Natural Products Induce Different Anti-Tumor Immune Responses in Murine Models of 4T1 Mammary Carcinoma and B16-F10 Melanoma
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Anamu-SC
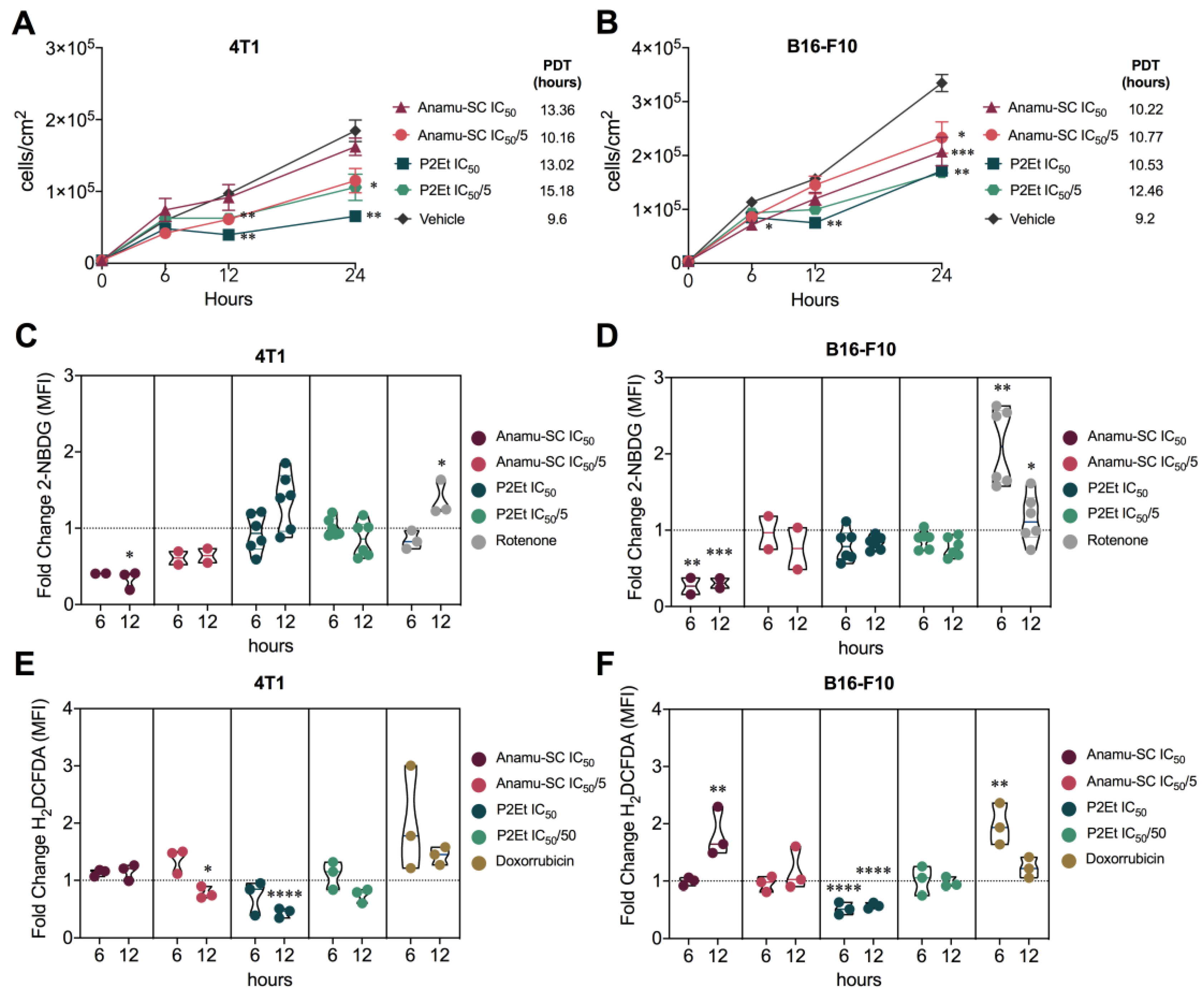
2.2. Comparative Anti-Tumor Activity
2.3. Anamu-SC Shows Specific Activity against 4T1 Murine Mammary Carcinoma Cells
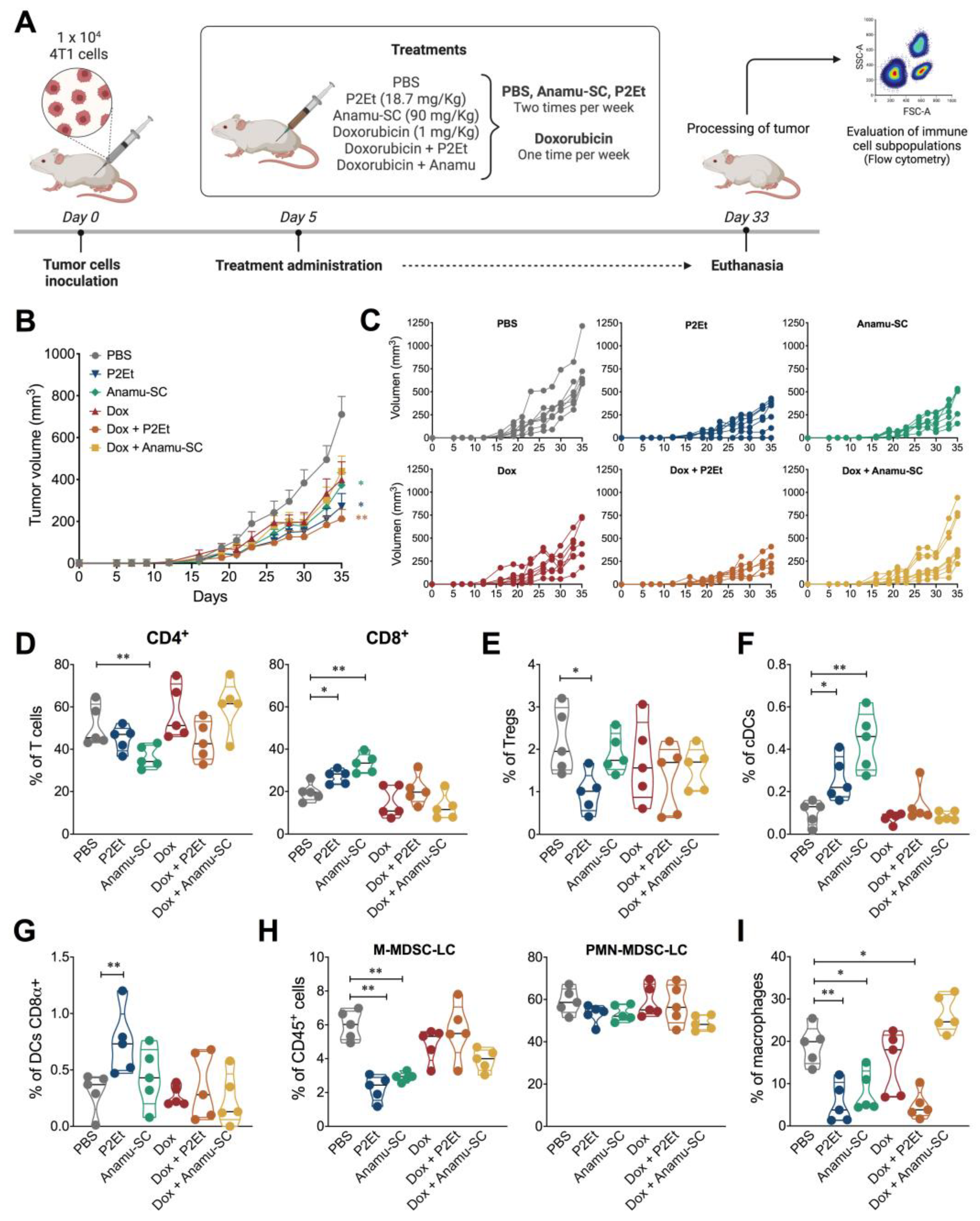
2.4. Intratumoral Immune Response Regulation in the Presence of Extracts
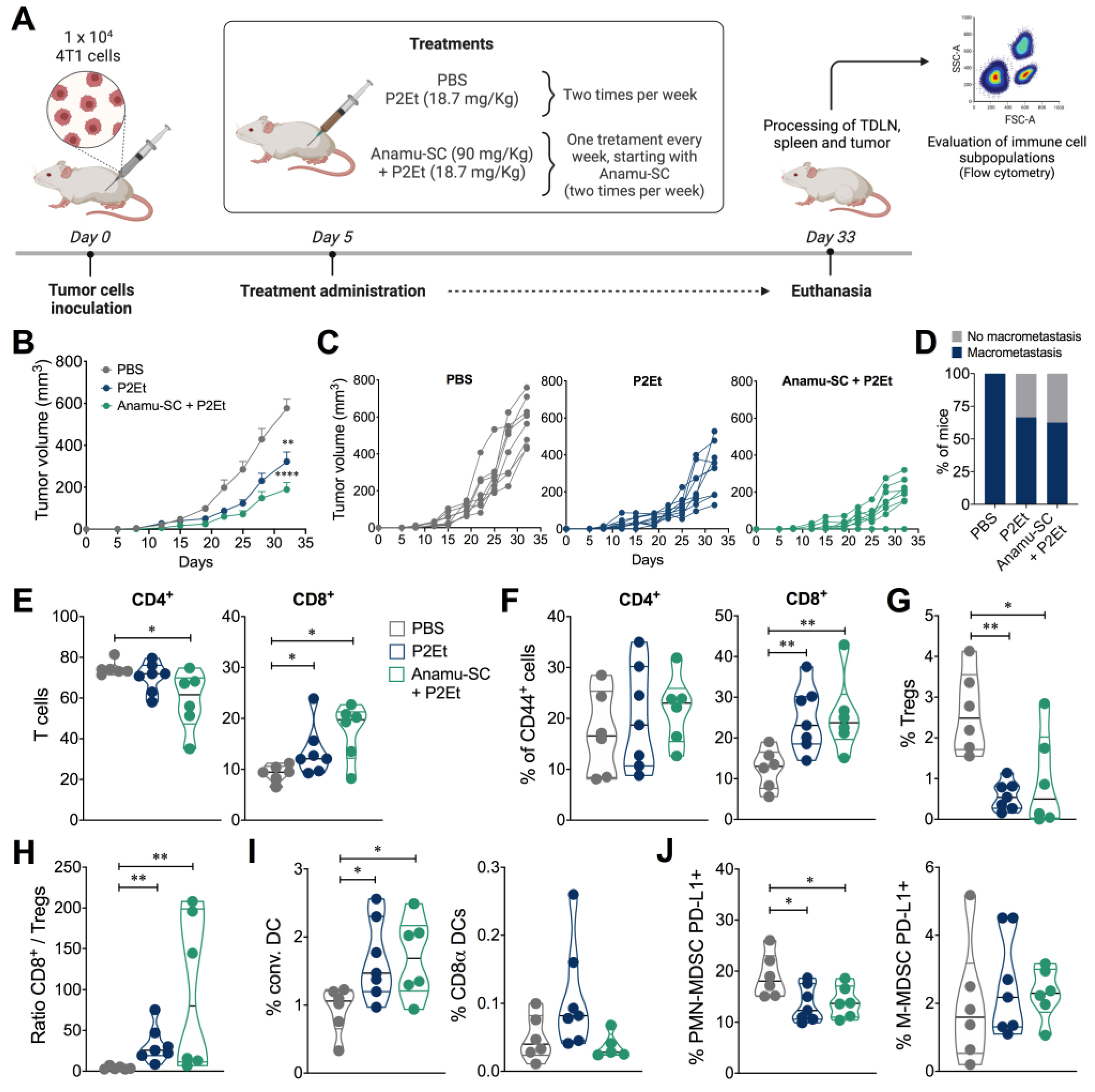
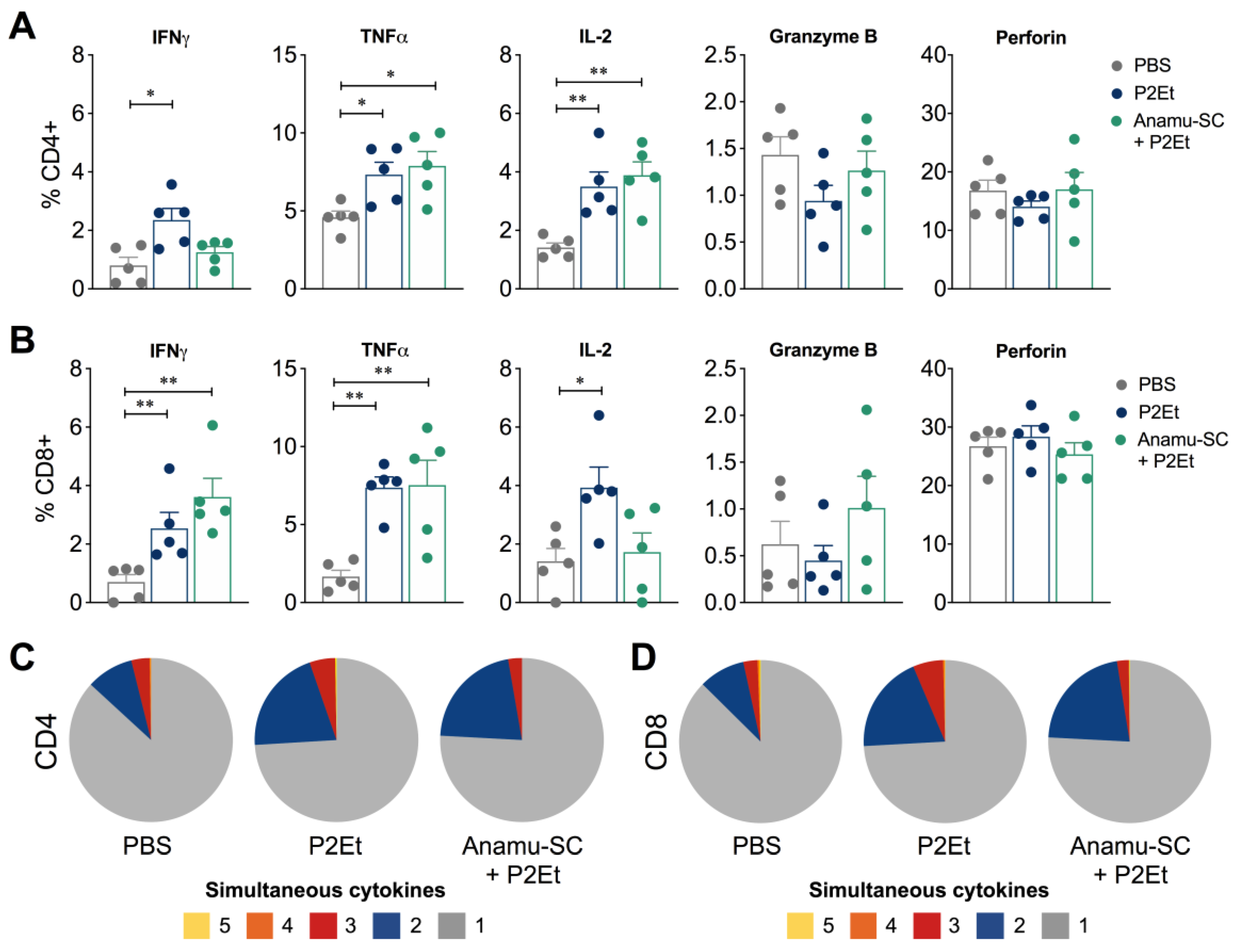
2.5. Anamu-SC Extract Subtly Potentiates the Antitumor Activity of P2Et Extract
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Ultra-High-Performance Liquid Chromatography (UPLC) Analysis
4.3. In Vitro Cytotoxicity Assays
4.4. Proliferation Assay
4.5. ROS Measurement
4.6. Glucose Uptake Assay
4.7. Mice and Tumor Cell Lines
4.8. Abs
4.9. Acute Toxicity Evaluation
4.10. In Vivo Tumor Development Experiments and Treatment
4.11. Evaluation of Immune Populations by Flow Cytometry
4.12. Evaluation of the Immune Response by Flow Cytometry
4.13. Cytokine Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, K.; Younger, N.; Aiken, W.; Brady-West, D.; Delgoda, R. Reliance on medicinal plant therapy among cancer patients in Jamaica. Cancer Causes Control 2017, 28, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Vandebroek, I.; Picking, D. Popular Medicinal Plants in Portland and Kingston, Jamaica; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Adesanya, E.O.; Oyesiku, O.O.; Adesanya, O.O.; Ogunlakin, A.D.; Odugbemi, A.I.; Egieyeh, S.A. Phytochemical components and GC–MS analysis of Petiveria alliaceae L. fractions and volatile oils. J. Phys. Sci. Rev. 2023, 1–13. [Google Scholar] [CrossRef]
