Single-Stage Microfluidic Synthesis Route for BaGdF5:Tb3+-Based Nanocomposite Materials: Synthesis, Characterization and Biodistribution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adapting Reaction Conditions in MF Mode
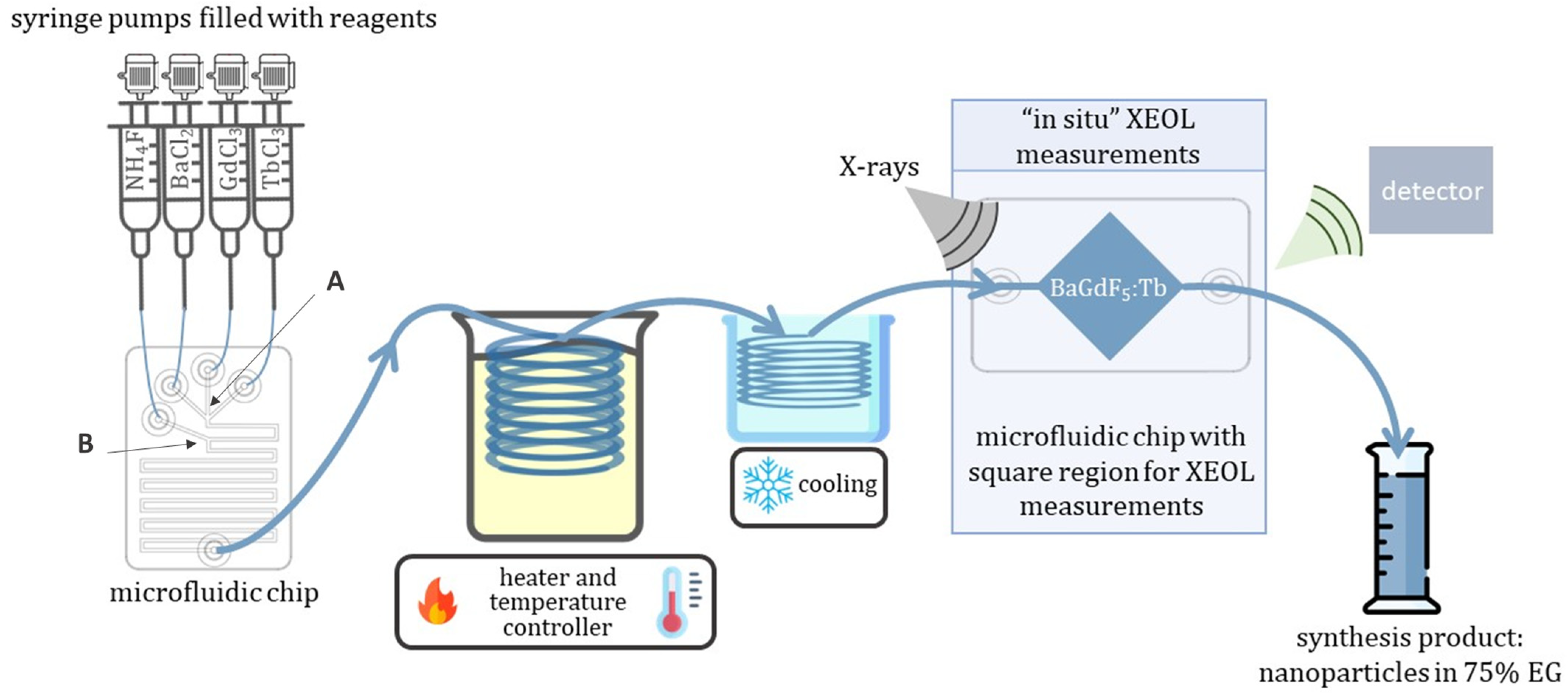
