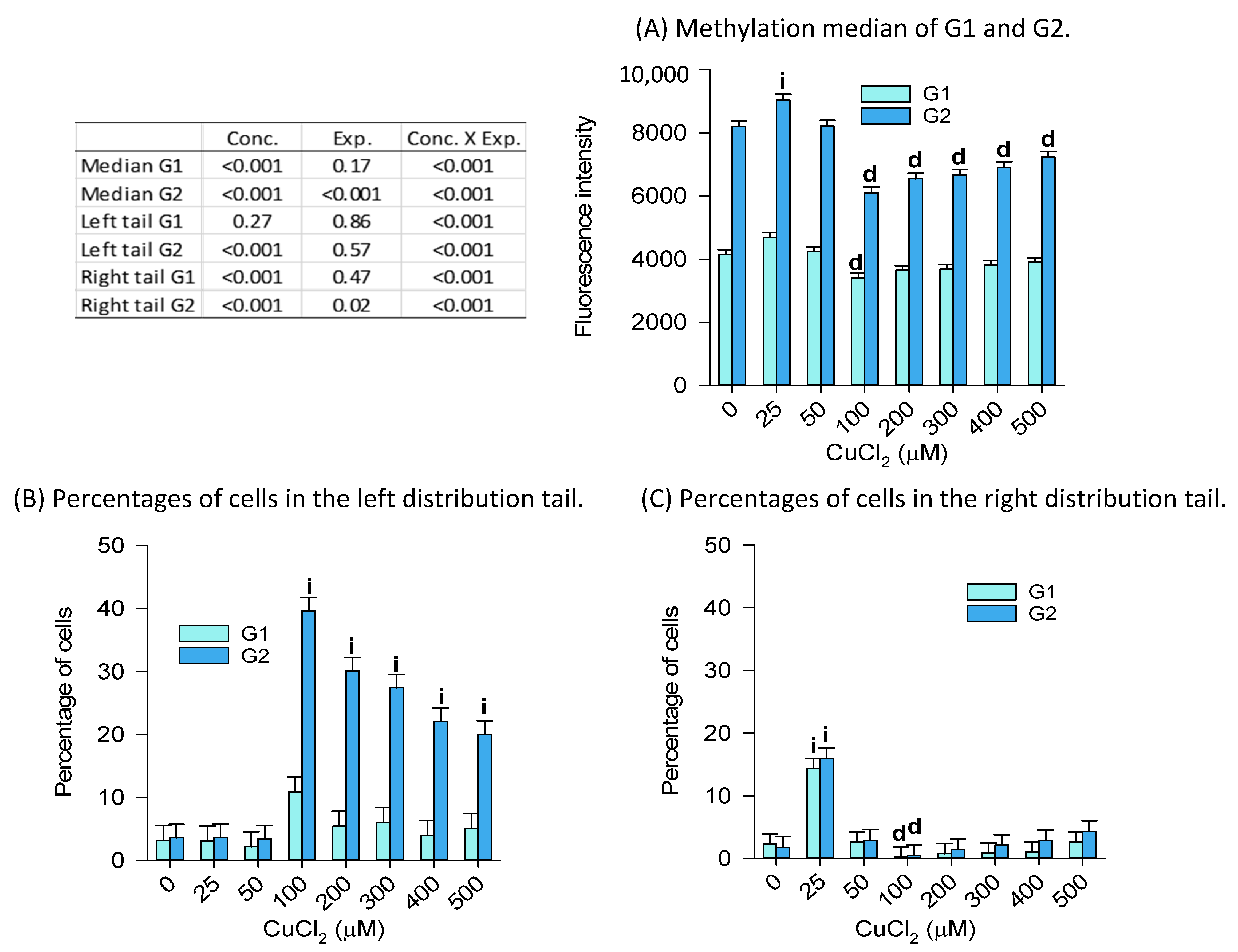
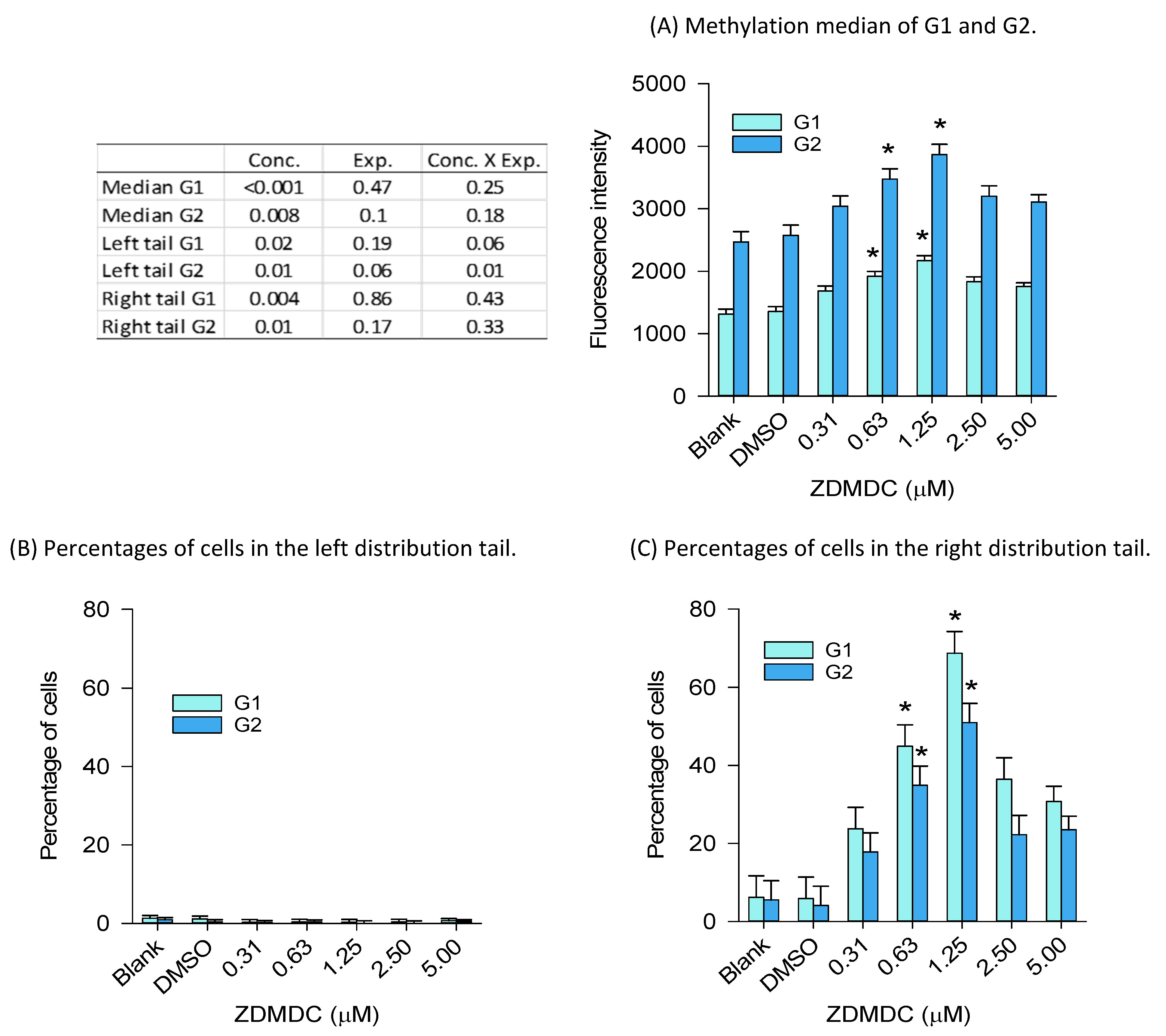
2.2.2. Global Genome DNA Methylation
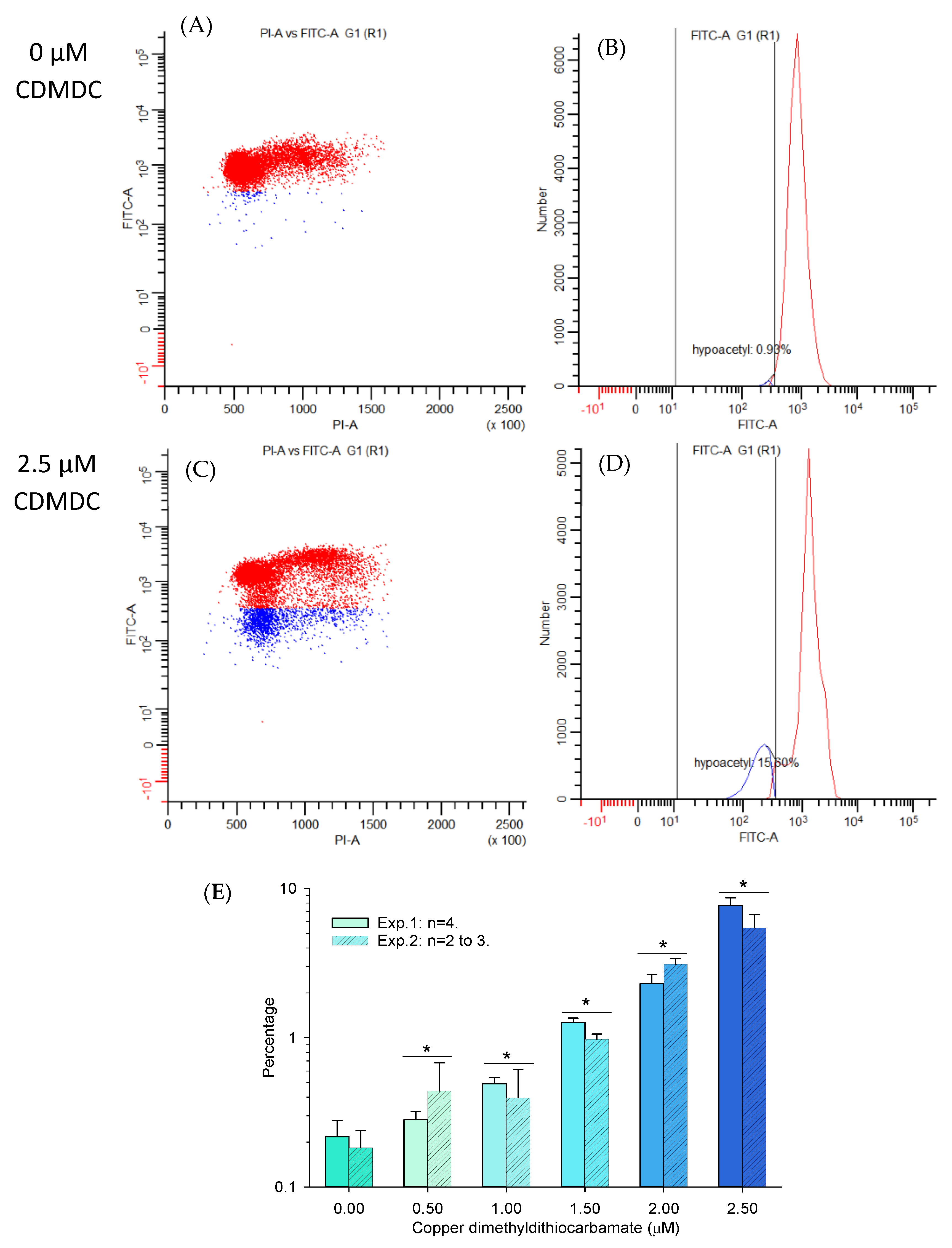
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. HC-04 Cell Line and Culture Conditions
4.3. DNA Damage Assays
4.3.1. γH2AX Assay
4.3.2. DNA Alkaline Unwinding Assay
4.4. Reactive Oxygen Species Assay
4.5. Epigenetic Assays
4.5.1. Screening for Histone Post-Translational Modifications Using Luminex Multiplex Bead Array
4.5.2. Flow Cytometry Analyses for the Quantification of H3K9 Acetylation
4.5.3. DNA Methylation by Flow Cytometry
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5aCdR | 5-aza-2’-deoxycytidine. |
| AFB1 | aflatoxin-B1. |
| BADZ | benzoic acid: 4-(1,1-dimethylethyl)-, zinc salt (2:1)*. |
| BDCN4CZ | benzenediazonium, 4-chloro-2-nitro-, tetrachlorozincate(2-) (2:1). Fast Red 3GL salt*. |
| BDMP3CZ | benzenediazonium,3-methyl-4-(1-pyrrolielinyl)-,trichlorozincate hydrate*. |
| BER | base excision repair. |
| CDMDC | copper(II) dimethyldithiocarbamate*. |
| CuBr2 | copper(II) bromide*. |
| CuCl2 | copper(II) chloride dehydrate*. |
| CuDg | copper(II) D-gluconate*. |
| CYP | cytochrome p450. |
| DTC | dithiocarbamate. |
| DSB | DNA double-strand breaks. |
| NER | nucleotide excision repair. |
| NQO | 4-nitroquinoline-oxide. |
| PSA | phenolsulfonic acid. |
| ROS | reactive oxygen species. |
| SDMDC | sodium dimethyldithiocarbamate. |
| SpTS | sodium p-toluenesulfonate. |
| SD | standard deviation. |
| SE | standard error. |
| SSB | DNA single-strand breaks. |
| Sudan | sudan I: 1-phenylazo-2-naphthol. |
| ZDEDC | zinc diethyldithiocarbamate*. |
| ZDMDC | zinc dimethyldithiocarbamate. |
| ZnCl2 | zinc chloride. |
| ZPS | zinc phenolsulfonate*. |
| ZpTS | zinc p-toluenesulfonate hydrate*. |
| * | identified as data-poor chemicals. |
References
- Shinde, S.D.; Sakla, A.P.; Shankaraiah, N. An insight into medicinal attributes of dithiocarbamates: Bird’s eye view. Bioorg. Chem. 2020, 105, 104346. [Google Scholar] [CrossRef] [PubMed]
- Menghani, S.V.; Sanchez-Rosario, Y.; Pok, C.; Liu, R.; Gao, F.; O’Brien, H.; Neubert, M.J.; Ochoa, K.; Durckel, M.; Hellinger, R.D.; et al. Novel dithiocarbamate derivatives are effective copper-dependent antimicrobials against Streptococcal species. Front. Microbiol. 2022, 13, 1099330. [Google Scholar] [CrossRef] [PubMed]
