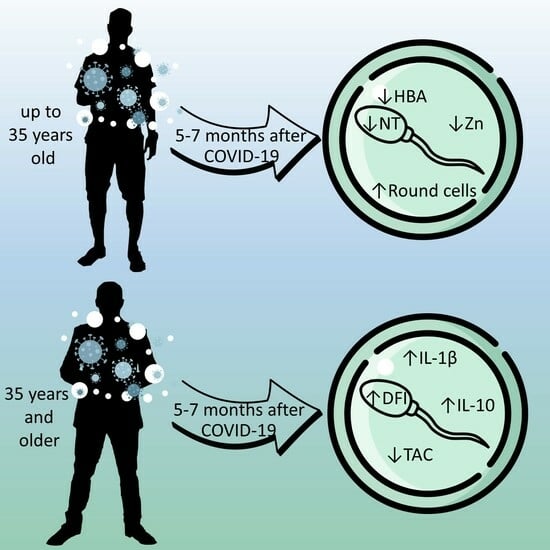
Age-Related COVID-19 Influence on Male Fertility
Abstract
:1. Introduction
2. Results
2.1. Semen Quality Parameters
2.2. Sperm DNA Fragmentation
2.3. Cytokines
2.4. Oxidative Stress Markers
2.5. Correlations between the Studied Variables of Cytokines
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Semen Analysis and Preparation
4.3. Sperm DNA Fragmentation
4.4. Hyaluronan Binding Assay
4.5. Assessment of Cytokines
4.6. Assessment of Total Antioxidant Capacity
4.7. Assessment of Nitrotyrosine Content
4.8. Zinc Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, K.; Eryilmaz, R.; Yokuş, A.; Körpe, K.; Gedük, N.; Özkan, M.; Aslan, R. Examining changes on testicular structure and sperm analysis of COVID-19 patients. Andrologia 2022, 54, e14609. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pallotti, F.; Colangelo, S.; Basilico, F.; Mazzuti, L.; Turriziani, O.; Antonelli, G.; Lenzi, A.; Lombardo, F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Investig. 2020, 43, 1819–1822. [Google Scholar] [CrossRef]
- Dipankar, S.P.; Kumar, T.; Itagi, A.B.H.; Naik, B.N.; Kumar, Y.; Sharma, M.; Sarfaraz, A.; Kumari, A. Semen Quality in Males Suffering From COVID-19: A Pilot Study. Cureus 2022, 14, e31776. [Google Scholar] [CrossRef] [PubMed]
- Falahieh, F.M.; Zarabadipour, M.; Mirani, M.; Abdiyan, M.; Dinparvar, M.; Alizadeh, H.; Paktinat, S.; Hosseinirad, H. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod. Fertil. Dev. 2021, 33, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Piroozmanesh, H.; Cheraghi, E.; Naserpoor, L.; Aghashahi, M.; Jannatifar, R. The Effect of COVID-19 Infection on Sperm Quality and Male Fertility. Jentashapir J. Cell. Mol. Biol. 2021, 12, e115390. [Google Scholar] [CrossRef]
- Stigliani, S.; Massarotti, C.; Bovis, F.; Maccarini, E.; Anserini, P.; Scaruffi, P. Semen parameters and male reproductive potential are not adversely affected after three or more months of recovery from COVID-19 disease. Front. Reprod. Health 2023, 4, 1114308. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Hu, B.; Liu, Z.; Liu, K.; Jiang, H.; Li, H.; Li, R.; Luan, Y.; Liu, X.; Yu, G.; et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 2020, 9, 99–106. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Shcherbitskaia, A.D.; Komarova, E.M.; Milyutina, Y.P.; Ishchuk, M.A.; Sagurova, Y.M.; Safaryan, G.K.; Lesik, E.A.; Gzgzyan, A.M.; Bespalova, O.N.; Kogan, I.Y. Oxidative Stress Markers and Sperm DNA Fragmentation in Men Recovered from COVID-19. Int. J. Mol. Sci. 2022, 23, 10060. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B. COVID-19 and male reproductive function: A prospective, longitudinal cohort study. Reproduction 2021, 161, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Quintana, L.; Vázquez-Lorente, H.; Gamarra-Morales, Y.; Molina-López, J.; Planells, E. Evolution of Status of Trace Elements and Metallothioneins in Patients with COVID-19: Relationship with Clinical, Biochemical, and Inflammatory Parameters. Metabolites 2023, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy–Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Abdlwahid, R.F.; Ali, K.M.; Mahmood, K.I.; Rashid, P.M.A.; Rostam, H.M. The influence of SARS-CoV-2 on male reproduction and men’s health. Eur. J. Clin. Investig. 2023; e14097, online ahead of print. [Google Scholar] [CrossRef]
