G6PD Orchestrates Genome-Wide DNA Methylation and Gene Expression in the Vascular Wall
Abstract
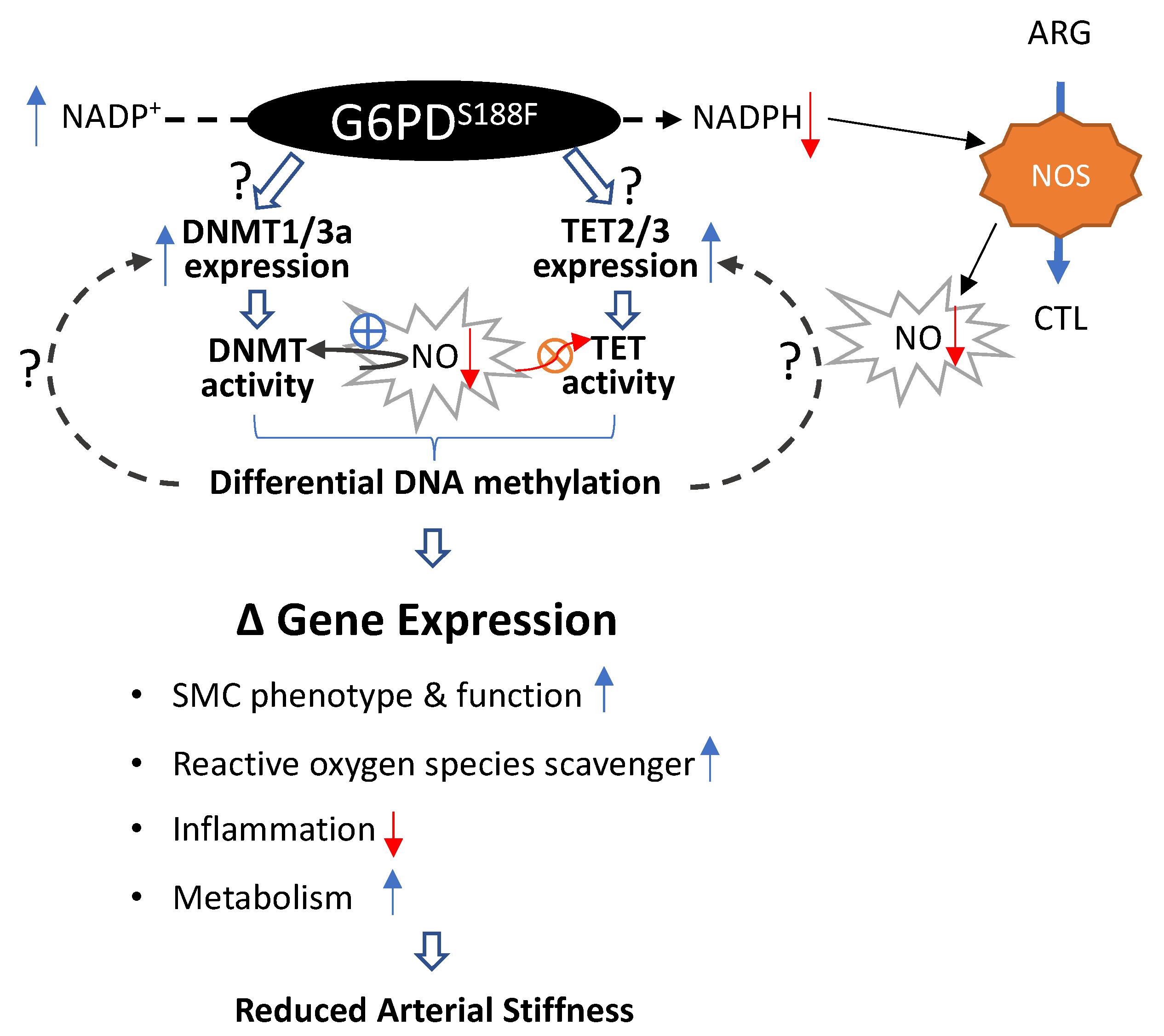
:1. Introduction
2. Results
2.1. The Loss-of-Function G6PDS188F Variant and Differential Gene Expression
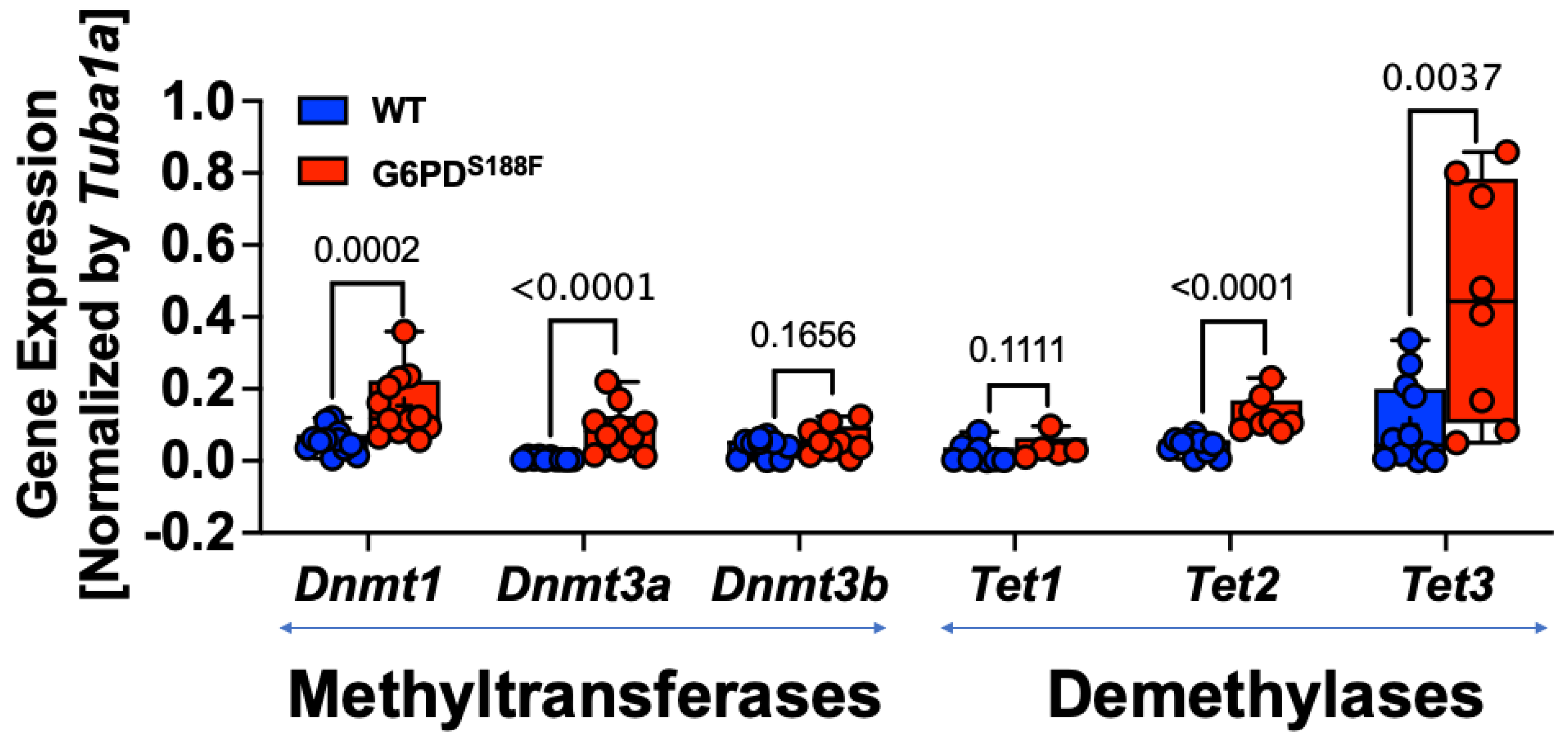
2.2. The Loss-of-Function G6PDS188F Variant Led to Augmented Expression of Genes Encoding DNA Methylases and Demethylases
2.3. DNMT and TET Activity Correlates with Nitric Oxide and Nitric Oxide Synthase Inhibitor Augmented DNMT and Attenuated TET Activity in Aorta
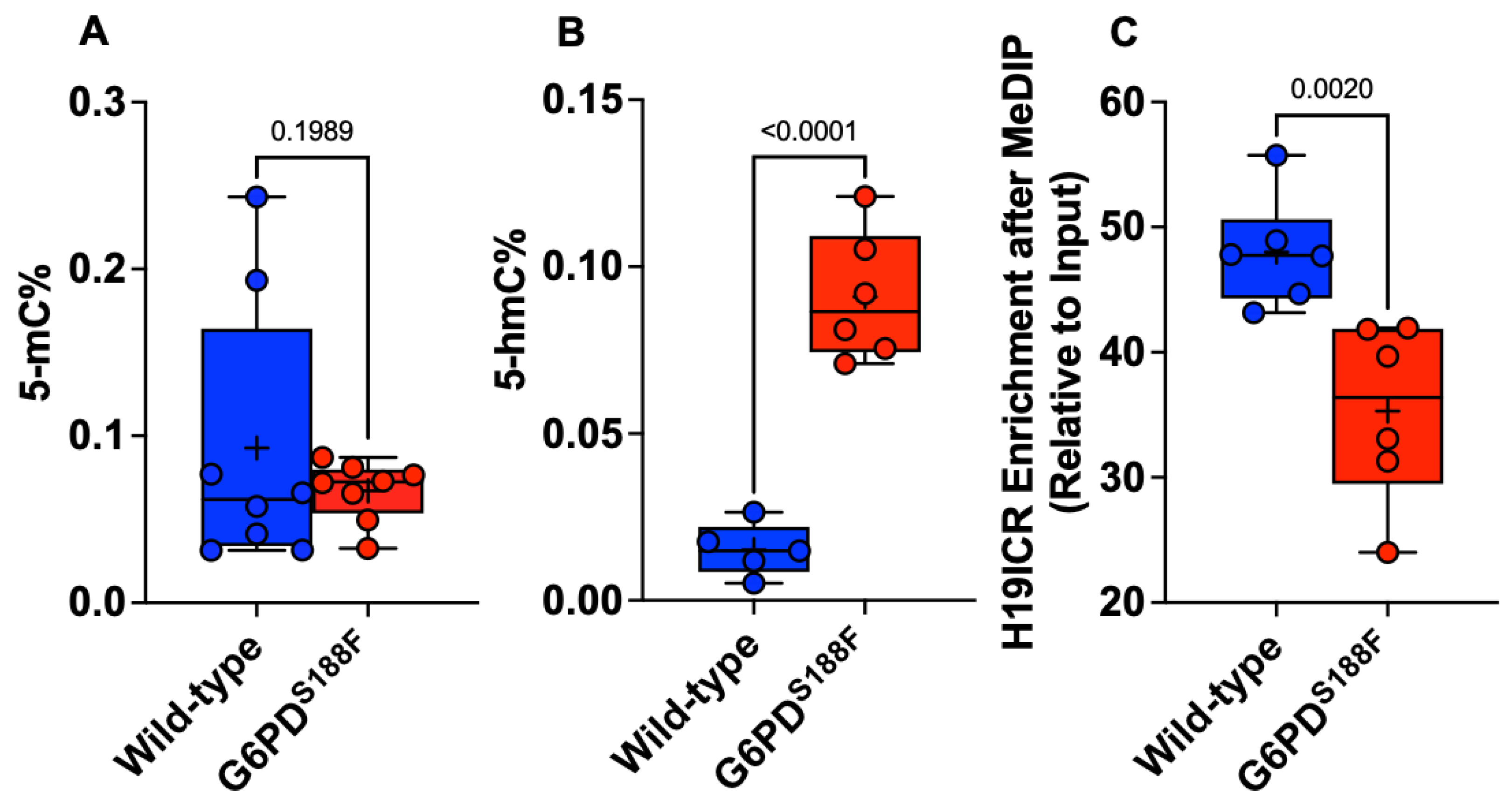
2.4. The Loss-of-Function G6PDS188F Variant Led to Decreases in Methylated Cytosine
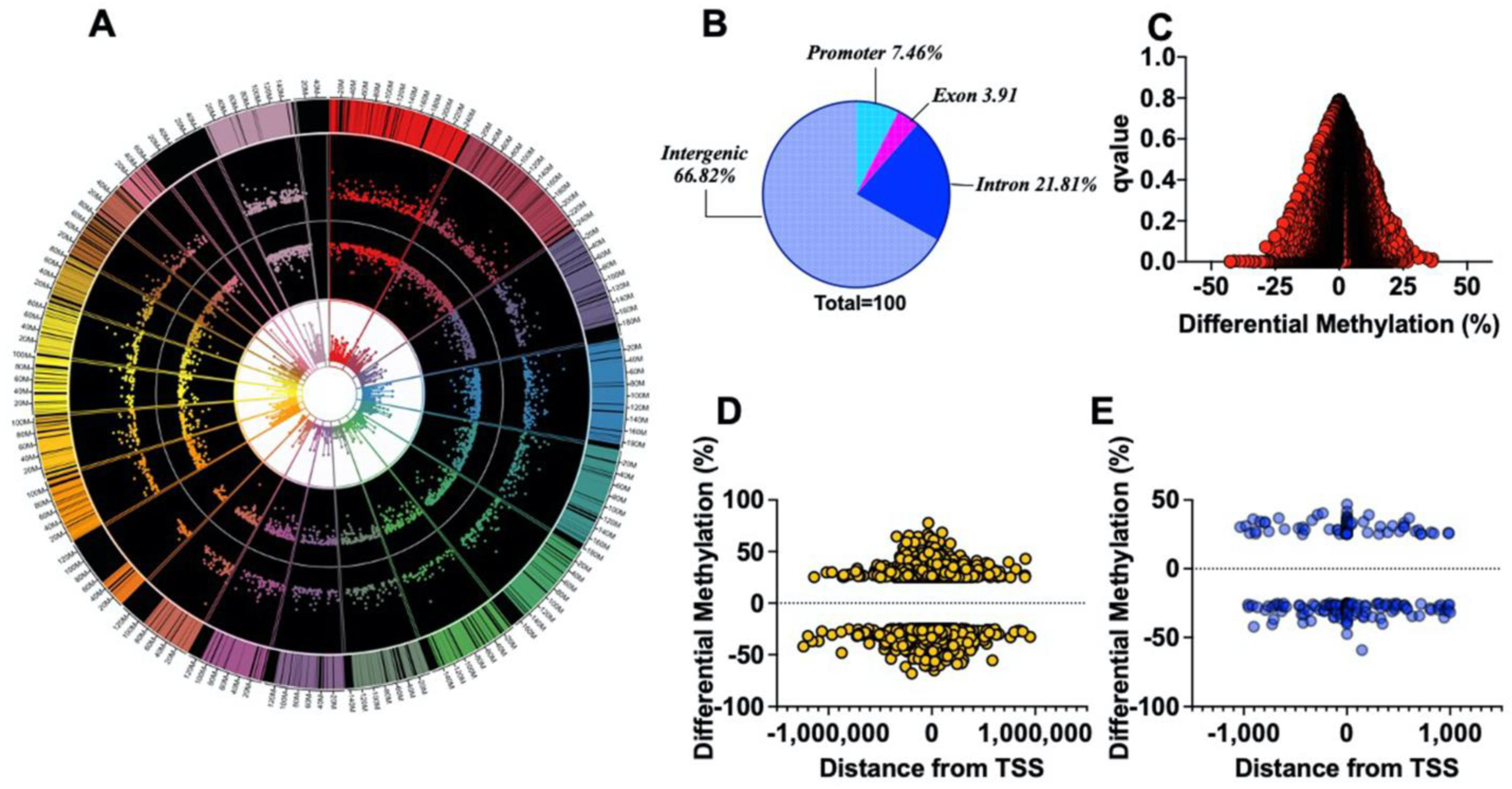
2.5. The Loss-of-Function G6PDS188F Variant Evoked Differential Methylation of DNA
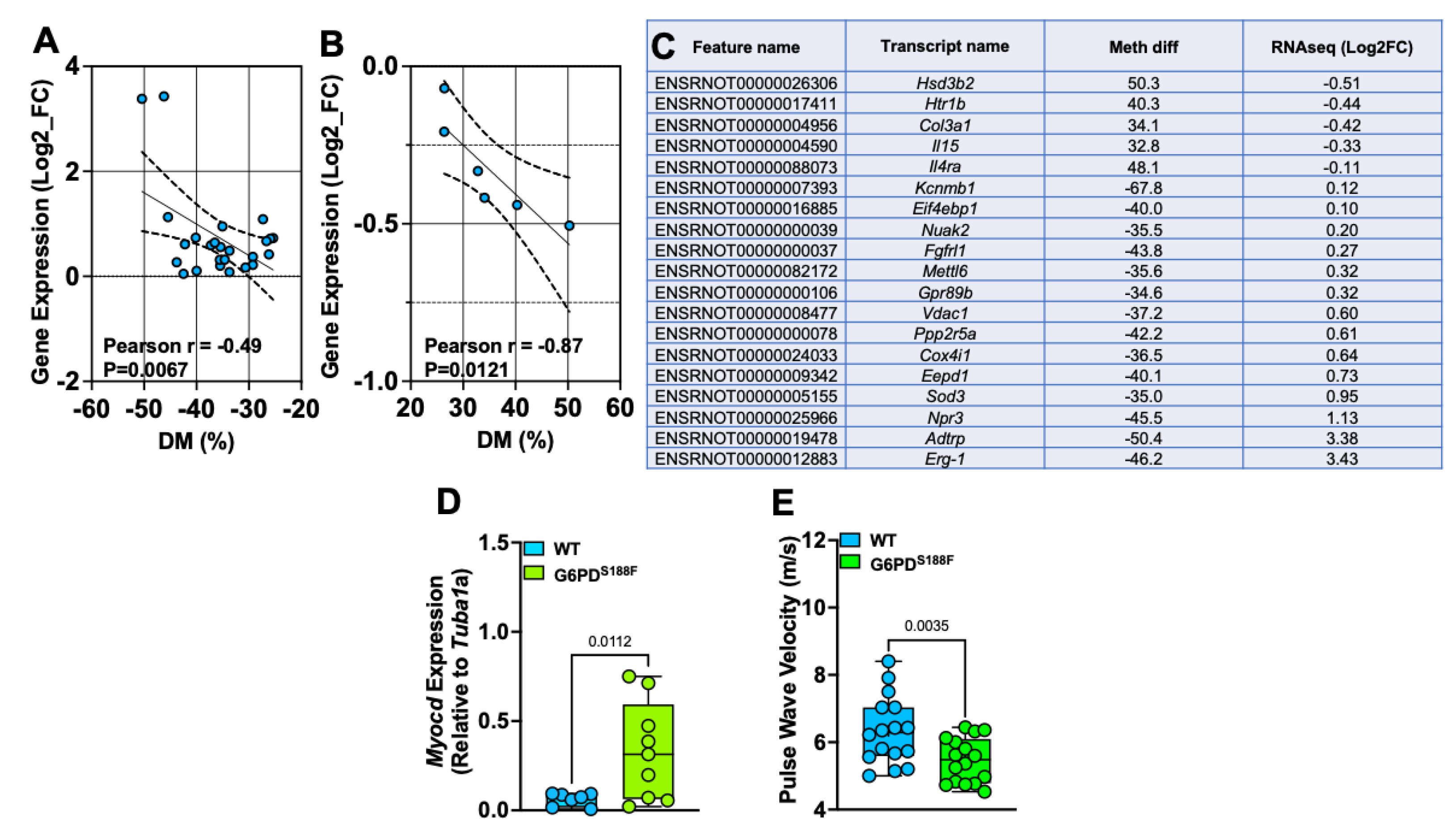
2.6. The Loss-of-Function G6PDS188F Variant Led to Hypomethylated and Augmented Genes Encoding Proteins Involved in Metabolic Processes, Cold Thermogenesis, and Reactive Oxygen Biosynthetic Processes
2.7. The Loss-of-Function G6PDS188F Variant Led to the Hypomethylation of Genes Encoding Negative Regulators of SMC Proliferation and Steroid Biosynthesis and Reduced Large Artery Stiffness
3. Discussion
4. Materials and Methods
4.1. Animal Models and Experimental Protocols
4.2. Echocardiography
4.3. Preparation of Nuclear Extracts and Measurement of Global DNA Methylation and Total DNMT and TET Activity
4.4. Methylated DNA Immunoprecipitation (MeDIP)
4.5. Whole Genome Bisulfite Sequencing
4.6. RNA-Seq
4.7. Quantitative Real-Time PCR
4.8. UHPLC-MS Metabolomics
4.9. Measurement of G6PD Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation through repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.M.; Kahles, F.K.; Bruemmer, D. Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Koldobskiy, M.A.; Göndör, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef]
- Meier, J.L. Metabolic mechanisms of epigenetic regulation. ACS Chem. Biol. 2013, 8, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Vogelauer, M.; Krall, A.S.; McBrian, M.A.; Li, J.-Y.; Kurdistani, S.K. Stimulation of histone deacetylase activity by metabolites of intermediary metabolism. J. Biol. Chem. 2012, 287, 32006–32016. [Google Scholar] [CrossRef] [PubMed]
