Oxidative Stress and Antioxidant Defense in the Heart, Liver, and Kidney of Bat Species with Different Feeding Habits
Abstract
:1. Introduction
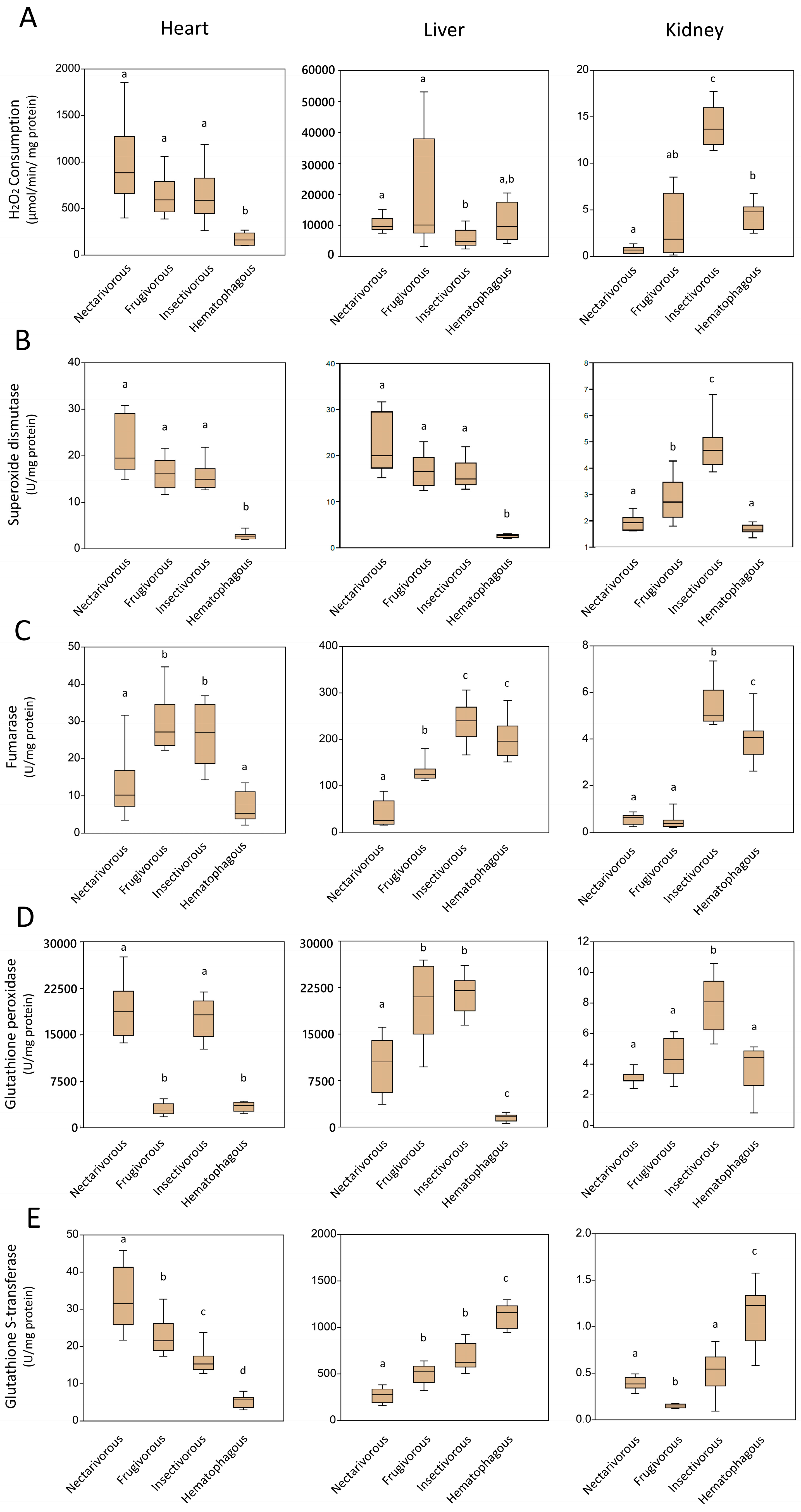
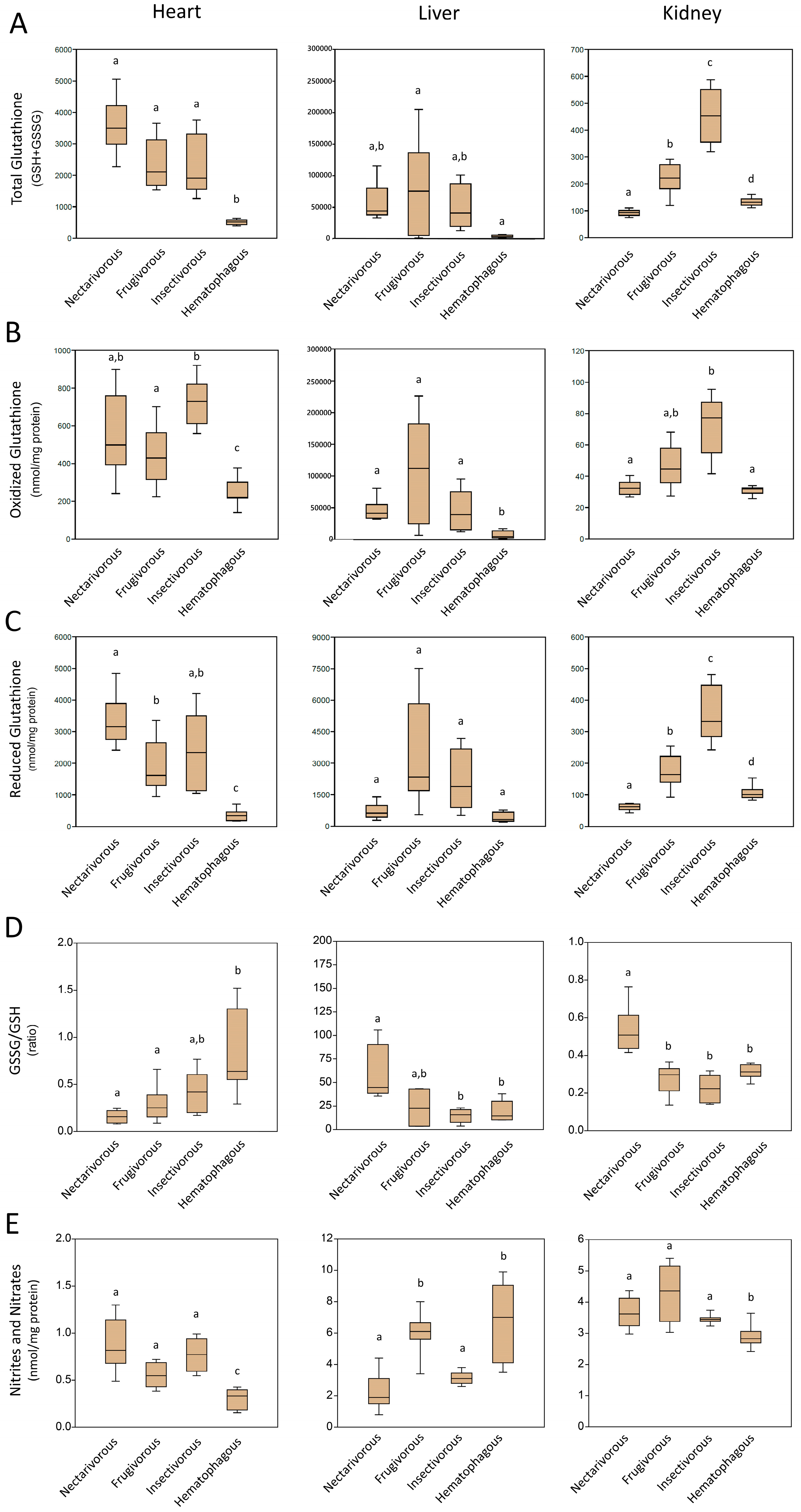
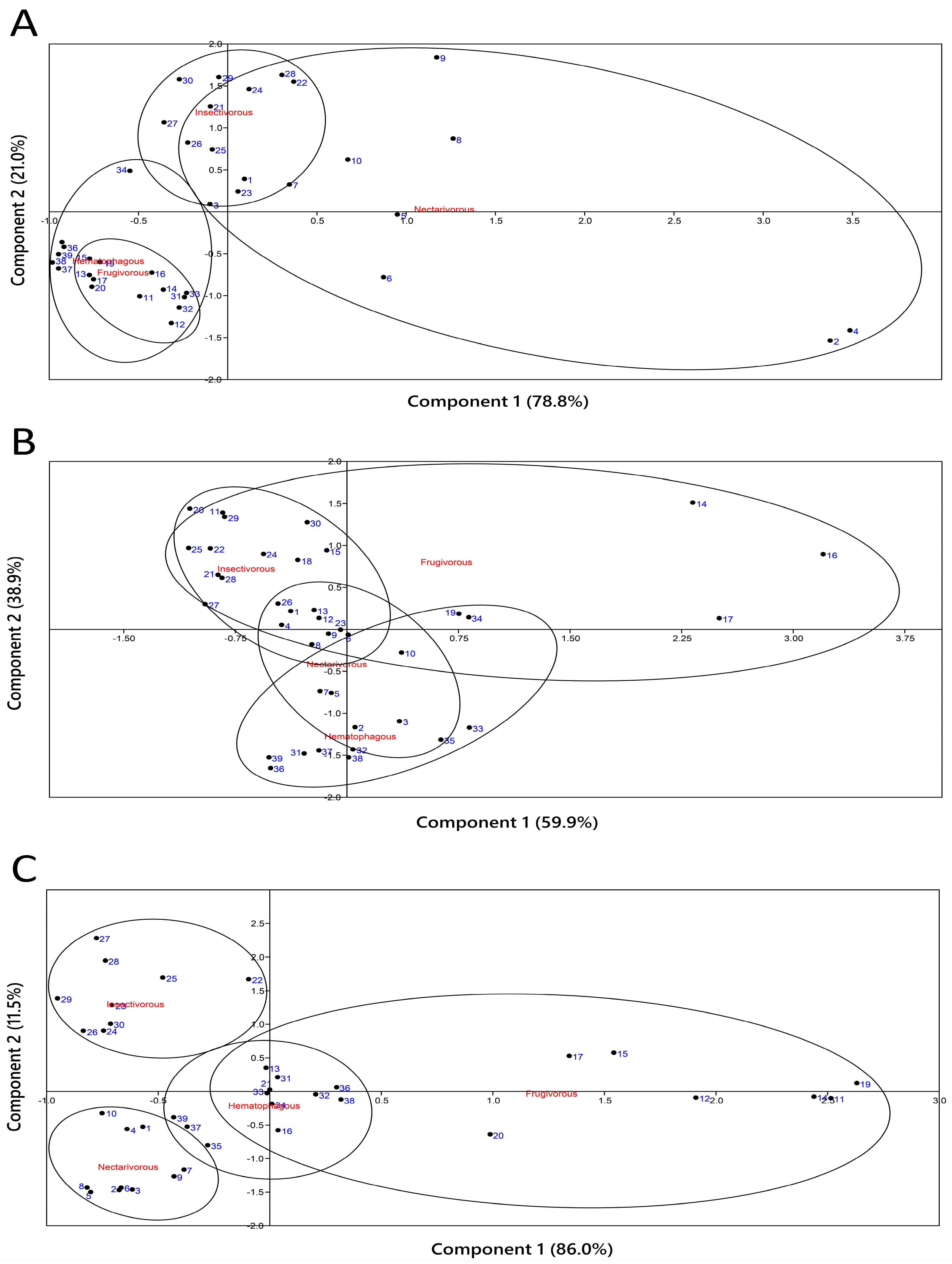
2. Results
2.1. Oxidative Damage
2.2. Antioxidant Enzymes
2.3. Non-Enzymatic Antioxidants
2.4. Principal Component Analysis
2.5. Correlation
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
4.2. Animals and Organ Collection
4.3. Organ Processing
4.4. Biochemical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.; McConkey, K.R.; Tanalgo, K.; Sritongchuay, T.; Low, M.-R.; Yong, J.Y.; Mildenstein, T.L.; Nuevo-Diego, C.E.; Lim, V.C.; Racey, P.A. The Critical Importance of Old World Fruit Bats for Healthy Ecosystems and Economies. Front. Ecol. Evol. 2021, 9, 641411. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; Chitranshi, N.; Malviya, R.; et al. Comprehensive Review of Methodology to Detect Reactive Oxygen Species (ROS) in Mammalian Species and Establish its Relationship with Antioxidants and Cancer. Antioxidants 2021, 10, 128. [Google Scholar] [CrossRef]
- Hunyadi, A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Orr, T.J.; Ortega, J.; Medellín, R.A.; Sánchez, C.D.; Hammond, K.A. Diet Choice in Frugivorous Bats: Gourmets or Operational Pragmatists? J. Mammal. 2016, 97, 1578–1588. [Google Scholar] [CrossRef]
- Schneeberger, K.; Czirják, G.Á.; Voigt, C.C. Frugivory Is Associated with Low Measures of Plasma Oxidative Stress and High Antioxidant Concentration in Free-Ranging Bats. Naturwissenschaften 2014, 101, 285–290. [Google Scholar] [CrossRef]
- Graça-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.; Paes, M.C.; Sorgine, M.H.; Oliveira, M.F.; Oliveira, P.L. Adaptations against Heme Toxicity in Blood-Feeding Arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335. [Google Scholar] [CrossRef]
- Oliveira, F.W.; Schindler, M.S.Z.; Cora, D.H.; Thiel, N.; Siebel, A.M.; Galiano, D. Oxidative State of the Frugivorous Bat Sturnira lilium (Chiroptera: Phyllostomidae) in Agricultural and Urban Areas of Southern Brazil. Environ. Sci. Pollut. Res. 2020, 27, 30868–30874. [Google Scholar] [CrossRef]