- Martinez, M.S.; Ferreyra, F.N.; Paira, D.A.; Rivero, V.E.; Olmedo, J.J.; Tissera, A.D.; Molina, R.I.; Motrich, R.D. COVID-19 associates with semen inflammation and sperm quality impairment that reverses in the short term after disease recovery. Front. Physiol. 2023, 14, 1220048. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.; Agarwal, A.; Assidi, M.; Abuzenadah, A.M.; Durairajanayagam, D.; Ayaz, A.; Sharma, R.; Sabanegh, E. Infertile men older than 40 years are at higher risk of sperm DNA damage. Reprod. Biol. Endocrinol. 2014, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Addai, J.; Smith, R.P.; Coward, R.M.; Lamb, D.J.; Lipshultz, L.I. The effects of advanced paternal age on fertility. Asian J. Androl. 2013, 15, 723–728. [Google Scholar] [CrossRef]
- Stone, B.A.; Alex, A.; Werlin, L.B.; Marrs, R.P. Age thresholds for changes in semen parameters in men. Fertil. Steril. 2013, 100, 952–958. [Google Scholar] [CrossRef]
- Slama, R.; Bouyer, J.; Windham, G.; Fenster, L.; Werwatz, A.; Swan, S.H. Influence of Paternal Age on the Risk of Spontaneous Abortion. Am. J. Epidemiol. 2005, 161, 816–823. [Google Scholar] [CrossRef]
- Mohammed, N.; Kamel, M.; Gadelkareem, R.A.; Zarzour, M.A.; Kurkar, A.; Abdel-Moniem, A.M.; Behnsawy, H. Semen quality changes during infection and recovery phases of mild-to-moderate COVID-19 in reproductive-aged patients: A prospective case series. Basic Clin. Androl. 2023, 33, 2. [Google Scholar] [CrossRef]
- Shi, S.; Hu, H.; Wang, J.; Huang, X.; Li, J.; Li, D. Evaluation of semen DNA integrity and related parameters with COVID-19 infection: A prospective cohort study. Virol. J. 2023, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Al-Awaida, W.J.a.; Garrouch, S.; Sallem, A.; Ben Fredj, M.; Kooli, R.; Bousabbeh, M.; Boughzala, I.; Sriha, A.; Hajjaji, A.; Mehdi, M. Deleterious impact of COVID-19 pandemic: Male fertility was not out of the bag. PLoS ONE 2023, 18, e0284489. [Google Scholar] [CrossRef]
- Aschauer, J.; Sima, M.; Imhof, M. Recovery of sperm quality after COVID-19 disease in male adults under the influence of a micronutrient combination: A prospective study. Arch. Ital. Urol. Androl. 2023, 95, 11157. [Google Scholar] [CrossRef] [PubMed]
- Can Balci, M.B.; Can Cilesiz, N. Investigation of the relationship between COVID-19 disease and semen parameters in idiopathic male infertility patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D.O.; Gultekin, D.M.; Aygun, P.G.; Kuskucu, D.M.A.; Akkus, P.E.; Ozkara, D.H. Changes of sperm parameters in men recovered from coronavirus disease 2019 (covid-19): A comparison between precovid and postcovid period. J. Sex. Med. 2022, 19, S316. [Google Scholar] [CrossRef]
- Allameh, F.; Kazemi, M.; Ajorlou, M.; Soroush, S.; Narouie, B.; Fatemi, A.; Dadpour, M. The Effect of SARS-Cov2 Infection on The Spermogram: A Prospective Study. Int. J. Fertil. Steril. 2023, 17, 259–263. [Google Scholar] [CrossRef] [PubMed]