- Solary, E.; Bernard, O.A.; Tefferi, A.; Fuks, F.; Vainchenker, W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia 2014, 28, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Kunimoto, H. TET2 as an epigenetic master regulator for normal and malignant hematopoiesis. Cancer Sci. 2014, 105, 1093–1099. [Google Scholar] [CrossRef]
- Secombe, J.; Eisenman, R.N. The function and regulation of the JARID1 family of histone H3 lysine 4 demethylases: The Myc connection. Cell Cycle 2007, 6, 1324–1328. [Google Scholar] [CrossRef]
- Puleston, D.J.; Villa, M.; Pearce, E.L. Ancillary Activity: Beyond Core Metabolism in Immune Cells. Cell Metab. 2017, 26, 131–141. [Google Scholar] [CrossRef]
- Joshi, S.R.; Kitagawa, A.; Jacob, C.; Hashimoto, R.; Dhagia, V.; Ramesh, A.; Zheng, C.; Zhang, H.; Jordan, A.; Waddell, I.; et al. Hypoxic activation of glucose-6-phosphate dehydrogenase controls the expression of genes involved in the pathogenesis of pulmonary hypertension through the regulation of DNA methylation. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L773–L786. [Google Scholar] [CrossRef]
- Reisz, J.A.; Tzounakas, V.L.; Nemkov, T.; Voulgaridou, A.I.; Papassideri, I.S.; Kriebardis, A.G.; D’Alessandro, A.; Antonelou, M.H. Metabolic Linkage and Correlations to Storage Capacity in Erythrocytes from Glucose 6-Phosphate Dehydrogenase-Deficient Donors. Front. Med. 2017, 4, 248. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Jacob, C.; Jordan, A.; Waddell, I.; McMurtry, I.F.; Gupte, S.A. Inhibition of Glucose-6-Phosphate Dehydrogenase Activity Attenuates Right Ventricle Pressure and Hypertrophy Elicited by VEGFR Inhibitor + Hypoxia. J. Pharmacol. Exp. Ther. 2021, 377, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Kitagawa, A.; Signoretti, C.; Dzieciatkowska, M.; D’alessandro, A.; Gupte, A.; Hossain, S.; D’addario, C.A.; Gupte, R.; Gupte, S.A. Mediterranean G6PD variant mitigates expression of DNA methyltransferases and right heart pressure in experimental model of pulmonary hypertension. J. Biol. Chem. 2022, 298, 102691. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Todde, P.; Fornera, S.; Manca, M.B.; Manca, P.; Sias, A.R. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood 1998, 91, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Meloni, L.; Manca, M.R.; Loddo, I.; Cioglia, G.; Cocco, P.; Schwartz, A.; Muntoni, S. Glucose-6-phosphate dehydrogenase deficiency protects against coronary heart disease. J. Inherit. Metab. Dis. 2008, 31, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Kizub, I.; Jacob, C.; Michael, K.; D’alessandro, A.; Reisz, J.A.; Grzybowski, M.; Geurts, A.M.; Rocic, P.; Gupte, R.; et al. CRISPR-Mediated Single Nucleotide Polymorphism Modeling in Rats Reveals Insight into Reduced Cardiovascular Risk Associated With Mediterranean G6PD Variant. Hypertension 2020, 76, 523–532. [Google Scholar] [CrossRef]
- Gupte, R.; Dhagia, V.; Rocic, P.; Ochi, R.; Gupte, S.A. Glucose-6-phosphate dehydrogenase increases Ca(2+) currents by interacting with Cav1.2 and reducing intrinsic inactivation of the L-type calcium channel. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H144–H158. [Google Scholar] [CrossRef]
- Spencer, N.Y.; Yan, Z.; Cong, L.; Zhang, Y.; Engelhardt, J.F.; Stanton, R.C. Definitive localization of intracellular proteins: Novel approach using CRISPR-Cas9 genome editing, with glucose 6-phosphate dehydrogenase as a model. Anal. Biochem. 2016, 494, 55–67. [Google Scholar] [CrossRef]
- Spencer, N.Y.; Yan, Z.; Boudreau, R.L.; Zhang, Y.; Luo, M.; Li, Q.; Tian, X.; Shah, A.M.; Davisson, R.L.; Davidson, B.; et al. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J. Biol. Chem. 2011, 286, 8977–8987. [Google Scholar] [CrossRef]
- Kuroda, J.; Nakagawa, K.; Yamasaki, T.; Nakamura, K.; Takeya, R.; Kuribayashi, F.; Imajoh-Ohmi, S.; Igarashi, K.; Shibata, Y.; Sueishi, K.; et al. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 2005, 10, 1139–1151. [Google Scholar] [CrossRef]
- Vulliamy, T.J.; D’Urso, M.; Battistuzzi, G.; Estrada, M.; Foulkes, N.S.; Martini, G.; Calabro, V.; Poggi, V.; Giordano, R.; Town, M. Diverse point mutations in the human glucose-6-phosphate dehydrogenase gene cause enzyme deficiency and mild or severe hemolytic anemia. Proc. Natl. Acad. Sci. USA 1988, 85, 5171–5175. [Google Scholar] [CrossRef]