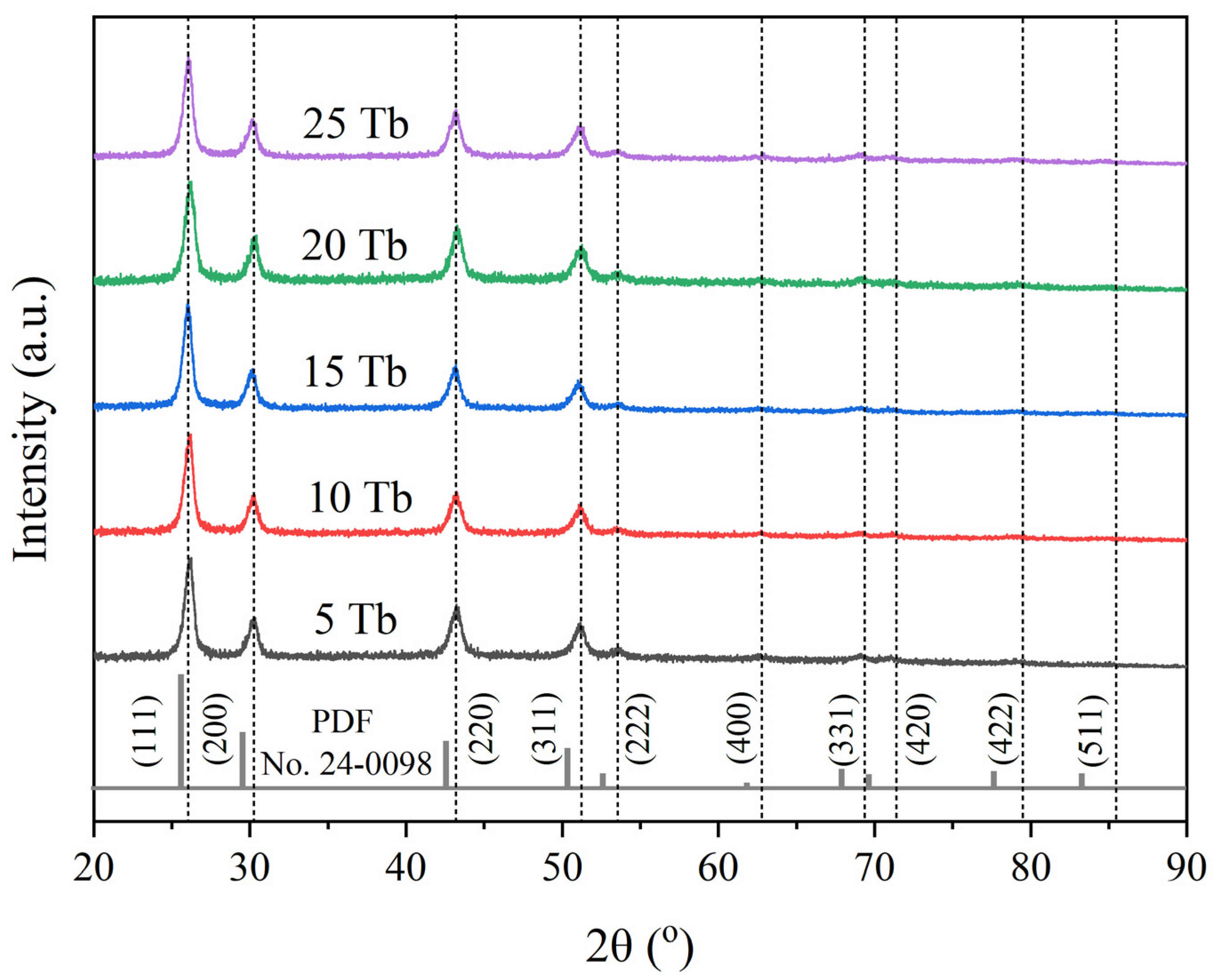
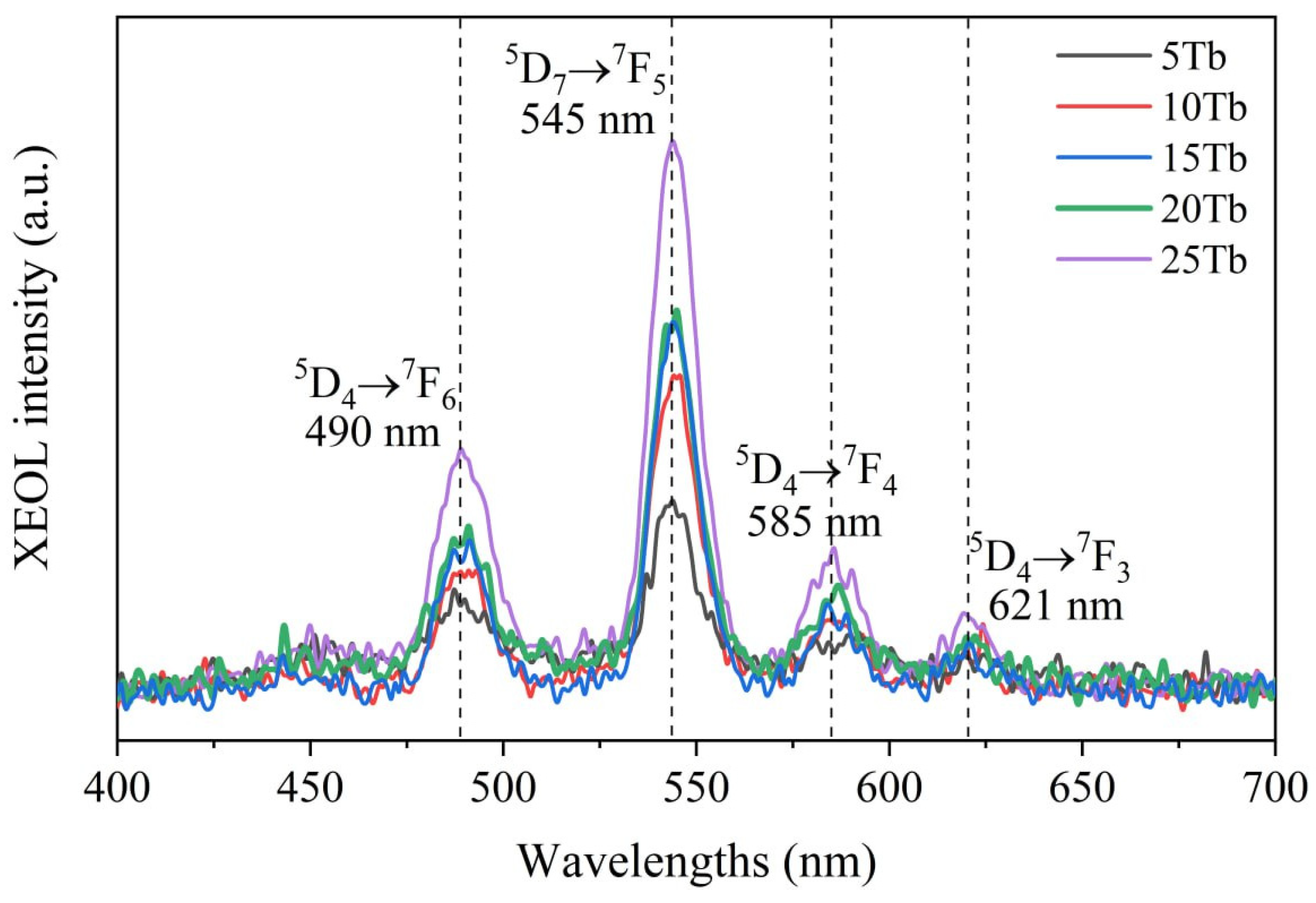
2.2. Synthesis of BaGd1−xF5:Tbx3+ Nanophosphors with In-Situ XEOL Acquisition