- GamalEl Din, S.F.; Nabil Ismail, N.; Kaddah, A.; Abdel Salam, M.A.; Korani, M.S.; Hamed, M.A. Effect of COVID-19 on sexual and reproductive functions of Egyptian males following recovery: A cross sectional study. Urol. J. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Aksak, T.; Satar, D.A.; Bağci, R.; Gültekin, E.O.; Coşkun, A.; Demirdelen, U. Investigation of the effect of COVID-19 on sperm count, motility, and morphology. J. Med. Virol. 2022, 94, 5201–5205. [Google Scholar] [CrossRef] [PubMed]
- Lyons, H.E.; Arman, B.M.; Robertson, S.A.; Sharkey, D.J. Immune regulatory cytokines in seminal plasma of healthy men: A scoping review and analysis of variance. Andrology 2023, 11, 1245–1266. [Google Scholar] [CrossRef]
- Yu, F.; He, H.; Huang, T.; Zhou, Y. Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data. Open Life Sci. 2023, 18, 20220661. [Google Scholar] [CrossRef]
- Pilatz, A.; Hudemann, C.; Wagenlehner, F.; Schuppe, H.C.; Diemer, T.; Weidner, W.; Renz, H.; Bschleipfer, T. Zytokine im Ejakulat. Der Urol. 2013, 52, 359–366. [Google Scholar] [CrossRef]
- Camejo, M.I. Relation between Immunosuppressive Pge 2 and Il-10 to Pro-Inflammatory Il-6 in Seminal Plasma of Infertile and Fertile Men. Arch. Androl. 2009, 49, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, R.; Salemi, M.; Vicari, L.O.; Vicari, E. Relationship of semen hyperviscosity with IL-6, TNF-α, IL-10 and ROS production in seminal plasma of infertile patients with prostatitis and prostato-vesiculitis. Andrologia 2014, 46, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015, 108, 98–104. [Google Scholar] [CrossRef]
- Aziz, N.; Agarwal, A.; Lewis-Jones, I.; Sharma, R.K.; Thomas, A.J. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil. Steril. 2004, 82, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, B.; Sun, A.; Guo, F. Interleukin-10 Family Cytokines Immunobiology and Structure. In Structural Immunology; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; pp. 79–96. [Google Scholar]
- Zalata, A. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol. Hum. Reprod. 1998, 4, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, M.E.; Beguerie, C.; De Zuñiga, I.; Martinez, G.; Frungieri, M.B. Impact of COVID-19 on sperm quality and the prostaglandin and polyamine systems in the seminal fluid. Andrology, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Kalezic, A.; Macanovic, B.; Garalejic, E.; Korac, A.; Otasevic, V.; Korac, B. Level of NO/nitrite and 3-nitrotyrosine in seminal plasma of infertile men: Correlation with sperm number, motility and morphology. Chem.-Biol. Interact. 2018, 291, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Zenzes, M.T. Detection of benzo[a]pyrene diol epoxide-DNA adducts in embryos from smoking couples: Evidence for transmission by spermatozoa. Mol. Hum. Reprod. 1999, 5, 125–131. [Google Scholar] [CrossRef]
- Simon, L.; Emery, B.; Carrell, D.T. Sperm DNA Fragmentation: Consequences for Reproduction. In Genetic Damage in Human Spermatozoa; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; pp. 87–105. [Google Scholar]
- Martin, J.H.; Aitken, R.J.; Bromfield, E.G.; Nixon, B. DNA damage and repair in the female germline: Contributions to ART. Hum. Reprod. Update 2019, 25, 180–201. [Google Scholar] [CrossRef]
- Horta, F.; Temple-Smith, P.; Vollenhoven, B.; Ramachandran, P.; Catt, S. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum. Reprod. 2020, 35, 529–544. [Google Scholar] [CrossRef]