- Padhye, L.P.; Kim, J.H.; Huang, C.H. Oxidation of dithiocarbamates to yield N-nitrosamines by water disinfection oxidants. Water Res. 2013, 47, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Trombetta, L.D. Toxicity and bioaccumulation of the wood preservative copper dimethyldithiocarbamate in tissues of Long-Evans rats. J. Toxicol. Environ. Health A 2008, 71, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Buac, D.; Schmitt, S.; Ventro, G.; Kona, F.R.; Dou, Q.P. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef]
- Goyer, R.A. Toxic effects of metals. In Casarett And Doull’s Toxicology: The Basic Science Of Poisons; Klaassen, C.D., Amdur, M.O., Doull, J., Eds.; McGraw-Hill: New York, NY, USA, 1996; pp. 691–736. [Google Scholar]
- Toth, B.; Patil, K.; Erickson, J.; Gannett, P. Carcinogenesis by benzenediazonium sulfate in mice. Vivo 1998, 12, 379–382. [Google Scholar]
- Stiborova, M.; Martinek, V.; Semanska, M.; Hodek, P.; Dracinsky, M.; Cvacka, J.; Schmeiser, H.H.; Frei, E. Oxidation of the carcinogenic non-aminoazo dye 1-phenylazo-2-hydroxy-naphthalene (Sudan I) by cytochromes P450 and peroxidases: A comparative study. Interdiscip. Toxicol. 2009, 2, 195–200. [Google Scholar] [CrossRef]
- Zhang, Y.; An, Y.; Jiang, L.; Geng, C.; Cao, J.; Jiang, L.; Zhong, L. The role of oxidative stress in Sudan IV-induced DNA damage in human liver-derived HepG2 cells. Environ. Toxicol. 2011, 26, 292–299. [Google Scholar] [CrossRef]
- An, Y.; Jiang, L.; Cao, J.; Geng, C.; Zhong, L. Sudan I induces genotoxic effects and oxidative DNA damage in HepG2 cells. Mutat. Res. 2007, 627, 164–170. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Krewski, D.; Chambers, A.; Stern, B.R.; Aggett, P.J.; Plunkett, L.; Rudenko, L. Development of a copper database for exposure-response analysis. J. Toxicol. Environ. Health A 2010, 73, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Chambers, A.; Birkett, N. The use of categorical regression in modeling copper exposure-response relationships. J. Toxicol. Environ. Health A 2010, 73, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- De Olivera, J.V.; Boufleur, L.A.; Dos Santos, C.E.; Dias, J.F.; Squeff, C.H.; Silva, G.R.; Ianistcki, M.; Benvegnu, V.C.; Da, S.J. Occupational genotoxicity among copper smelters. Toxicol. Ind. Health 2012, 28, 789–795. [Google Scholar] [CrossRef]
- Kumar, S.; Khaliq, F.; Singh, S.; Ahmed, R.; Kumar, R.; Deshmukh, P.S.; Banerjee, B.D. Pulmonary Functions, Oxidative Stress and DNA Damage in Workers of a Copper Processing Industry. Int. J. Occup. Environ. Med. 2016, 7, 107–115. [Google Scholar] [CrossRef]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef]
- Formigari, A.; Irato, P.; Santon, A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 443–459. [Google Scholar] [CrossRef]
