Murine Bone Marrow Erythroid Cells Have Two Branches of Differentiation Defined by the Presence of CD45 and a Different Immune Transcriptome Than Fetal Liver Erythroid Cells
Abstract
:1. Introduction
2. Results
2.1. Usage of CD71 as a Murine Erythroid Cell Enrichment Marker for Bulk Immunomics Results in B-Cell Contamination, as Well as Artificially Lower Gene Expression of Cytokines Compared to Ter-119
2.2. Murine Bone Marrow Erythroid Cells Have an Antimicrobial Gene Expression Signature, While Murine Fetal Liver Erythroid Cells Have an Antiviral Gene Expression Signature
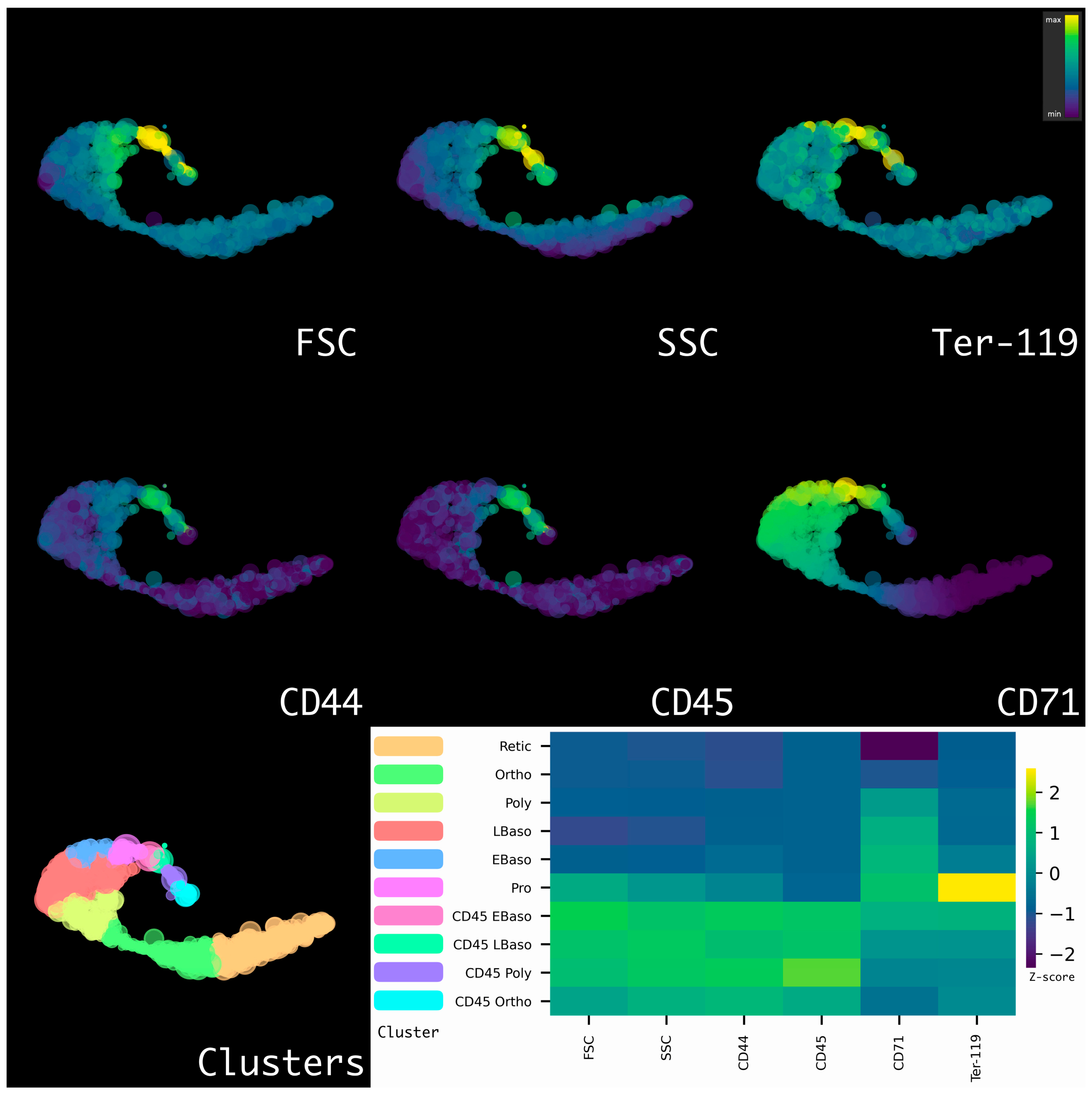
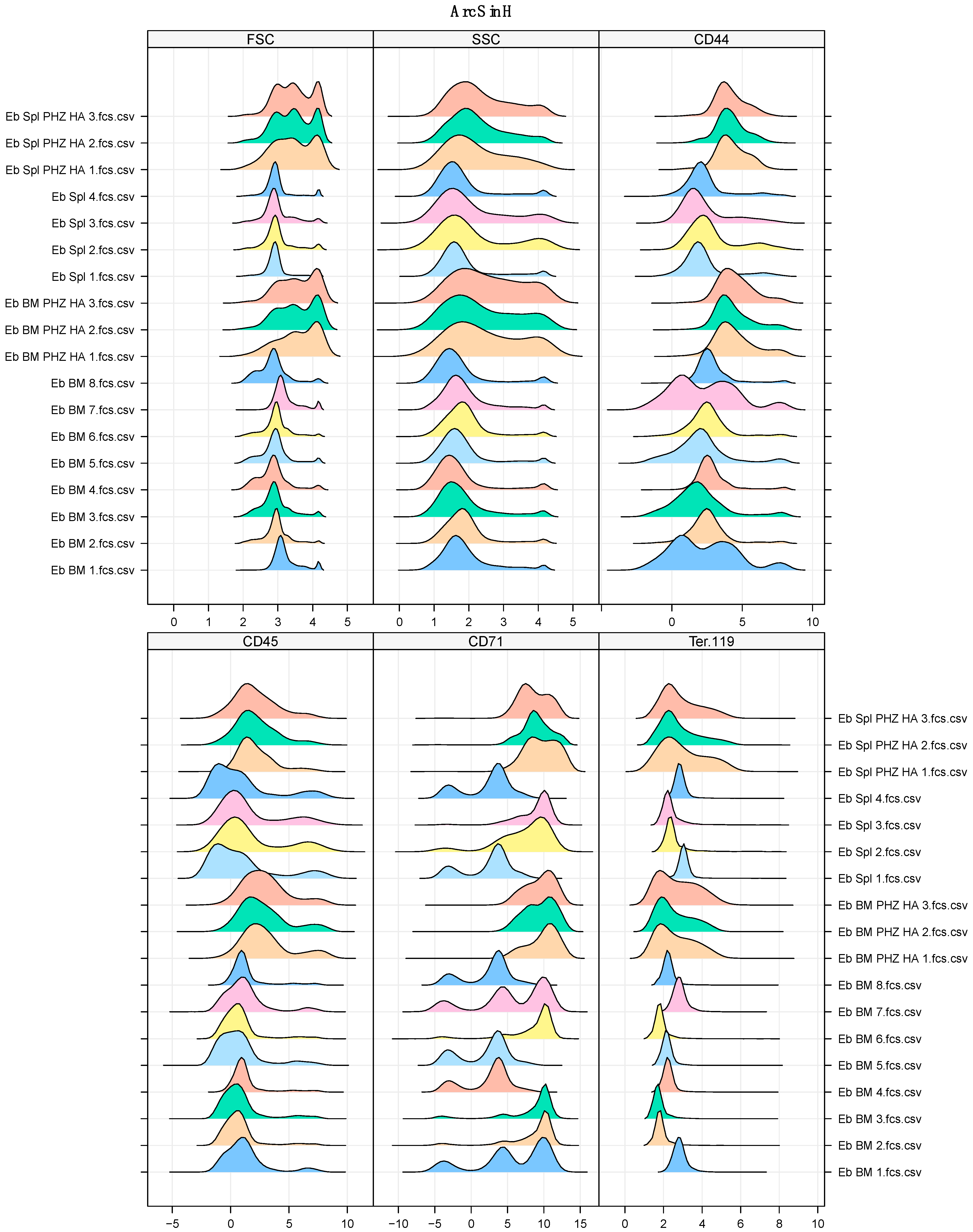
2.3. Murine Bone Marrow Erythroid Cells Have Two Distinct Branches of Erythropoiesis
2.4. Phenylhydrazine-Induced Hemolytic Anemia Distorts Both Branches of Murine Erythropoiesis
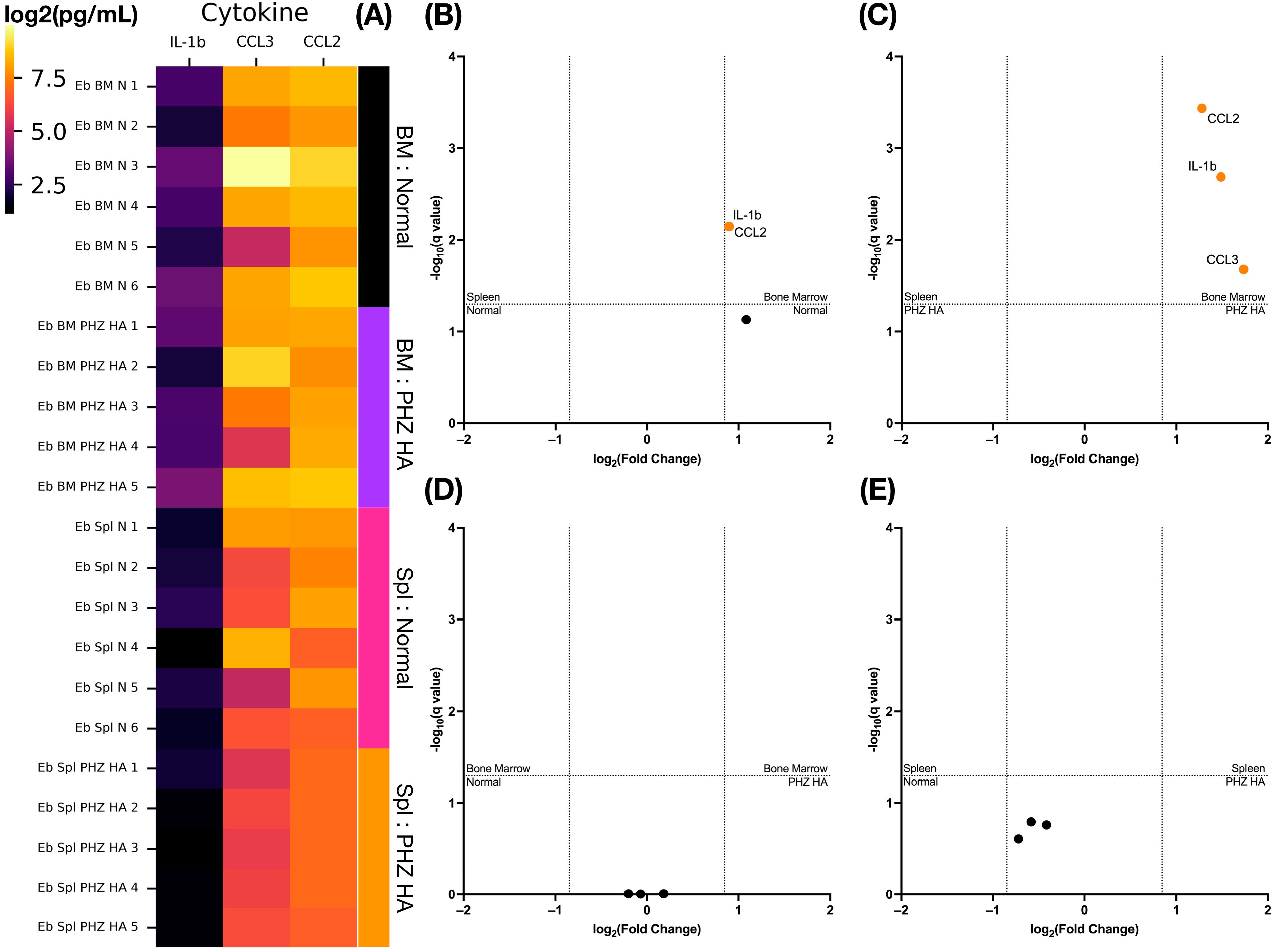
2.5. Phenylhydrazine-Induced Hemolytic Anemia Causes a Decrease in Cytokine Secretion by Murine Splenic Erythroid Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Hemolytic Anemia Model
4.3. Cell Isolation
4.4. Magnetic Separation
4.5. Viability Staining
4.6. Cell Culturing
4.7. Harvesting the Conditioned Media of Erythroid Cells
4.8. Cytokine Quantification in a Culture Medium Using Bio-Plex
4.9. Total RNA Extraction
4.10. Nanostring Gene Expression Profiling
4.11. Differential Expression and Secretion Testing
4.12. Flow Cytometry
4.13. Gating of Erythroid Cells
4.14. Arcsinh-Data Transformation and Data Normalization
4.15. HSNE Dimensionality Reduction and Clustering