- Lutsky, D.L.; Mahmudov, R.M.; Lutskaya, A.M.; Vybornov, S.V.; Nikolaev, A.A.; Kalashnikov, E.S.; Nikulina, D.M.; Lozovskii, V.V.; Lozovskiy, V.V.; Shishkina, L.M. The impact of asymptomatic and mild COVID-19 on sperm characteristics. J. Clin. Pract. 2022, 13, 17–24. [Google Scholar] [CrossRef]
- Son, W.Y. Specific expression of heat shock protein HspA2 in human male germ cells. Mol. Hum. Reprod. 1999, 5, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Cayli, S. Cellular maturity and apoptosis in human sperm: Creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Mol. Hum. Reprod. 2004, 10, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]




| Parameters | <35, COVID− | <35, COVID+ | >35, COVID− | >35, COVID+ |
|---|---|---|---|---|
| Progressive motility (PR, %) | 61.50 [49.00–70.00] | 57.00 [50.00–62.00] | 63.00 [55.00–72.00] | 61.00 [50.00–69.00] |
| Nonprogressive motility (NP, %) | 7.00 [6.00–11.00] | 8.00 [6.00–12.00] | 8.00 [5.00–9.00] | 7.00 [6.00–10.00] |
| Immotility (IM, %) | 30.00 [21.00–39.00] | 31.00 [28.00–42.00] | 28.00 [21.00–41.00] | 30.00 [22.00–42.00] |
| Round cells (106 cells/mL) | 0.30 [0.10–0.60] | 1.00 [0.70–2.00] ** | 0.40 [0.00–1.00] | 0.40 [0.10–4.00] |
| Normal morphology (%) | 3.50 [2.00–5.00] | 3.00 [1.00–4.00] | 3.00 [2.00–5.00] | 3 [1.00–4.00] |
| Head defects (%) | 91.00 [87.00–93.00] | 91.00 [89.00–94.00] | 90.00 [87.00–94.00] | 92.00 [88.00–94.00] |
| Midpiece defects (%) | 1.00 [0.00–1.00] | 1.00 [0.00–1.00] | 1.00 [0.00–1.50] | 1.00 [0.00–1.00] |
| Tail defects (%) | 0.00 [0.00–1.00] | 1.00 [0.00–1.00] | 0.00 [0.00–1.00] | 0.00 [0.00–1.00] |
| HBA (%) | 89.50 [88.00–93.00] | 70.00 [50.00–92.00] * | 83.00 [25.00–88.00] | 89.00 [71.00–90.00] |
| Parameters | <35, COVID− | <35, COVID+ | >35, COVID− | >35, COVID+ |
|---|---|---|---|---|
| n | 39 | 20 | 38 | 40 |
| Age (years) | 31 [29–33] | 33 [21–34] | 38.5 [37–40] | 37.5 [36–40] |
| BMI | 25 [24–27] | 25 [23–27] | 26 [24–30] | 26 [23–28] |
| Months after COVID | NA | 7 [4–12] | NA | 5 [3–11] |
| Paternity (%) | 11 | 5 | 35 | 44 |
| Smoking (%) | 30 | 32 | 32 | 28 |
| Alcohol consumption (%) | 22 | 32 | 27 | 38 |
| Volume (mL) | 4 [3–5] | 3.5 [3–4.75] | 3.25 [2.50–4.00] | 3.25 [3–4.75] |
| Sperm concentration (mln/mL) | 82 [61–136] | 70 [50–110] | 112.5 [69.00–171] | 104.5 [69–157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbitskaia, A.D.; Komarova, E.M.; Milyutina, Y.P.; Sagurova, Y.M.; Ishchuk, M.A.; Mikhel, A.V.; Ob’edkova, K.V.; Lesik, E.A.; Gzgzyan, A.M.; Tapilskaya, N.I.; et al. Age-Related COVID-19 Influence on Male Fertility. Int. J. Mol. Sci. 2023, 24, 15742. https://doi.org/10.3390/ijms242115742
Shcherbitskaia AD, Komarova EM, Milyutina YP, Sagurova YM, Ishchuk MA, Mikhel AV, Ob’edkova KV, Lesik EA, Gzgzyan AM, Tapilskaya NI, et al. Age-Related COVID-19 Influence on Male Fertility. International Journal of Molecular Sciences. 2023; 24(21):15742. https://doi.org/10.3390/ijms242115742
Chicago/Turabian StyleShcherbitskaia, Anastasiia D., Evgeniia M. Komarova, Yulia P. Milyutina, Yanina M. Sagurova, Mariia A. Ishchuk, Anastasiia V. Mikhel, Ksenia V. Ob’edkova, Elena A. Lesik, Alexander M. Gzgzyan, Natalya I. Tapilskaya, and et al. 2023. "Age-Related COVID-19 Influence on Male Fertility" International Journal of Molecular Sciences 24, no. 21: 15742. https://doi.org/10.3390/ijms242115742