- Loh, S.N. The missing zinc: p53 misfolding and cancer. Metallomics 2010, 2, 442–449. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Rusyn, I.; Chiu, W.A.; Corpet, D.E.; van den Berg, M.; Ross, M.K.; Christiani, D.C.; Beland, F.A.; Smith, M.T. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 2018, 39, 614–622. [Google Scholar] [CrossRef]
- Saghafinia, S.; Mina, M.; Riggi, N.; Hanahan, D.; Ciriello, G. Pan-Cancer Landscape of Aberrant DNA Methylation across Human Tumors. Cell Rep. 2018, 25, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.F.; Choudhuri, S.; Muldoon-Jacobs, K. Epigenetic targets of some toxicologically relevant metals: A review of the literature. J. Appl. Toxicol. 2012, 32, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. The relationship of copper to DNA damage and damage prevention in humans. Mutat. Res. 2012, 733, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chen, J.; Shi, Y.; Jia, J.; Wang, Z. Histone hypoacetylation is involved in 1,10-phenanthroline-Cu2+-induced human hepatoma cell apoptosis. J. Biol. Inorg. Chem. 2005, 10, 190–198. [Google Scholar] [CrossRef]
- Chen, J.; Du, C.; Kang, J.; Wang, J. Cu2+ is required for pyrrolidine dithiocarbamate to inhibit histone acetylation and induce human leukemia cell apoptosis. Chem. Biol. Interact. 2008, 171, 26–36. [Google Scholar] [CrossRef]
- Lin, C.; Kang, J.; Zheng, R. Oxidative stress is involved in inhibition of copper on histone acetylation in cells. Chem. Biol. Interact. 2005, 151, 167–176. [Google Scholar] [CrossRef]
- Medici, V.; Shibata, N.M.; Kharbanda, K.K.; LaSalle, J.M.; Woods, R.; Liu, S.; Engelberg, J.A.; Devaraj, S.; Torok, N.J.; Jiang, J.X.; et al. Wilson’s disease: Changes in methionine metabolism and inflammation affect global DNA methylation in early liver disease. Hepatology 2013, 57, 555–565. [Google Scholar] [CrossRef]
- Svetlova, M.P.; Solovjeva, L.V.; Tomilin, N.V. Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks. Mutat. Res. 2010, 685, 54–60. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Eltze, T.; Dressler, D.; Bernhardt, J.; Hirsch, C.; Wick, P.; Von, S.G.; Lex, K.; Burkle, A. The automated FADU-assay, a potential high-throughput in vitro method for early screening of DNA breakage. ALTEX-Altern. Anim. Exp. 2011, 28, 295–303. [Google Scholar]
- Papamargaritis, D.; Aasheim, E.T.; Sampson, B.; le Roux, C.W. Copper, selenium and zinc levels after bariatric surgery in patients recommended to take multivitamin-mineral supplementation. J. Trace Elem. Med. Biol. 2015, 31, 167–172. [Google Scholar] [CrossRef]