- Sung, H.Y.; Guan, H.; Czibula, A.; King, A.R.; Eder, K.; Heath, E.; Suvarna, S.K.; Dower, S.K.; Wilson, A.G.; Francis, S.E.; et al. Human tribbles-1 controls proliferation and chemotaxis of smooth muscle cells via MAPK signaling pathways. J. Biol. Chem. 2007, 282, 18379–18387. [Google Scholar] [CrossRef]
- Khambata, R.S.; Panayiotou, C.M.; Hobbs, A.J. Natriuretic peptide receptor-3 underpins the disparate regulation of endothelial and vascular smooth muscle cell proliferation by C-type natriuretic peptide. Br. J. Pharmacol. 2011, 164, 584–597. [Google Scholar] [CrossRef]
- Hervouet, E.; Peixoto, P.; Delage-Mourroux, R.; Boyer-Guittaut, M.; Cartron, P.-F. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenet. 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Bovee, R.C.; Thomas, D.D. Nitric oxide, the new architect of epigenetic landscapes. Nitric Oxide 2016, 59, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Zhang, Y.-Y.; Scribner, A.W.; Stanton, R.C.; Loscalzo, J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arter. Thromb. Vasc. Biol. 2003, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Kondo, Y.; Guo, Y.; Zhang, J.; Zhang, L.; Ahmed, S.; Shu, J.; Chen, X.; Waterland, R.A.; Issa, J.P.J. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 2007, 3, 2023–2036. [Google Scholar] [CrossRef]
- Fouse, S.D.; Shen, Y.; Pellegrini, M.; Cole, S.; Meissner, A.; Van Neste, L.; Jaenisch, R.; Fan, G. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2008, 2, 160–169. [Google Scholar] [CrossRef]
- Mohn, F.; Weber, M.; Rebhan, M.; Roloff, T.C.; Richter, J.; Stadler, M.B.; Bibel, M.; Schübeler, D. Lineage-specific polycomb targets and de novo dna methylation define restriction and potential of neuronal progenitors. Mol. Cell 2008, 30, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; Mikkelsen, T.S.; Gu, H.; Wernig, M.; Hanna, J.; Sivachenko, A.; Zhang, X.; Bernstein, B.E.; Nusbaum, C.; Jaffe, D.B.; et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 2008, 454, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’neill, J.S.; Reddy, A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.V.; Bogenpohl, J.W.; Howell, M.P.; Wevrick, R.; Panda, S.; Hogenesch, J.B.; Muglia, L.J.; Van Gelder, R.N.; Herzog, E.D.; Stewart, C.L. The imprinted gene Magel2 regulates normal circadian output. Nat. Genet. 