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From erythroblasts to mature red blood cells: Organelle clearance in mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Socolovsky, M.; Nam, H.S.; Fleming, M.D.; Haase, V.H.; Brugnara, C.; Lodish, H.F. Ineffective erythropoiesis in Stat5a−/− 5b−/− mice due to decreased survival of early erythroblasts. Blood J. Am. Soc. Hematol. 2001, 98, 3261–3273. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Q.; Hou, J.; Gu, Y.; Zhang, Y.; Chen, Z.; Fan, J.; Zhou, W.; Qiu, S.; Zhang, Y.; et al. Tumor-induced generation of splenic erythroblast-like Ter-cells promotes tumor progression. Cell 2018, 173, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Kina, T.; Ikuta, K.; Takayama, E.; Wada, K.; Majumdar, A.S.; Weissman, I.L.; Katsura, Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 2000, 109, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef] [PubMed]
- Marsee, D.K.; Pinkus, G.S.; Yu, H. CD71 (transferrin receptor) an effective marker for erythroid precursors in bone marrow biopsy specimens. Am. J. Clin. Pathol. 2010, 134, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V.; Samarin, D.M.; Taraban, V.Y.; Sennikov, S.V.; Kozlov, V.A. Characterization of erythroid cell-derived natural suppressor activity. Immunobiology 1998, 198, 361–374. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Nowis, D.; Golab, J. The role of CD71+ erythroid cells in the regulation of the immune response. Pharmacol. Ther. 2021, 228, 107927. [Google Scholar] [CrossRef]