- Burns, J.; Paterson, C.R. Effect of iron-folate supplementation on serum copper concentration in late pregnancy. Acta Obstet. Gynecol. Scand. 1993, 72, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, V.; Ali, M.; Small, B.; Rajendran, G.; Elzhenni, S.; Taj, H.; Wang, W.; Dou, Q.P. Recent Advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents. Front. Mol. Biosci. 2021, 8, 741316. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Allensworth, J.L.; Evans, M.K.; Bertucci, F.; Aldrich, A.J.; Festa, R.A.; Finetti, P.; Ueno, N.T.; Safi, R.; McDonnell, D.P.; Thiele, D.J.; et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol. 2015, 9, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Ogo, O.A.; Tyson, J.; Cockell, S.J.; Howard, A.; Valentine, R.A.; Ford, D. The zinc finger protein ZNF658 regulates the transcription of genes involved in zinc homeostasis and affects ribosome biogenesis through the zinc transcriptional regulatory element. Mol. Cell Biol. 2015, 35, 977–987. [Google Scholar] [CrossRef]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef]
- Urani, C.; Melchioretto, P.; Morazzoni, F.; Canevali, C.; Camatini, M. Copper and zinc uptake and hsp70 expression in HepG2 cells. Toxicol In Vitro 2001, 15, 497–502. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Urani, C.; Melchioretto, P.; Bruschi, M.; Fabbri, M.; Sacco, M.G.; Gribaldo, L. Impact of Cadmium on Intracellular Zinc Levels in HepG2 Cells: Quantitative Evaluations and Molecular Effects. Biomed. Res. Int. 2015, 2015, 949514. [Google Scholar] [CrossRef] [PubMed]
- Ecobichon, D.J. Toxic effects of pesticides. In Casarett And Doull’s Toxicology: The Basic Science of Poisons; Klaassen, C.D., Amdur, M.O., Doull, J., Eds.; McGraw-Hill: New York, NY, USA, 1995; pp. 643–689. [Google Scholar]
- Rigas, D.A.; Eginitis-Rigas, C.; Head, C. Biphasic toxicity of diethyldithiocarbamate, a metal chelation, to T lymphocytes and polymorphonuclear granulocytes: Reversal by zinc and copper. Biochem. Biophys. Res. Commun. 1979, 88, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lobera, M.; Madauss, K.P.; Pohlhaus, D.T.; Wright, Q.G.; Trocha, M.; Schmidt, D.R.; Baloglu, E.; Trump, R.P.; Head, M.S.; Hofmann, G.A.; et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat. Chem. Biol. 2013, 9, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef]
- Rahman, S.; Housein, Z.; Dabrowska, A.; Mayan, M.D.; Boobis, A.R.; Hajji, N. E2F1-mediated FOS induction in arsenic trioxide-induced cellular transformation: Effects of global H3K9 hypoacetylation and promoter-specific hyperacetylation in vitro. Environ. Health Perspect. 2015, 123, 484–492. [Google Scholar] [CrossRef]