- Silva, J.; do Nascimento, S.; Okabe, D.; Pinto, A.; de Oliveira, F.; da Paixão, T.; Siqueira, M.; Baetas, A.; de Andrade, M. Antimicrobial and anticancer potential of Petiveria alliacea L.(Herb to” tame the master”): A review. Pharmacogn. Rev. 2018, 12, 85–93. [Google Scholar] [CrossRef]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Ferraz Maia, C.S.; Fontes-Junior, E.A. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef]
- Murray, J.; Picking, D.; Lamm, A.; McKenzie, J.; Hartley, S.; Watson, C.; Williams, L.; Lowe, H.; Delgoda, R. Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions. Fitoterapia 2016, 111, 138–146. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castaneda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea extracts uses multiple mechanisms to inhibit growth of human and mouse tumoral cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Barreto, A.; Fioretino, S.; Jandus, C. Immune system activation by natural products and complex fractions: A network pharmacology approach in cancer treatment. Cell Stress 2020, 4, 154–166. [Google Scholar] [CrossRef]
- Ballesteros-Ramirez, R.; Aldana, E.; Herrera, M.V.; Uruena, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid. Based Complement. Altern. Med. 2020, 2020, 4736206. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Wang, S.; Long, S.; Deng, Z.; Wu, W. Positive Role of Chinese Herbal Medicine in Cancer Immune Regulation. Am. J. Chin. Med. 2020, 48, 1577–1592. [Google Scholar] [CrossRef]