- Dong, H.Y.; Wilkes, S.; Yang, H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: A comparative immunochemical study with glycophorin A and hemoglobin A. Am. J. Surg. Pathol. 2011, 35, 723–732. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Rydzynska, Z.; Lazniewski, M.; Plewczynski, D.; Klicka, K.; Malecka-Gieldowska, M.; Rodziewicz-Lurzynska, A.; Ciepiela, O.; Justyniarska, M.; et al. Potent but transient immunosuppression of T-cells is a general feature of CD71+ erythroid cells. Commun. Biol. 2021, 4, 1384. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Xu, L.; Elahi, S. Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: The kinetics of expression of T cell activation markers. J. Immunol. Methods 2016, 437, 43–52. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Jackson, K.J.; Kissick, H.T.; Nakaya, H.I.; Davis, C.W.; Roskin, K.M.; McElroy, A.K.; Oshansky, C.M.; Elbein, R.; Thomas, S.; et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016, 17, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Allden, S.J.; Ogger, P.P.; Ghai, P.; McErlean, P.; Hewitt, R.; Toshner, R.; Walker, S.A.; Saunders, P.; Kingston, S.; Molyneaux, P.L.; et al. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 209–219. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Ginzburg, Y.; Li, H.; Xue, F.; De Franceschi, L.; Chasis, J.A.; Mohandas, N.; An, X. Quantitative analysis of murine terminal erythroid differentiation in vivo: Novel method to study normal and disordered erythropoiesis. Blood J. Am. Soc. Hematol. 2013, 121, e43–e49. [Google Scholar] [CrossRef]
- Wangen, J.R.; Eidenschink Brodersen, L.; Stolk, T.T.; Wells, D.A.; Loken, M.R. Assessment of normal erythropoiesis by flow cytometry: Important considerations for specimen preparation. Int. J. Lab. Hematol. 2014, 36, 184–196. [Google Scholar] [CrossRef]
