Radiobiological Assessment of Targeted Radionuclide Therapy with [177Lu]Lu-PSMA-I&T in 2D vs. 3D Cell Culture Models
Abstract
:1. Introduction
2. Results
2.1. Verification of PSMA Expression via Western Blot
2.2. Trypan Blue Exclusion Assay: Short-Term Cell Viability and Replicability in Monolayers
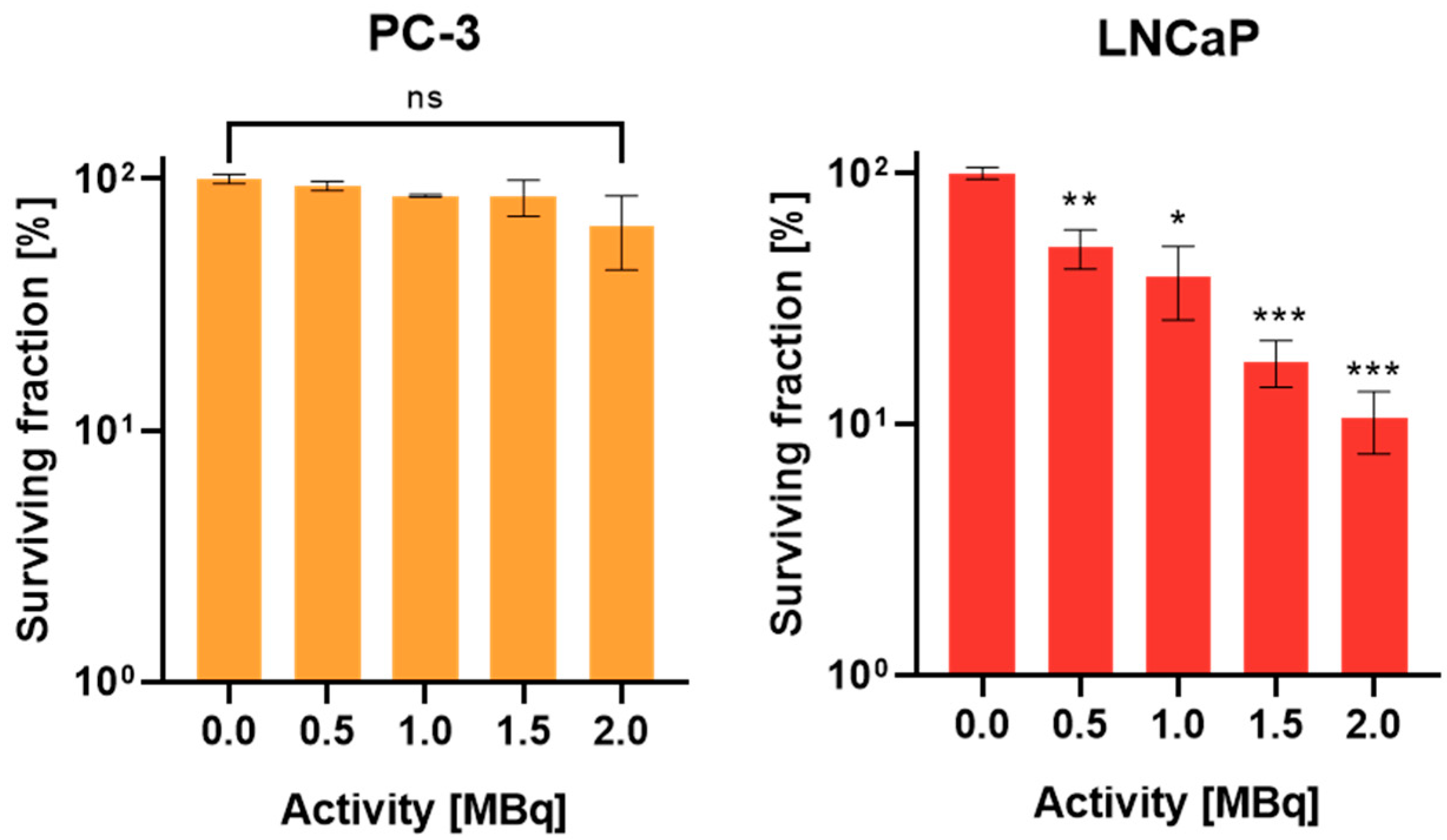
2.3. Evaluation of Reproductive Potential in Monolayers by Standard Clonogenic Assay