- Zhang, X.; Kluz, T.; Gesumaria, L.; Matsui, M.S.; Costa, M.; Sun, H. Solar Simulated Ultraviolet Radiation Induces Global Histone Hypoacetylation in Human Keratinocytes. PLoS ONE 2016, 11, e0150175. [Google Scholar] [CrossRef]
- Chen, D.; Fang, L.; Mei, S.; Li, H.; Xu, X.; Des Marais, T.L.; Lu, K.; Liu, X.S.; Jin, C. Regulation of Chromatin Assembly and Cell Transformation by Formaldehyde Exposure in Human Cells. Environ. Health Perspect. 2017, 125, 097019. [Google Scholar] [CrossRef]
- Giorgio, M.; Dellino, G.I.; Gambino, V.; Roda, N.; Pelicci, P.G. On the epigenetic role of guanosine oxidation. Redox Biol. 2020, 29, 101398. [Google Scholar] [CrossRef]
- Zarakowska, E.; Gackowski, D.; Foksinski, M.; Olinski, R. Are 8-oxoguanine (8-oxoGua) and 5-hydroxymethyluracil (5-hmUra) oxidatively damaged DNA bases or transcription (epigenetic) marks? Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 764, 58–63. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuang, Z.; Wang, W.; He, L.; Wu, H.; Cao, Y.; Pan, F.; Zhao, J.; Hu, Z.; Sekhar, C.; et al. OGG1 is essential in oxidative stress induced DNA demethylation. Cell Signal 2016, 28, 1163–1171. [Google Scholar] [CrossRef]
- Lukashevich, O.V.; Baskunov, V.B.; Darii, M.V.; Kolbanovskiy, A.; Baykov, A.A.; Gromova, E.S. Dnmt3a-CD is less susceptible to bulky benzo[a]pyrene diol epoxide-derived DNA lesions than prokaryotic DNA methyltransferases. Biochemistry 2011, 50, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.V.; Baykov, A.A.; Jeltsch, A.; Gromova, E.S. Impact of 7,8-dihydro-8-oxoguanine on methylation of the CpG site by Dnmt3a. Biochemistry 2009, 48, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Segovia, N.; Crovetto, G.; Lardelli, P.; Espigares, M. In vitro toxicity of several dithiocarbamates and structure-activity relationships. J. Appl. Toxicol. 2002, 22, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Yourick, J.J.; Faiman, M.D. Diethyldithiocarbamic acid-methyl ester: A metabolite of disulfiram and its alcohol sensitizing properties in the disulfiram-ethanol reaction. Alcohol. 1987, 4, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Raynal, N.J.; Lee, J.T.; Wang, Y.; Beaudry, A.; Madireddi, P.; Garriga, J.; Malouf, G.G.; Dumont, S.; Dettman, E.J.; Gharibyan, V.; et al. Targeting Calcium Signaling Induces Epigenetic Reactivation of Tumor Suppressor Genes in Cancer. Cancer Res. 2016, 76, 1494–1505. [Google Scholar] [CrossRef]
- Sekirnik, R.; Rose, N.R.; Thalhammer, A.; Seden, P.T.; Mecinovic, J.; Schofield, C.J. Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(II). Chem. Commun. 2009, 42, 6376–6378. [Google Scholar] [CrossRef]