- Wilhelm, D.; Althoff, S.L.; Dafre, A.L.; Boveris, A. Antioxidant Defenses, Longevity and Ecophysiology of South American Bats. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2007, 146, 214–220. [Google Scholar] [CrossRef]
- Hanadhita, D.; Rahma, A.; Prawira, A.Y.; Mayasari, N.; Satyaningtijas, A.S.; Hondo, E.; Agungpriyono, S. The Spleen Morphophysiology of Fruit Bats. Anat. Histol. Embryol. 2019, 48, 315–324. [Google Scholar] [CrossRef]
- Mena Canata, D.A.; Benfato, M.S.; Pereira, F.D.; Pereira, M.J.R.; Rampelotto, P.H. Vitamin C Levels in Different Organs of Bat Species from Different Food Groups. Life 2022, 12, 2121. [Google Scholar] [CrossRef] [PubMed]
- Lemke, T.O. Foraging Ecology of the Long-Nosed Bat, Glossophaga soricina, with Respect to Resource Availability. Ecology 1984, 65, 538–548. [Google Scholar] [CrossRef]
- Jacomassa, F.A.F.; Bernardi, I.P.; Passos, F.C. Seasonal Diet Variation, Preferences and Availability of Resources Consumed by Sturnira lilium (É. Geoffroy St.-Hilaire, 1810) (Chiroptera: Phyllostomidae) in Brazilian Seasonal Deciduous Forest. An. Acad. Bras. Cienc. 2021, 93, e20201571. [Google Scholar] [CrossRef] [PubMed]
- Willig, M.R.; Camilo, G.R.; Noble, S.J. Dietary Overlap in Frugivorous and Insectivorous Bats from Edaphic Cerrado Habitats of Brazil. J. Mammal. 1993, 74, 117–128. [Google Scholar] [CrossRef]
- Mantovan, K.B.; Menozzi, B.D.; Paiz, L.M.; Sevá, A.P.; Brandão, P.E.; Langoni, H. Geographic Distribution of Common Vampire Bat Desmodus rotundus (Chiroptera: Phyllostomidae) Shelters: Implications for the Spread of Rabies Virus to Cattle in Southeastern Brazil. Pathogens 2022, 11, 942. [Google Scholar] [CrossRef]
- Freitas, R.M.P.; Oliveira, M.; Castro, D.L.J.; Sarandy, M.M.; Gonçalves, R.V.; Freitas, M.B. The Antioxidant Status of Three Neotropical Bat Species with Different Feeding Habits. Acta Chiropt. 2020, 21, 395–402. [Google Scholar] [CrossRef]