- Boulais, P.E.; Mizoguchi, T.; Zimmerman, S.; Nakahara, F.; Vivié, J.; Mar, J.C.; van Oudenaarden, A.; Frenette, P.S. The majority of CD45–Ter119–CD31–bone marrow cell fraction is of hematopoietic origin and contains erythroid and lymphoid progenitors. Immunity 2018, 49, 627–639. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Justyniarska, M.; Nowis, D.; Golab, J. Tumor immune evasion induced by dysregulation of erythroid progenitor cells development. Cancers 2021, 13, 870. [Google Scholar] [CrossRef]
- Zhao, G.J.; Jiang, D.W.; Cai, W.C.; Chen, X.Y.; Dong, W.; Chen, L.W.; Hong, G.L.; Wu, B.; Yao, Y.M.; Lu, Z.Q. CD71+ Erythroid Cell Expansion in Adult Sepsis: Potential Causes and Role in Prognosis and Nosocomial Infection Prediction. Front. Immunol. 2022, 13, 830025. [Google Scholar] [CrossRef]
- Mashhouri, S.; Koleva, P.; Huynh, M.; Okoye, I.; Shahbaz, S.; Elahi, S. Sex matters: Physiological abundance of immuno-regulatory CD71+ erythroid cells impair immunity in females. Front. Immunol. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Elahi, S.; Vega-López, M.A.; Herman-Miguel, V.; Ramírez-Estudillo, C.; Mancilla-Ramírez, J.; Motyka, B.; West, L.; Oyegbami, O. CD71+ erythroid cells in human neonates exhibit immunosuppressive properties and compromise immune response against systemic infection in neonatal mice. Front. Immunol. 2020, 11, 597433. [Google Scholar] [CrossRef] [PubMed]