- Rodriguez-Carrillo, A.; D’Cruz, S.C.; Mustieles, V.; Suarez, B.; Smagulova, F.; David, A.; Peinado, F.; Artacho-Cordon, F.; Lopez, L.C.; Arrebola, J.P.; et al. Exposure to non-persistent pesticides, BDNF, and behavioral function in adolescent males: Exploring a novel effect biomarker approach. Environ. Res. 2022, 211, 113115. [Google Scholar] [CrossRef]
- Sattabongkot, J.; Yimamnuaychoke, N.; Leelaudomlipi, S.; Rasameesoraj, M.; Jenwithisuk, R.; Coleman, R.E.; Udomsangpetch, R.; Cui, L.; Brewer, T.G. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 2006, 74, 708–715. [Google Scholar] [CrossRef]
- Lim, P.L.; Tan, W.; Latchoumycandane, C.; Mok, W.C.; Khoo, Y.M.; Lee, H.S.; Sattabongkot, J.; Beerheide, W.; Lim, S.G.; Tan, T.M.; et al. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol. In Vitro 2007, 21, 1390–1401. [Google Scholar] [CrossRef]
- Gursoy-Yuzugullu, O.; Ayrapetov, M.K.; Price, B.D. Histone chaperone Anp32e removes H2A.Z from DNA double-strand breaks and promotes nucleosome reorganization and DNA repair. Proc. Natl. Acad. Sci. USA 2015, 112, 7507–7512. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.L.; Chen, X.; Walters, M.A.; Zhang, Z. Histone-modifying enzymes, histone modifications and histone chaperones in nucleosome assembly: Lessons learned from Rtt109 histone acetyltransferases. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Redon, C.E.; Weyemi, U.; Parekh, P.R.; Huang, D.; Burrell, A.S.; Bonner, W.M. gamma-H2AX and other histone post-translational modifications in the clinic. Biochim. Biophys. Acta 2012, 1819, 743–756. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Pfeiffer, R.; Sindlinger, T.; Leake, A.; Muller, M.; Kirkwood, T.B.; Burkle, A. A modified and automated version of the ‘Fluorimetric Detection of Alkaline DNA Unwinding’ method to quantify formation and repair of DNA strand breaks. BMC Biotechnol. 2009, 9, 39. [Google Scholar] [CrossRef]
- Baumstark-Khan, C.; Horneck, G. Results from the “Technical workshop on genotoxicity biosensing” on the micro-scale fluorometric assay of deoxyribonucleic acid unwinding. Anal. Chim. Acta 2007, 593, 75–81. [Google Scholar] [CrossRef]
- Gealy, R.; Wright-Bourque, J.L.; Kraynak, A.R.; McKelvey, T.W.; Barnum, J.E.; Storer, R.D. Validation of a high-throughput in vitro alkaline elution/rat hepatocyte assay for DNA damage. Mutat. Res. 2007, 629, 49–63. [Google Scholar] [CrossRef]
- Enciso, J.M.; Sanchez, O.; Lopez de, C.A.; Azqueta, A. Does the duration of lysis affect the sensitivity of the in vitro alkaline comet assay? Mutagenesis 2015, 30, 21–28. [Google Scholar] [CrossRef]