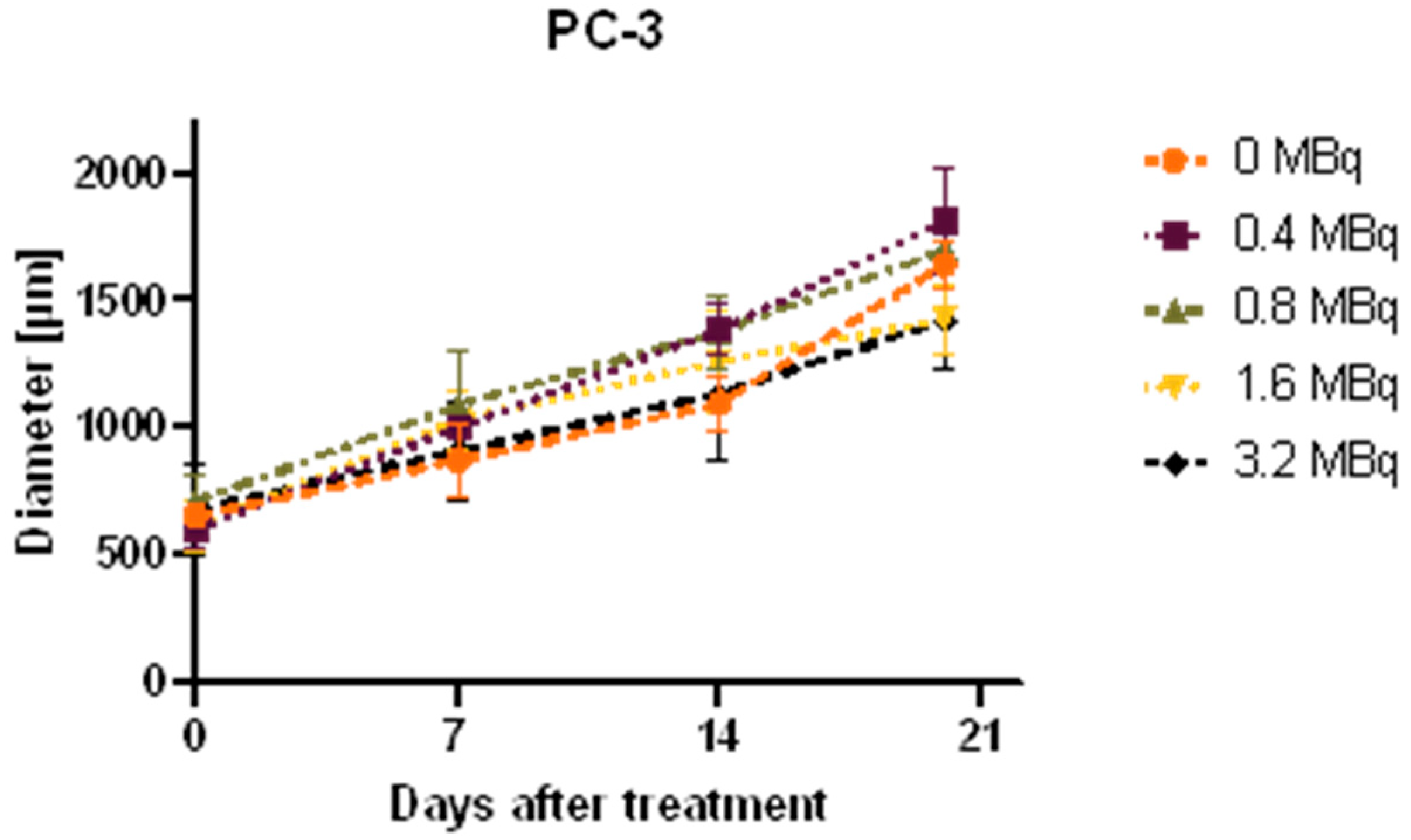
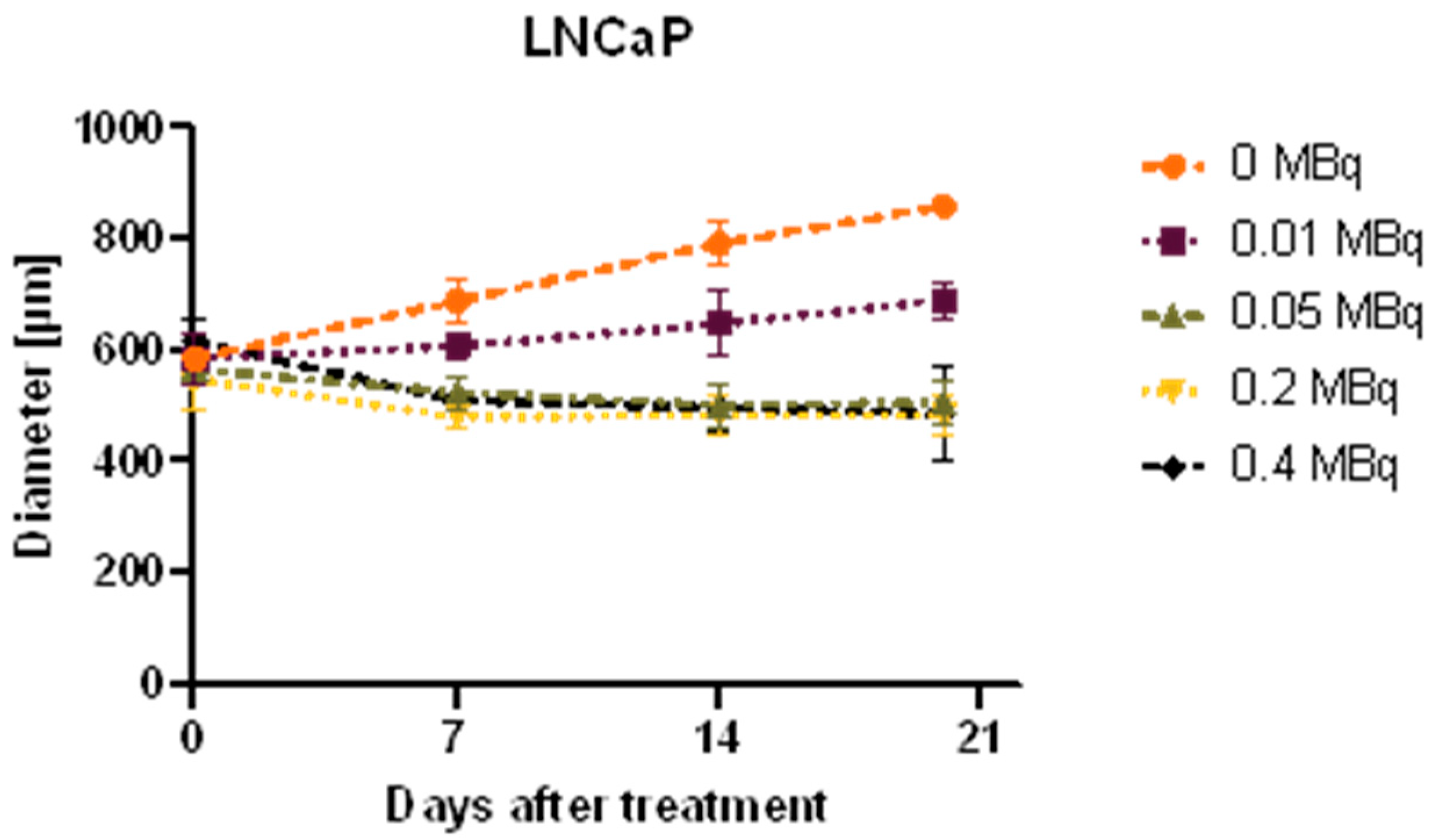
2.4. Growth Analysis of Spheroids
2.5. Long-Term Cell Viability Determination Using Cell Titer Glo®: 2D vs. 3D
2.6. Dosimetry Assessment Using Monte Carlo Simulations
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Generation of Spheroids
4.4. Western Blot
4.5. Treatment Procedure
4.6. Microscopic Growth Analysis
4.7. Clonogenic Assay
4.8. Trypan Blue Exclusion Assay
4.9. Viability Assessment Using Cell Titer Glo® 3D
4.10. Dosimetry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saloni Dattani, Fiona Spooner, Hannah Ritchie, and Max Roser (2018)-“Causes of Death”. Available online: https://ourworldindata.org/causes-of-death (accessed on 26 November 2023).
- Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 26 November 2023).
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow (accessed on 26 November 2023).
- Abdel-Wahab, M.; Gondhowiardjo, S.S.; Rosa, A.A.; Lievens, Y.; El-Haj, N.; Rubio, J.A.P.; Prajogi, G.B.; Helgadottir, H.; Zubizarreta, E.; Meghzifene, A.; et al. Global Radiotherapy: Current Status and Future Directions-White Paper. JCO Glob. Oncol. 2021, 7, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; Jaffray, D.A.; Barton, M.B.; Bray, F.; Baumann, M.; Vikram, B.; Hanna, T.P.; Knaul, F.M.; Lievens, Y.; Lui, T.Y.M.; et al. Expanding Global Access to Radiotherapy. Lancet Oncol. 2015, 16, 1153–1186. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. Molecular Imaging Radiotherapy: Theranostics for Personalized Patient Management of Neuroendocrine Tumors (NETs). Theranostics 2012, 2, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Philips, W.; Baldock, C. The Future of Radiotherapy Is Molecular. Phys. Eng. Sci. Med. 2020, 43, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, E.A.M.; Van Weerden, W.M.; Nonnekens, J.; De Jong, M. The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research. Pharmaceutics 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Nonnekens, J.; Konijnenberg, M.W.; de Jong, M. To Go Where No One Has Gone before: The Necessity of Radiobiology Studies for Exploration beyond the Limits of the “Holy Gray” in Radionuclide Therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2680–2682. [Google Scholar] [CrossRef] [PubMed]
- Urbański, B. The Future of Radiation Oncology: Considerations of Young Medical Doctor. Rep. Pract. Oncol. Radiother. 2012, 17, 288–293. [Google Scholar] [CrossRef]
- Aerts, A.; Eberlein, U.; Holm, S.; Hustinx, R.; Konijnenberg, M.; Strigari, L.; Van Leeuwen, F.W.B.; Glatting, G.; Lassmann, M. EANM Position Paper on the Role of Radiobiology in Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3365–3377. [Google Scholar] [CrossRef]
- Holtfreter, J. A Study of the Mechanics of Gastrulation. J. Exp. Zool. 1943, 94, 171–212. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Santini, M.T.; Rainaldi, G.; Indovina, P.L. Multicellular Tumour Spheroids in Radiation Biology. Int. J. Radiat. Biol. 1999, 75, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Balber, T.; Tran, L.; Benčurová, K.; Raitanen, J.; Egger, G.; Mitterhauser, M. Experimental Nuclear Medicine Meets Tumor Biology. Pharmaceuticals 2022, 15, 227. [Google Scholar] [CrossRef]