- Sennikov, S.V.; Eremina, L.V.; Samarin, D.M.; Avdeev, I.V.; Kozlov, V.A. Cytokine gene expression in erythroid cells. Eur. Cytokine Netw. 1996, 7, 771–774. [Google Scholar] [PubMed]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines. Int. J. Mol. Sci. 2023, 24, 8130. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, J.A.; Perik-Zavodskii, R.Y.; Nazarov, K.V.; Denisova, V.V.; Perik-Zavodskaya, O.Y.; Philippova, Y.G.; Alsalloum, A.; Sennikov, S.V. Immunoregulatory properties of erythroid nucleated cells induced from CD34+ progenitors from bone marrow. PLoS ONE 2023, 18, e0287793. [Google Scholar] [CrossRef] [PubMed]
- Sennikov, S.V.; Injelevskaya, T.V.; Krysov, S.V.; Silkov, A.N.; Kovinev, I.B.; Dyachkova, N.J.; Zenkov, A.N.; Loseva, M.I.; Kozlov, V.A. Production of hemo-and immunoregulatory cytokines by erythroblast antigen+ and glycophorin A+ cells from human bone marrow. BMC Cell Biol. 2004, 5, 39. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, M.; Chen, Y.; Liu, J.; Qi, S.; Ma, J.; Zhang, Z.; Xu, Y. EPO-dependent induction of erythroferrone drives hepcidin suppression and systematic iron absorption under phenylhydrazine-induced hemolytic anemia. Blood Cells Mol. Dis. 2016, 58, 45–51. [Google Scholar] [CrossRef]
- Ousaaid, D.; Ghouizi, A.E.; Laaroussi, H.; Bakour, M.; Mechchate, H.; Es-Safi, I.; Kamaly, O.A.; Saleh, A.; Conte, R.; Lyoussi, B.; et al. Anti-Anemic Effect of Antioxidant-Rich Apple Vinegar against Phenylhydrazine-Induced Hemolytic Anemia in Rats. Life 2022, 12, 239. [Google Scholar] [CrossRef]
- Augusto, O.; Kunze, K.L.; Ortiz de Montellano, P.R. N-Phenylprotoporphyrin IX formation in the hemoglobin-phenylhydrazine reaction. Evidence for a protein-stabilized iron-phenyl intermediate. J. Biol. Chem. 1982, 257, 6231–6241. [Google Scholar] [CrossRef]