- Ullmann, K.; Muller, C.; Steinberg, P. Two essential modifications strongly improve the performance of the Fast Micromethod to identify DNA single- and double-strand breaks. Arch. Toxicol. 2008, 82, 861–867. [Google Scholar] [CrossRef]
- Schroder, H.C.; Batel, R.; Schwertner, H.; Boreiko, O.; Muller, W.E. Fast micromethod DNA single-strand-break assay. Methods Mol. Biol. 2006, 314, 287–305. [Google Scholar]
- Batel, R.; Jaksic, Z.; Bihari, N.; Hamer, B.; Fafandel, M.; Chauvin, C.; Schroder, H.C.; Muller, W.E.; Zahn, R.K. A microplate assay for DNA damage determination (fast micromethod). Anal. Biochem. 1999, 270, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ronzoni, S.; Faretta, M.; Ballarini, M.; Pelicci, P.; Minucci, S. New method to detect histone acetylation levels by flow cytometry. Cytometry A 2005, 66, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Chow, S.; Barsyte, D.; Arrowsmith, C.; Shankey, T.V.; Minden, M.; Hedley, D. The study of epigenetic mechanisms based on the analysis of histone modification patterns by flow cytometry. Cytometry A 2014, 85, 78–87. [Google Scholar] [CrossRef]
- Wersto, R.P.; Chrest, F.J.; Leary, J.F.; Morris, C.; Stetler-Stevenson, M.A.; Gabrielson, E. Doublet discrimination in DNA cell-cycle analysis. Cytometry 2001, 46, 296–306. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981, 35, 124–129. [Google Scholar]
- Heo, K.; Kim, H.; Choi, S.H.; Choi, J.; Kim, K.; Gu, J.; Lieber, M.R.; Yang, A.S.; An, W. FACT-mediated exchange of histone variant H2AX regulated by phosphorylation of H2AX and ADP-ribosylation of Spt16. Mol. Cell 2008, 30, 86–97. [Google Scholar] [CrossRef]
- Begg, A.C.; Mooren, E. Rapid fluorescence-based assay for radiosensitivity and chemosensitivity testing in mammalian cells in vitro. Cancer Res. 1989, 49, 565–569. [Google Scholar]
- McCabe, M.T.; Ott, H.M.; Ganji, G.; Korenchuk, S.; Thompson, C.; Van Aller, G.S.; Liu, Y.; Graves, A.P.; Della, P.A., III; Diaz, E.; et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 2012, 492, 108–112. [Google Scholar] [CrossRef]



| Name | Supplier | CAS | Abbreviation | Water Soluble |
|---|---|---|---|---|
| Inorganic copper and zinc chemicals | ||||
| Copper(II) chloride dihydrate | Sigma-Aldrich, Oakville, ON, Canada | 10125-13-0 | CuCl2* | Yes |
| Copper(II) bromide | Sigma-Aldrich | 7789-45-9 | CuBr2* | Yes |
| Zinc Chloride a | Sigma-Aldrich | 7646-85-7 | ZnCl2 | Yes |
| Organic chemicals | ||||
| Dietary supplement | ||||
| Copper(II) D-gluconate | Sigma-Aldrich | 527-09-3 | CuDg* | Yes |
| Rubber vulcanization, fungicide wood preservative (dithiocarbamate related, they can form nitrosamines) | ||||