- Lasso, P.; Rojas, L.; Arevalo, C.; Uruena, C.; Murillo, N.; Barreto, A.; Costa, G.M.; Fiorentino, S. Tillandsia usneoides Extract Decreases the Primary Tumor in a Murine Breast Cancer Model but Not in Melanoma. Cancers 2022, 14, 5383. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Kawaguchi, Y.; Yamazaki, M.; Hirata, N.; Naruto, S.; Asanuma, Y.; Kaihatsu, T.; Kubo, M. Melanogenesis stimulation in murine B16 melanoma cells by Piper nigrum leaf extract and its lignan constituents. Biol. Pharm. Bull. 2004, 27, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.S.; Choo, G.S.; Kim, S.H.; Woo, J.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Park, B.K.; Cho, S.D.; et al. Antitumor and Apoptosis-inducing Effects of Piperine on Human Melanoma Cells. Anticancer Res. 2019, 39, 1883–1892. [Google Scholar] [CrossRef]
- Cohen, G.; Chandran, P.; Lorsung, R.M.; Aydin, O.; Tomlinson, L.E.; Rosenblatt, R.B.; Burks, S.R.; Frank, J.A. Pulsed-Focused Ultrasound Slows B16 Melanoma and 4T1 Breast Tumor Growth through Differential Tumor Microenvironmental Changes. Cancers 2021, 13, 1546. [Google Scholar] [CrossRef]
- Visus, C.; Wang, Y.; Lozano-Leon, A.; Ferris, R.L.; Silver, S.; Szczepanski, M.J.; Brand, R.E.; Ferrone, C.R.; Whiteside, T.L.; Ferrone, S.; et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin. Cancer Res. 2011, 17, 6174–6184. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Q.; Liu, S.; Ning, N.; Zhang, X.; Xu, Y.; Chang, A.E.; Wicha, M.S. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells 2015, 33, 2085–2092. [Google Scholar] [CrossRef]