- Shetlar, M.D.; Hill, H.A. Reactions of hemoglobin with phenylhydrazine: A review of selected aspects. Environ. Health Perspect. 1985, 64, 265. [Google Scholar] [CrossRef]
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood J. Am. Soc. Hematol. 2010, 115, e10–e19. [Google Scholar] [CrossRef]
- Gautier, E.F.; Ducamp, S.; Leduc, M.; Salnot, V.; Guillonneau, F.; Dussiot, M.; Hale, J.; Giarratana, M.C.; Raimbault, A.; Douay, L.; et al. Comprehensive proteomic analysis of human erythropoiesis. Cell Rep. 2016, 16, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Shevchenko, J.; Denisova, V.; Alrhmoun, S.; Volynets, M.; Tereshchenko, V.; Zaitsev, K.; Sennikov, S. Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing. Cells 2022, 11, 3537. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Piyadasa, H.; Mostafa, D.; Chow, L.N.; Halayko, A.J.; Mookherjee, N. Cathelicidin and calprotectin are disparately altered in murine models of inflammatory arthritis and airway inflammation. Front. Immunol. 2020, 11, 1932. [Google Scholar] [CrossRef]
- Zeiner, P.S.; Zinke, J.; Kowalewski, D.J.; Bernatz, S.; Tichy, J.; Ronellenfitsch, M.W.; Thorsen, F.; Berger, A.; Forster, M.T.; Muller, A.; et al. CD74 regulates complexity of tumor cell HLA class II peptidome in brain metastasis and is a positive prognostic marker for patient survival. Acta Neuropathol. Commun. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Jiang, S.; Tsukada, Y.; Tsujimoto, H.; Kimura, T. IFN-Alpha1 antisense RNA represses human influenza A virus growth in a guinea pig system. Front. Biosci.-Landmark. 2019, 24, 798–818. [Google Scholar] [CrossRef]