2007, 39, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Carias, K.V.; Zoeteman, M.; Seewald, A.; Sanderson, M.R.; Bischof, J.M.; Wevrick, R. A MAGEL2-deubiquitinase complex modulates the ubiquitination of circadian rhythm protein CRY1. PLoS ONE 2020, 15, e0230874. [Google Scholar] [CrossRef]
- Anea, C.B.; Zhang, M.; Stepp, D.W.; Simkins, G.B.; Reed, G.; Fulton, D.J.; Rudic, R.D. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009, 119, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Gupte, R.; Rawat, D.; Gebb, S.A.; McMurtry, I.F.; Gupte, S.A.; Hensley, M.K.; Levine, A.; Gladwin, M.T.; Lai, Y.-C.; et al. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: Implication in pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L287–L300. [Google Scholar] [CrossRef]
- Chettimada, S.; Joshi, S.R.; Dhagia, V.; Aiezza, A.; Lincoln, T.M.; Gupte, R.; Miano, J.M.; Gupte, S.A. Vascular smooth muscle cell contractile protein expression is increased through protein kinase G-dependent and -independent pathways by glucose-6-phosphate dehydrogenase inhibition and deficiency. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H904–H912. [Google Scholar] [CrossRef]
- Dhagia, V.; Kitagawa, A.; Jacob, C.; Zheng, C.; D’alessandro, A.; Edwards, J.G.; Rocic, P.; Gupte, R.; Gupte, S.A. G6PD activity contributes to the regulation of histone acetylation and gene expression in smooth muscle cells and to the pathogenesis of vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H999–H1016. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, B.S.; He, C. TET family proteins: Oxidation activity, interacting molecules, and functions in diseases. Chem. Rev. 2015, 115, 2225–2239. [Google Scholar] [CrossRef]
- Melamed, P.; Yosefzon, Y.; David, C.; Tsukerman, A.; Pnueli, L. Tet Enzymes, Variants, and Differential Effects on Function. Front. Cell Dev. Biol. 2018, 6, 22. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M.; Kim, J.H.; Lee, M.-R.; Hong, Y.-C.; Choquet, H.; Trapani, E.; Goitre, L.; Trabalzini, L.; Akers, A.; et al. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.S.; Vengrenyuk, Y.; Shameer, K.; Maehara, A.; Purushothaman, M.; Yoshimura, T.; Matsumura, M.; Aquino, M.; Haider, N.; Johnson, K.W.; et al. Intracoronary Imaging, Cholesterol Efflux, and Transcriptomes After Intensive Statin Treatment: The YELLOW II Study. J. Am. Coll. Cardiol. 2017, 69, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Ng, F.L.; Warren, H.R.; Witkowska, K.; Baron, M.; Jia, Z.; Cabrera, C.; Zhang, R.; Mifsud, B.; Munroe, P.B.; et al. The biological impact of blood pressure-associated genetic variants in the natriuretic peptide receptor C gene on human vascular smooth muscle. Hum. Mol. Genet. 2017, 27, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lupu, C.; Patel, M.M.; Lupu, F. Insights into the Functional Role of ADTRP (Androgen-Dependent TFPI-Regulating Protein) in Health and Disease. Int. J. Mol. Sci. 2021, 22, 4451. [Google Scholar] [CrossRef] [PubMed]
- Lupu, C.; Zhu, H.; Popescu, N.I.; Wren, J.D.; Lupu, F. Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood 2011, 118, 4463–4471. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jin, Y.; Tang, W.H.; Qin, L.; Zhang, X.; Tellides, G.; Hwa, J.; Yu, J.; Martin, K.A. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 2013, 128, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Ostriker, A.C.; Xie, Y.; Chakraborty, R.; Sizer, A.J.; Bai, Y.; Ding, M.; Song, W.-L.; Huttner, A.; Hwa, J.; Martin, K.A. TET2 Protects Against Vascular Smooth Muscle Cell Apoptosis and Intimal Thickening in Transplant Vasculopathy. Circulation 2021, 144, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Compere, S.J.; Palmiter, R.D. DNA methylation controls the inducibility of the mouse metallothionein-I gene in lymphoid cells. Cell 1981, 25, 233–240. [Google Scholar] [CrossRef]
- Matsumura, S.; D’Addiaro, C.; Slivano, O.J.; De Miguel, C.; Stier, C.; Gupte, R.; Miano, J.M.; Gupte, S.A. Mediterranean G6PD variant rats are protected from Angiotensin II-induced hypertension and kidney damage, but not from inflammation and arterial stiffness. Vasc. Pharmacol. 2022, 145, 107002. [Google Scholar] [CrossRef]