- Sutherland, R.; Carlsson, J.; Durand, R.; Yuhas, J. Spheroids in Cancer Research. Cancer Res. 1981, 41, 2980–2984. [Google Scholar]
- Abramenkovs, A.; Hariri, M.; Spiegelberg, D.; Nilsson, S.; Stenerlöw, B. Ra-223 Induces Clustered DNA Damage and Inhibits Cell Survival in Several Prostate Cancer Cell Lines. Transl. Oncol. 2022, 26, 101543. [Google Scholar] [CrossRef]
- Salerno, D.; Howe, A.; Bhatavdekar, O.; Josefsson, A.; Pacheco-Torres, J.; Bhujwalla, Z.M.; Gabrielson, K.L.; Sofou, S. Two Diverse Carriers Are Better than One: A Case Study in α-Particle Therapy for Prostate Specific Membrane Antigen-Expressing Prostate Cancers. Bioeng. Transl. Med. 2022, 7, e10266. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Larsen, R.H.; Ma, L.W.; Peng, Q.; Juzenas, P.; Bruland, Ø.S.; Juzeniene, A. Evaluation of the Psma-Binding Ligand212pb-Ng001 in Multicellular Tumour Spheroid and Mouse Models of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4815. [Google Scholar] [CrossRef]
- Pinto, C.I.G.; Bucar, S.; Alves, V.; Fonseca, A.; Abrunhosa, A.J.; da Silva, C.L.; Guerreiro, J.F.; Mendes, F. Copper-64 Chloride Exhibits Therapeutic Potential in Three-Dimensional Cellular Models of Prostate Cancer. Front. Mol. Biosci. 2020, 7, 609172. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Petrić, T.; Sabol, M. Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 5293. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Recent Progress in Monte Carlo Simulation on Gold Nanoparticle Radiosensitization. AIMS Biophys. 2018, 5, 231–244. [Google Scholar] [CrossRef]
- Raitanen, J.; Barta, B.; Hacker, M.; Georg, D.; Balber, T.; Mitterhauser, M. Comparison of Radiation Response between 2D and 3D Cell Culture Models of Different Human Cancer Cell Lines. Cells 2023, 12, 360. [Google Scholar] [CrossRef]
- MycoAlertTM Mycoplasma Detection Kit Manual. Available online: https://bioscience.lonza.com/lonza_bs/AT/en/Cell-analysis/p/000000000000186475/MycoAlert-Mycoplasma-Detection-Kit-%2850-Tests%29 (accessed on 26 November 2023).