- Hahne, F.; Khodabakhshi, A.H.; Bashashati, A.; Wong, C.J.; Gascoyne, R.D.; Weng, A.P.; Seyfert-Margolis, V.; Bourcier, K.; Asare, A.; Lumley, T.; et al. Per-channel basis normalization methods for flow cytometry data. Cytom. Part A J. Int. Soc. Adv. Cytom. 2010, 77, 121–131. [Google Scholar] [CrossRef]
- Melsen, J.E.; van Ostaijen-Ten Dam, M.M.; Lankester, A.C.; Schilham, M.W.; van den Akker, E.B. A comprehensive workflow for applying single-cell clustering and pseudotime analysis to flow cytometry data. J. Immunol. 2020, 205, 864–871. [Google Scholar] [CrossRef]
- Höllt, T.; Pezzotti, N.; van Unen, V.; Koning, F.; Eisemann, E.; Lelieveldt, B.; Vilanova, A. Cytosplore: Interactive immune cell phenotyping for large single-cell datasets. Comput. Graph. Forum 2016, 35, 171–180. [Google Scholar] [CrossRef]

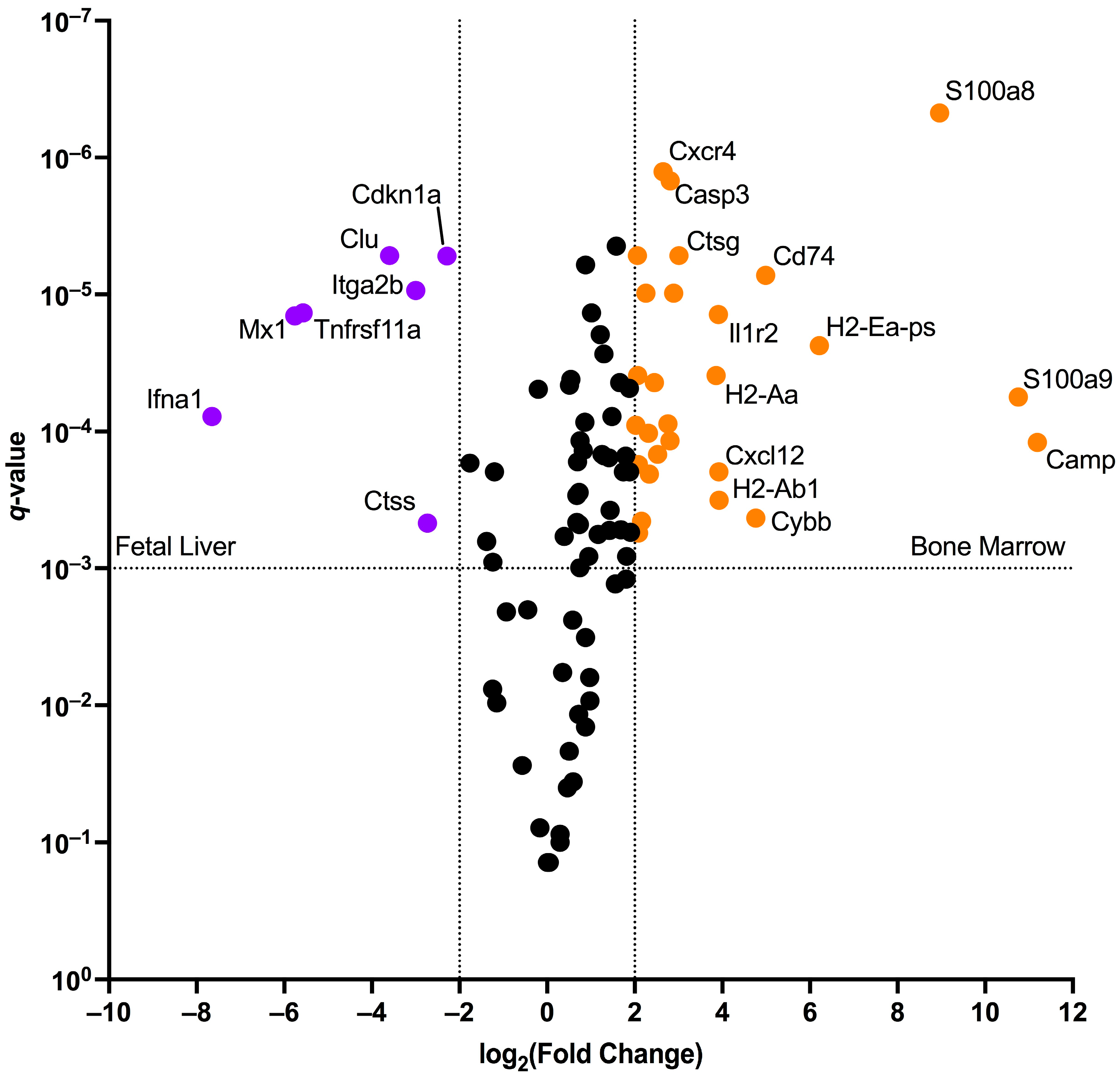




| Term | Overlap | q-Value | Score | Genes |
|---|---|---|---|---|
| Positive Regulation Of Response To External Stimulus | 4/155 | 0.003 | 224.76 | NFKBIZ, S100A9, ETS1, S100A8 |
| Response To Lipopolysaccharide | 4/159 | 0.003 | 216.71 | TNFAIP3, CTSG, S100A9, S100A8 |
| Peptidyl-Cysteine Modification | 2/9 | 0.003 | 2207.74 | S100A9, S100A8 |
| Response To Molecule Of Bacterial Origin | 3/69 | 0.004 | 344.24 | TNFAIP3, S100A9, S100A8 |
| Neutrophil Chemotaxis | 3/70 | 0.004 | 337.49 | S100A9, TREM1, S100A8 |
| Granulocyte Activation | 2/12 | 0.004 | 1448.78 | CTSG, CAMP |
| Granulocyte Chemotaxis | 3/73 | 0.004 | 318.53 | S100A9, TREM1, S100A8 |