- Hmadcha, A.; Bedoya, F.J.; Sobrino, F.; Pintado, E. Methylation-dependent gene silencing induced by interleukin 1beta via nitric oxide production. J. Exp. Med. 1999, 190, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ning, S.; Scicinski, J.; Oronsky, B.; Knox, S.J.; Peehl, D.M. Epigenetic effects of RRx-001: A possible unifying mechanism of anticancer activity. Oncotarget 2015, 6, 43172–43181. [Google Scholar] [CrossRef] [PubMed]
- van der Wijst, M.G.P.; Venkiteswaran, M.; Chen, H.; Xu, G.-L.; Plösch, T.; Rots, M.G. Local chromatin microenvironment determines DNMT activity: From DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase. Epigenetics 2015, 10, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wang, K.-Y.; Shen, C.-K.J. DNA 5-methylcytosine demethylation activities of the mammalian DNA methyltransferases. J. Biol. Chem. 2013, 288, 9084–9091. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Ata, H.; Rawat, D.; Abe, M.; Taylor, M.S.; Ochi, R.; Gupte, S.A.; Yao, C.; Yu, J.; Taylor, L.; et al. Glucose-6-phosphate dehydrogenase is a regulator of vascular smooth muscle contraction. Antioxid. Redox Signal. 2011, 14, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Facchinello, N.; Astone, M.; Audano, M.; Oberkersch, R.E.; Spizzotin, M.; Calura, E.; Marques, M.; Crisan, M.; Mitro, N.; Santoro, M.M. Oxidative pentose phosphate pathway controls vascular mural cell coverage by regulating extracellular matrix composition. Nat. Metab. 2022, 4, 123–140. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Yang, H.-C.; Wu, Y.-H.; Yen, W.-C.; Liu, H.-Y.; Hwang, T.-L.; Stern, A.; Chiu, D.T.-Y. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef]
- Gupte, S.A.; Kaminski, P.M.; Floyd, B.; Agarwal, R.; Ali, N.; Ahmad, M.; Edwards, J.; Wolin, M.S. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am. J. Physiol. Circ. Physiol. 2005, 288, H13–H21. [Google Scholar] [CrossRef]
- Chettimada, S.; Rawat, D.K.; Dey, N.; Kobelja, R.; Simms, Z.; Wolin, M.S.; Lincoln, T.M.; Gupte, S.A.; Yu, H.; Alruwaili, N.; et al. Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L64–L74. [Google Scholar] [CrossRef]