- Cham, C.M.; Gajewski, T.F. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005, 174, 4670–4677. [Google Scholar] [CrossRef]
- Maciver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef]
- Renner, K.; Geiselhoringer, A.L.; Fante, M.; Bruss, C.; Farber, S.; Schonhammer, G.; Peter, K.; Singer, K.; Andreesen, R.; Hoffmann, P.; et al. Metabolic plasticity of human T cells: Preserved cytokine production under glucose deprivation or mitochondrial restriction, but 2-deoxy-glucose affects effector functions. Eur. J. Immunol. 2015, 45, 2504–2516. [Google Scholar] [CrossRef]
- Oluwa, A.; Avoseh, O.; Omikorede, O.; Ogunwande, I.; Lawal, O. Study on the chemical constituents and anti-inflammatory activity of essential oil of Petiveria alliacea L. Br. J. Pharm. Res. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Vania Azalia Gunawan, V.; Harlina Soetjipto, H.; Arifa Mustika, A. Hypoglicemic and Antioxidant Activity of Petiveria alliacea in Diabetic Rat Models. Biomol. Health Sci. J. 2020, 3, 19. [Google Scholar]
- Mustika, A.; Fatimah, N.; Meinar Sari, G. The self-nanoemulsifying drug delivery system of Petiveria alliacea extract reduced the homeostatic model assessment-insulin resistance value, interleukin-6, and tumor necrosis factor-alpha level in diabetic rat models. Vet. World 2021, 14, 3229–3234. [Google Scholar] [CrossRef]