| Defense Response To Gram-negative Bacterium | 3/75 | 0.004 | 306.87 | CTSG, TREM1, CAMP |
| Neutrophil Migration | 3/77 | 0.004 | 295.92 | S100A9, TREM1, S100A8 |
| Defense Response To Bacterium | 4/204 | 0.004 | 150.99 | CTSG, S100A9, S100A8, CAMP |
| Positive Regulation Of Immune Response | 3/80 | 0.004 | 280.68 | CD74, PTPRC, ITGAM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perik-Zavodskaia, O.; Perik-Zavodskii, R.; Nazarov, K.; Volynets, M.; Alrhmoun, S.; Shevchenko, J.; Sennikov, S. Murine Bone Marrow Erythroid Cells Have Two Branches of Differentiation Defined by the Presence of CD45 and a Different Immune Transcriptome Than Fetal Liver Erythroid Cells. Int. J. Mol. Sci. 2023, 24, 15752. https://doi.org/10.3390/ijms242115752
Perik-Zavodskaia O, Perik-Zavodskii R, Nazarov K, Volynets M, Alrhmoun S, Shevchenko J, Sennikov S. Murine Bone Marrow Erythroid Cells Have Two Branches of Differentiation Defined by the Presence of CD45 and a Different Immune Transcriptome Than Fetal Liver Erythroid Cells. International Journal of Molecular Sciences. 2023; 24(21):15752. https://doi.org/10.3390/ijms242115752
Chicago/Turabian StylePerik-Zavodskaia, Olga, Roman Perik-Zavodskii, Kirill Nazarov, Marina Volynets, Saleh Alrhmoun, Julia Shevchenko, and Sergey Sennikov. 2023. "Murine Bone Marrow Erythroid Cells Have Two Branches of Differentiation Defined by the Presence of CD45 and a Different Immune Transcriptome Than Fetal Liver Erythroid Cells" International Journal of Molecular Sciences 24, no. 21: 15752. https://doi.org/10.3390/ijms242115752