- Golpa, P. Morphological Adaptations in the Kidney and Urine Concentrating Ability in Relation to Dietary Habit in the Three Species of Bats. World J. Zool. 2013, 8, 198–205. [Google Scholar]
- van Deel, E.D.; Lu, Z.; Xu, X.; Zhu, G.; Hu, X.; Oury, T.D.; Bache, R.J.; Duncker, D.J.; Chen, Y. Extracellular Superoxide Dismutase Protects the Heart against Oxidative Stress and Hypertrophy after Myocardial Infarction. Free Radic. Biol. Med. 2008, 44, 1305–1313. [Google Scholar] [CrossRef]
- Schondube, J.E.; Herrera, M.L.G.; Martínez del Rio, C. Diet and the Evolution of Digestion and Renal Function in Phyllostomid Bats. Zoology 2001, 104, 59–73. [Google Scholar] [CrossRef]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 5028181. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular Redox Compartmentation and ROS-Related Communication in Regulation and Signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, I.M.; Smith, D.A.; Ganz, T.; Crawshaw, G.J.; Hammermueller, J.D.; Bienzle, D.; Lillie, B.N. Iron Storage Disease (Hemochromatosis) and Hepcidin Response to Iron Load in Two Species of Pteropodid Fruit Bats Relative to the Common Vampire Bat. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2018, 188, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Ge, H.; Liao, C.C.; Liu, D.; Zhang, S.; Pan, Y.H. Antioxidant Defenses in the Brains of Bats during Hibernation. PLoS ONE 2016, 11, e0152135. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione System Enhancement for Cardiac Protection: Pharmacological Options against Oxidative Stress and Ferroptosis. Cell Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox Status Expressed as GSH:GSSG Ratio as a Marker for Oxidative Stress in Pediatric Tumour Patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Martín-Grau, M.; Pardo-Tendero, M.; Casanova, P.; Dromant, M.; Marrachelli, V.G.; Morales, J.M.; Borrás, C.; Pisoni, S.; Maestrini, S.; Di Blasio, A.M.; et al. Altered Lipid Moieties and Carbonyls in a Wistar Rat Dietary Model of Subclinical Fatty Liver: Potential Sex-Specific Biomarkers of Early Fatty Liver Disease? Antioxidants 2023, 12, 1808. [Google Scholar] [CrossRef]
- Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Oxidative Stress Parameters in the Liver of Growing Male Rats Receiving Various Alcoholic Beverages. Nutrients 2020, 12, 158. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney, and Heart of High Fat Diet-Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Zhang, Y.; Zhang, Y.; Wang, W.; Han, H.; Yang, C.; Dong, X. Superoxide dismutase ameliorates oxidative stress and regulates liver transcriptomics to provide therapeutic benefits in hepatic inflammation. PeerJ 2023, 11, e15829. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox State and Methods to Evaluate Oxidative Stress in Liver Damage: From Bench to Bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [PubMed]
- Miyata, T.; Kurokawa, K.; van Ypersele de Strihou, C. Relevance of Oxidative and Carbonyl Stress to Long-term Uremic Complications. Kidney Int. 2000, 76, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Van Den Branden, C.; Ceyssens, B.; De Craemer, D.; De Bleser, P.; Hellemans, K.; Geerts, A.; Verbeelen, D. Antioxidant Enzyme Gene Expression in Rats with Remnant Kidney Induced Chronic Renal failure. Exp. Nephrol. 2000, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, L.M. Adapt or Live: Adaptation, Convergent Evolution, and Plesiomorphy. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., Mello, M.A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar] [CrossRef]
- Cruz-Neto, A.P.; Herrera, L.G. The Relationship between Physiology and Diet. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., Mello, M.A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar] [CrossRef]
- Saldaña-Vázquez, R.A.; Fleming, T.H. The Frugivores: Evolution, Functional Traits, and Their Role in Seed Dispersal. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., Mello, M.A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar] [CrossRef]
- Brown, N.; Escobar, L.E. A Review of the Diet of the Common Vampire Bat (Desmodus rotundus) in the Context of Anthropogenic Change. Mamm. Biol. 2023, 103, 433–453. [Google Scholar] [CrossRef]