- Uehara, T.; Eikawa, S.; Nishida, M.; Kunisada, Y.; Yoshida, A.; Fujiwara, T.; Kunisada, T.; Ozaki, T.; Udono, H. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: Implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int. Immunol. 2019, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Pan, T.; Wang, S.; Li, P.; Men, Y.; Tan, R.; Zhong, Z.; Wang, Y. Immunometabolism modulation, a new trick of edible and medicinal plants in cancer treatment. Food Chem. 2021, 376, 131860. [Google Scholar] [CrossRef]
- Urueña, C.; Ballesteros-Ramirez, R.; Gomez-Cadena, A.; Barreto, A.; Prieto, K.; Quijano, S.; Aschner, P.; Martinez, C.; Zapata-Cardona, M.I.; El-Ahanidi, H.; et al. Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et). Front. Med. 2022, 9, 991873. [Google Scholar] [CrossRef]
- Dias, A.S.; Helguero, L.; Almeida, C.R.; Duarte, I.F. Natural Compounds as Metabolic Modulators of the Tumor Microenvironment. Molecules 2021, 26, 3494. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, C.; Rizza, S.; Christiansen, H.; Schmidt, S.; Dahl, C.; Abdul-Al, A.; Christensen, A.; Filomeni, G.; Guldberg, P. Screening of metabolic modulators identifies new strategies to target metabolic reprogramming in melanoma. Sci. Rep. 2021, 11, 4390. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Gori, S.S.; Tsukamoto, T.; Rais, R.; Slusher, B.S. Clinical development of metabolic inhibitors for oncology. J. Clin. Investig. 2022, 132, e148550. [Google Scholar] [CrossRef]
- Zyad, A.; Leouifoudi, I.; Tilaoui, M.; Mouse, H.A.; Khouchani, M.; Jaafari, A. Natural products as cytotoxic agents in chemotherapy against cancer. In Cytotoxicity, 1st ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 65–88. [Google Scholar]