- Neo, B.H.; Patel, D.; Kandhi, S.; Wolin, M.S.; Lakhkar, A.; Dhagia, V.; Joshi, S.R.; Gotlinger, K.; Sun, D.; Schwartzman, M.L.; et al. Roles for cytosolic NADPH redox in regulating pulmonary artery relaxation by thiol oxidation-elicited subunit dimerization of protein kinase G1α. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H330–H343. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Bhatnagar, A.; Sadoshima, J. Overview of Pyridine nucleotides review series. Circ. Res. 2012, 111, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Gao, S.; Ho, D.; Vatner, D.E.; Vatner, S.F. Echocardiography in Mice. Curr. Protoc. Mouse Biol. 2011, 1, 71–83. [Google Scholar] [CrossRef]
- Okuda, K.; Nakahara, K.; Ito, A.; Iijima, Y.; Nomura, R.; Kumar, A.; Fujikawa, K.; Adachi, K.; Shimada, Y.; Fujio, S.; et al. Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis. Nat. Commun. 2023, 14, 621. [Google Scholar] [CrossRef]
- Merlin, J.; Ivanov, S.; Dumont, A.; Sergushichev, A.; Gall, J.; Stunault, M.; Ayrault, M.; Vaillant, N.; Castiglione, A.; Swain, A.; et al. Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation. Nat. Metab. 2021, 3, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Cho, H.-J.; Eykyn, T.R.; Lavender, P.; Eaton, P. NOS2 and S -nitrosothiol signaling induces DNA hypomethylation and LINE-1 retrotransposon expression. Proc. Natl. Acad. Sci. USA 2022, 119, e2200022119. [Google Scholar] [CrossRef]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef]
- Krueger, F.; Andrews, S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Nemkov, T.; Hansen, K.C.; D’Alessandro, A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. 2017, 31, 663–673. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signoretti, C.; Gupte, S.A. G6PD Orchestrates Genome-Wide DNA Methylation and Gene Expression in the Vascular Wall. Int. J. Mol. Sci. 2023, 24, 16727. https://doi.org/10.3390/ijms242316727
Signoretti C, Gupte SA. G6PD Orchestrates Genome-Wide DNA Methylation and Gene Expression in the Vascular Wall. International Journal of Molecular Sciences. 2023; 24(23):16727. https://doi.org/10.3390/ijms242316727
Chicago/Turabian StyleSignoretti, Christina, and Sachin A. Gupte. 2023. "G6PD Orchestrates Genome-Wide DNA Methylation and Gene Expression in the Vascular Wall" International Journal of Molecular Sciences 24, no. 23: 16727. https://doi.org/10.3390/ijms242316727