| Copper(II) Dimethyldithiocarbamate | Spectrum Chemical MFG Corp, New Brunswick, NJ, USA, and Tokyo Chemical Industry (TCI), Cambridge, MA, USA | 137-29-1 | CDMDC* | 0.05% DMSO |
| Zinc diethyldithiocarbamate | Sigma-Aldrich | 14324-55-1 | ZDEDC* | 0.05% DMSO |
| Zinc dimethyldithiocarbamate (Ziram) a | Sigma-Aldrich | 137-30-4 | ZDMDC | 0.05% DMSO |
| Sodium Dimethyldithiocarbamate (Dibam) a | Sigma-Aldrich | 128-04-1 | SDMDC | Yes |
| Dyes (benzenediazonium related) | ||||
| Benzenediazonium,3-methyl-4-(1-pyrrolielinyl)-,Trichlorozincate hydrate | Waterstone Technology Carmel, IN, USA | 52572-38-0 | BDMP3CZ* | Yes |
| Benzenediazonium, 4-chloro-2-nitro-, tetrachlorozincate(2-) (2:1). Fast Red 3GL salt | Molekula Irvine, CA, USA | 14263-89-9 | BDCN4CZ* | Yes |
| Sudan I, 1-Phenylazo-2-naphthol a | Sigma-Aldrich | 842-07-9 | Sudan | 0.05% DMSO |
| Mouthwash, biocide, soap | ||||
| Zinc Phenolsulfonate | Spectrum Chemical MFG Corp, New Brunswick, NJ, USA | 127-82-2 | ZPS* | Yes |
| Phenolsulfonic acid a | Sigma-Aldrich | 98-67-9 | PSA | Yes |
| Zinc p-toluenesulfonate hydrate | Sigma-Aldrich | 13438-45-4 | ZpTS* | Yes |
| Sodium p-toluenesulfonate a | Sigma-Aldrich | 657-84-01 | SpTS | Yes |
| Benzoic acid, 4-(1,1-dimethylethyl)-, zinc salt (2:1) | BOC Sciences, Shirley, NY, USA | 4980-54-5 | BADZ* | 0.05% DMSO |
| Positive controls | ||||
| Aflatoxin-B1 | Sigma-Aldrich | 1162-65-8 | AFB1 | 0.05% DMSO |
| 4-nitroquinoline-oxide | Sigma-Aldrich | 56-57-5 | NQO | 0.05% DMSO |
| Hydrogen peroxide | Sigma-Aldrich | 7722-84-1 | H2O2 | Yes |
| Trichostatin-A | Sigma-Aldrich | 58880-19-6 | TSA | 0.5% DMSO |
| GSK126 | Sigma-Aldrich | 1346574-57-9 | GSK126 | 0.5% DMSO |
| 5-aza-2′-deoxycytidine | Sigma-Aldrich | 2353-33-5 | 5aCdR | 0.5% DMSO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desaulniers, D.; Zhou, G.; Stalker, A.; Cummings-Lorbetskie, C. Effects of Copper or Zinc Organometallics on Cytotoxicity, DNA Damage and Epigenetic Changes in the HC-04 Human Liver Cell Line. Int. J. Mol. Sci. 2023, 24, 15580. https://doi.org/10.3390/ijms242115580
Desaulniers D, Zhou G, Stalker A, Cummings-Lorbetskie C. Effects of Copper or Zinc Organometallics on Cytotoxicity, DNA Damage and Epigenetic Changes in the HC-04 Human Liver Cell Line. International Journal of Molecular Sciences. 2023; 24(21):15580. https://doi.org/10.3390/ijms242115580
Chicago/Turabian StyleDesaulniers, Daniel, Gu Zhou, Andrew Stalker, and Cathy Cummings-Lorbetskie. 2023. "Effects of Copper or Zinc Organometallics on Cytotoxicity, DNA Damage and Epigenetic Changes in the HC-04 Human Liver Cell Line" International Journal of Molecular Sciences 24, no. 21: 15580. https://doi.org/10.3390/ijms242115580