- Galli, F.; Aguilera, J.V.; Palermo, B.; Markovic, S.N.; Nistico, P.; Signore, A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 89. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Chandan, K.; Sarwat, M. Natural products and their derivatives as immune check point inhibitors: Targeting cytokine/chemokine signalling in cancer. Semin. Cancer Biol. 2022, 86, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The Role of Natural Products and Their Multitargeted Approach to Treat Solid Cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Vaidya, A.D. Reverse pharmacology-A paradigm shift for drug discovery and development. Curr. Res. Drug Discov. 2014, 1, 39–44. [Google Scholar]
- Roederer, M.; Nozzi, J.L.; Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011, 79, 167–174. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Fiorentino, S. Natural Products Induce Different Anti-Tumor Immune Responses in Murine Models of 4T1 Mammary Carcinoma and B16-F10 Melanoma. Int. J. Mol. Sci. 2023, 24, 16698. https://doi.org/10.3390/ijms242316698
Lasso P, Rojas L, Arévalo C, Urueña C, Murillo N, Fiorentino S. Natural Products Induce Different Anti-Tumor Immune Responses in Murine Models of 4T1 Mammary Carcinoma and B16-F10 Melanoma. International Journal of Molecular Sciences. 2023; 24(23):16698. https://doi.org/10.3390/ijms242316698
Chicago/Turabian StyleLasso, Paola, Laura Rojas, Cindy Arévalo, Claudia Urueña, Natalia Murillo, and Susana Fiorentino. 2023. "Natural Products Induce Different Anti-Tumor Immune Responses in Murine Models of 4T1 Mammary Carcinoma and B16-F10 Melanoma" International Journal of Molecular Sciences 24, no. 23: 16698. https://doi.org/10.3390/ijms242316698