- Riskin, D.K.; Carter, G.G. The Evolution of Sanguivory in Vampire Bats: Origins and Convergences. Can. J. Zool. 2023, 101, 207–221. [Google Scholar] [CrossRef]
- Barros, M.A.S.; Pessoa, D.M.A.; Rui, A.M. Habitat Use and Seasonal Activity of Insectivorous Bats (Mammalia: Chiroptera) in the Grasslands of Southern Brazil. Zoologia 2014, 31, 153–161. [Google Scholar] [CrossRef]
- Frick, W.F.; Shipley, J.R.; Kelly, J.F.; Heady, P.A.; Kay, K.M. Seasonal Reliance on Nectar by an Insectivorous Bat Revealed by Stable Isotopes. Oecologia 2014, 174, 55–65. [Google Scholar] [CrossRef]
- Aliperti, J.R.; Kelt, D.A.; Heady, P.A.; Frick, W.F. Using Behavioral and Stable Isotope Data to Quantify Rare Dietary Plasticity in a Temperate Bat. J. Mammal. 2017, 98, 340–349. [Google Scholar] [CrossRef]
- Vallejo, N.; Aihartza, J.; Olasagasti, L.; Aldasoro, M.; Goiti, U.; Garin, I. Seasonal Shift in the Diet of the Notched-eared Bat (Myotis emarginatus) in the Basque Country: From Flies to Spiders. Mamm. Biol. 2023, 103, 419–431. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Giannakos, N.R.O.; Mena Canata, D.A.; Pereira, F.D.; Hackenhaar, F.S.; Pereira, M.J.R.; Benfato, M.S. Oxidative Stress and Antioxidant Defense in the Brain of Bat Species with Different Feeding Habits. Int. J. Mol. Sci. 2023, 24, 12162. [Google Scholar] [CrossRef]
- Racey, P.A. Ageing and Assessment of Reproductive Status of Pipistrelle bats, Pipistrellus pipistrellus. J. Zool. 1974, 173, 264–271. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. In Oxygen Radicals and Biological Systems Part B; Academic Press: New York, NY, USA, 1990; pp. 464–478. [Google Scholar]
- Karatepe, M. Simultaneous Determination of Ascorbic Acid and Free Malondialdehyde in Human Serum by HPLC-UV. LC-GC N. Am. 2004, 22, 362–365. [Google Scholar]
- Mescam, M.; Vinnakota, K.C.; Beard, D.A. Identification of the Catalytic Mechanism and Estimation of Kinetic Parameters for Fumarase. J. Biol. Chem. 2011, 286, 21100–21109. [Google Scholar] [CrossRef]