- Thermo Fisher Scientific Pierce ECL Western Blotting Substrate (Pub. No. MAN0011536 B.0). Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0011536_Pierce_ECL_West_Blot_Subs_UG.pdf (accessed on 26 November 2023).
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- 3D Cell Culture. Promega CellTiter-Glo® 3D Cell Viability Assay. Available online: https://at.promega.com/products/cell-health-assays/cell-viability-and-cytotoxicity-assays/celltiter-glo-3d-cell-viability-assay/?catNum=G9681#protocols (accessed on 26 November 2023).
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE -Geant4 Application for Tomographic Emission: A Simulation Toolkit for PET and SPECT. Phys. Med. Biol. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef]
- Sarrut, D.; Arbor, N.; Baudier, T.; Borys, D.; Etxebeste, A.; Fuchs, H.; Gajewski, J.; Grevillot, L.; Jan, S.; Kagadis, G.C.; et al. The OpenGATE Ecosystem for Monte Carlo Simulation in Medical Physics. Phys. Med. Biol. 2022, 67, 184001. [Google Scholar] [CrossRef]

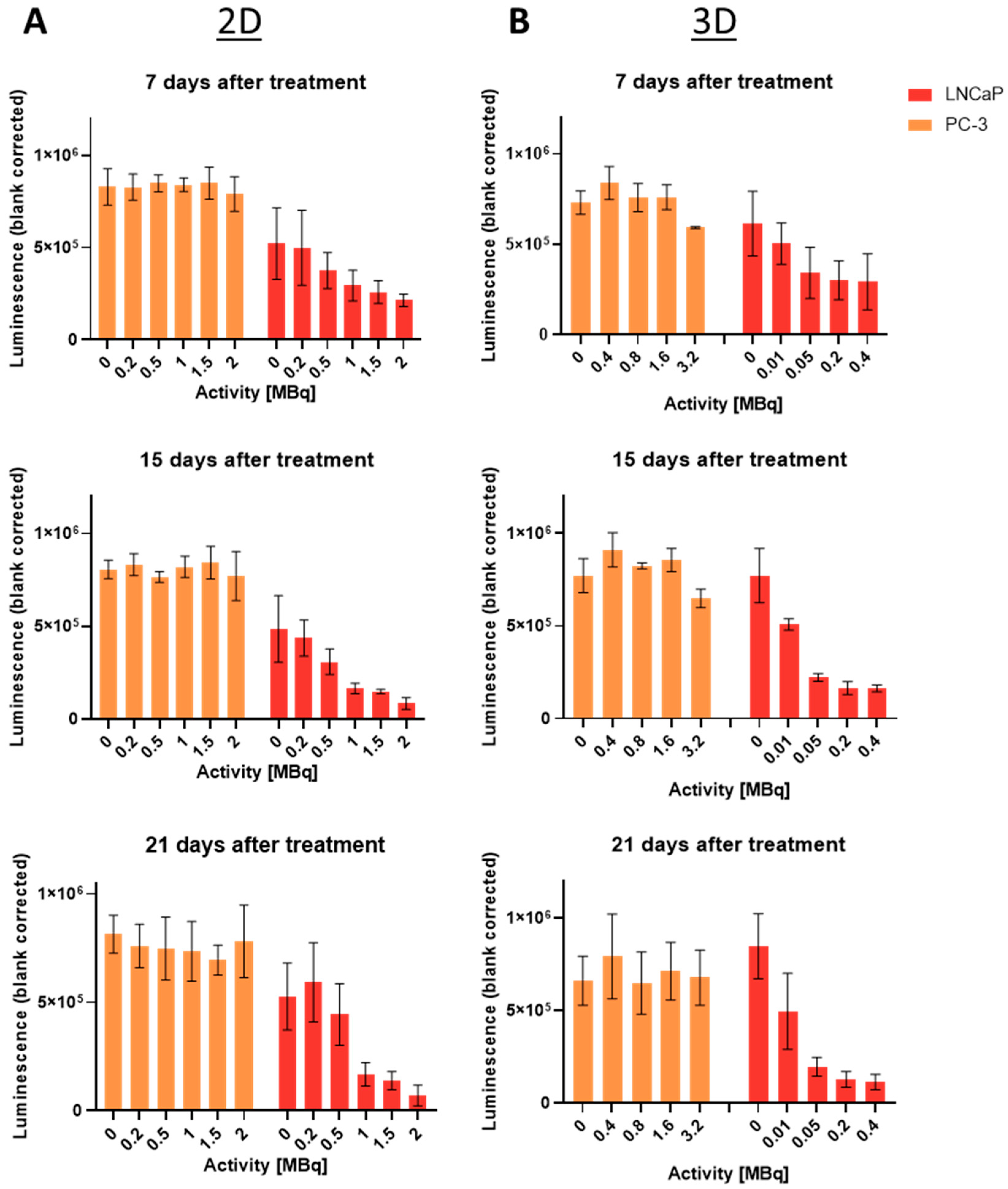

| Days after Treatment | X-Fold Difference (2D/3D) |
|---|---|
| 7 | 2.4 |
| 14 | 2.9 |
| 21 | 4.5 |
| Parameter | Value |
|---|---|
| Physics list | QGSP_BIC_HP_EMZ |
| Ionization potential of water | 78 eV |
| Production and stopping cuts in scoring region | 0.01 mm |
| Maximum step size in scoring region | 0.01 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raitanen, J.; Barta, B.; Fuchs, H.; Hacker, M.; Balber, T.; Georg, D.; Mitterhauser, M. Radiobiological Assessment of Targeted Radionuclide Therapy with [177Lu]Lu-PSMA-I&T in 2D vs. 3D Cell Culture Models. Int. J. Mol. Sci. 2023, 24, 17015. https://doi.org/10.3390/ijms242317015
Raitanen J, Barta B, Fuchs H, Hacker M, Balber T, Georg D, Mitterhauser M. Radiobiological Assessment of Targeted Radionuclide Therapy with [177Lu]Lu-PSMA-I&T in 2D vs. 3D Cell Culture Models. International Journal of Molecular Sciences. 2023; 24(23):17015. https://doi.org/10.3390/ijms242317015
Chicago/Turabian StyleRaitanen, Julia, Bernadette Barta, Hermann Fuchs, Marcus Hacker, Theresa Balber, Dietmar Georg, and Markus Mitterhauser. 2023. "Radiobiological Assessment of Targeted Radionuclide Therapy with [177Lu]Lu-PSMA-I&T in 2D vs. 3D Cell Culture Models" International Journal of Molecular Sciences 24, no. 23: 17015. https://doi.org/10.3390/ijms242317015