- Taniguchi, N.; Gutteridge, J. Experimental Protocols for Reactive Oxygen and Nitrogen Species, 1st ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Li, Y.; Schellhorn, H.E. Rapid Kinetic Microassay for Catalase Activity. J. Biomol. Tech. 2007, 18, 185–187. [Google Scholar]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of Nitrate and Nitrite in Extracellular Fluids. In Nitric Oxide Part A Sources Detect. NO; NO Synthase; Academic Press: New York, NY, USA, 1996; pp. 237–246. [Google Scholar]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]


| Bat Species | Feeding Habit | Season | Body Weight (g) | Heart (g) | Liver (g) | Kidneys (g) |
|---|---|---|---|---|---|---|
| Glossophaga soricina | Nectarivorous | Autumn (2019) | 16.81 ± 1.33 | 0.46 ± 0.07 | 0.34 ± 0.06 | 0.13 ± 0.01 |
| Sturnira lilium | Frugivorous | Winter (2019) | 21.60 ± 1.57 | 0.41 ± 0.08 | 0.81 ± 0.11 | 0.29 ± 0.06 |
| Molossus molossus | Insectivorous | Summer (2018) | 18.09 ± 1.80 | 0.41 ± 0.04 | 0.55 ± 0.05 | 0.14 ± 0.01 |
| Desmodus rotundus | Hematophagous | Summer (2018) | 40.85 ± 3.15 | 0.79 ± 0.18 | 1.84 ± 0.23 | 0.48 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.D.; Mena Canata, D.A.; Salomon, T.B.; Hackenhaar, F.S.; Pereira, M.J.R.; Benfato, M.S.; Rampelotto, P.H. Oxidative Stress and Antioxidant Defense in the Heart, Liver, and Kidney of Bat Species with Different Feeding Habits. Int. J. Mol. Sci. 2023, 24, 16369. https://doi.org/10.3390/ijms242216369
Pereira FD, Mena Canata DA, Salomon TB, Hackenhaar FS, Pereira MJR, Benfato MS, Rampelotto PH. Oxidative Stress and Antioxidant Defense in the Heart, Liver, and Kidney of Bat Species with Different Feeding Habits. International Journal of Molecular Sciences. 2023; 24(22):16369. https://doi.org/10.3390/ijms242216369
Chicago/Turabian StylePereira, Francielly Dias, Diego Antonio Mena Canata, Tiago Boeira Salomon, Fernanda Schäfer Hackenhaar, María João Ramos Pereira, Mara Silveira Benfato, and Pabulo Henrique Rampelotto. 2023. "Oxidative Stress and Antioxidant Defense in the Heart, Liver, and Kidney of Bat Species with Different Feeding Habits" International Journal of Molecular Sciences 24, no. 22: 16369. https://doi.org/10.3390/ijms242216369