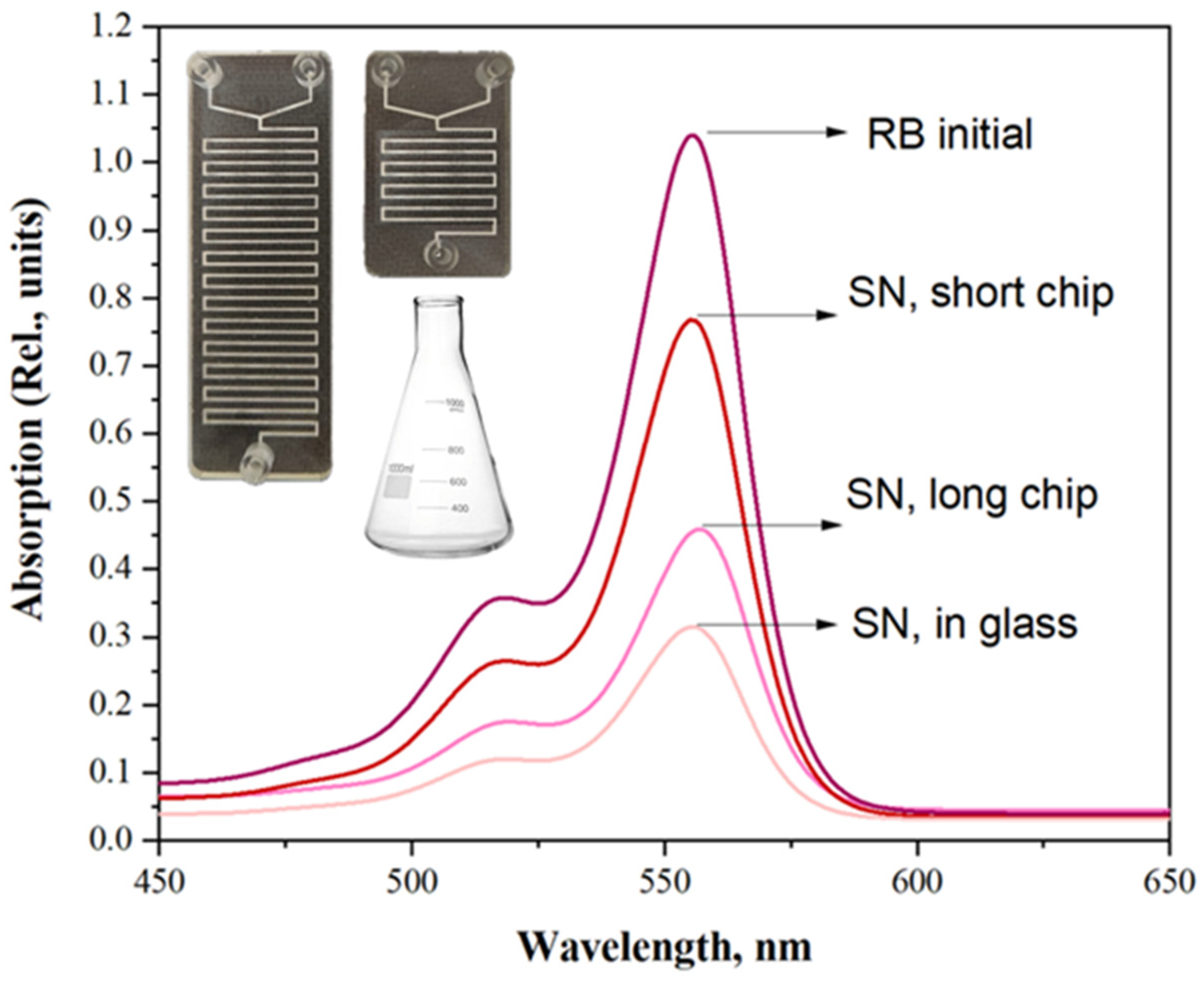
2.3. Single-Stage Microfluidic Synthesis of BaGdF5:Tb@RoseBengal Nanocomposite
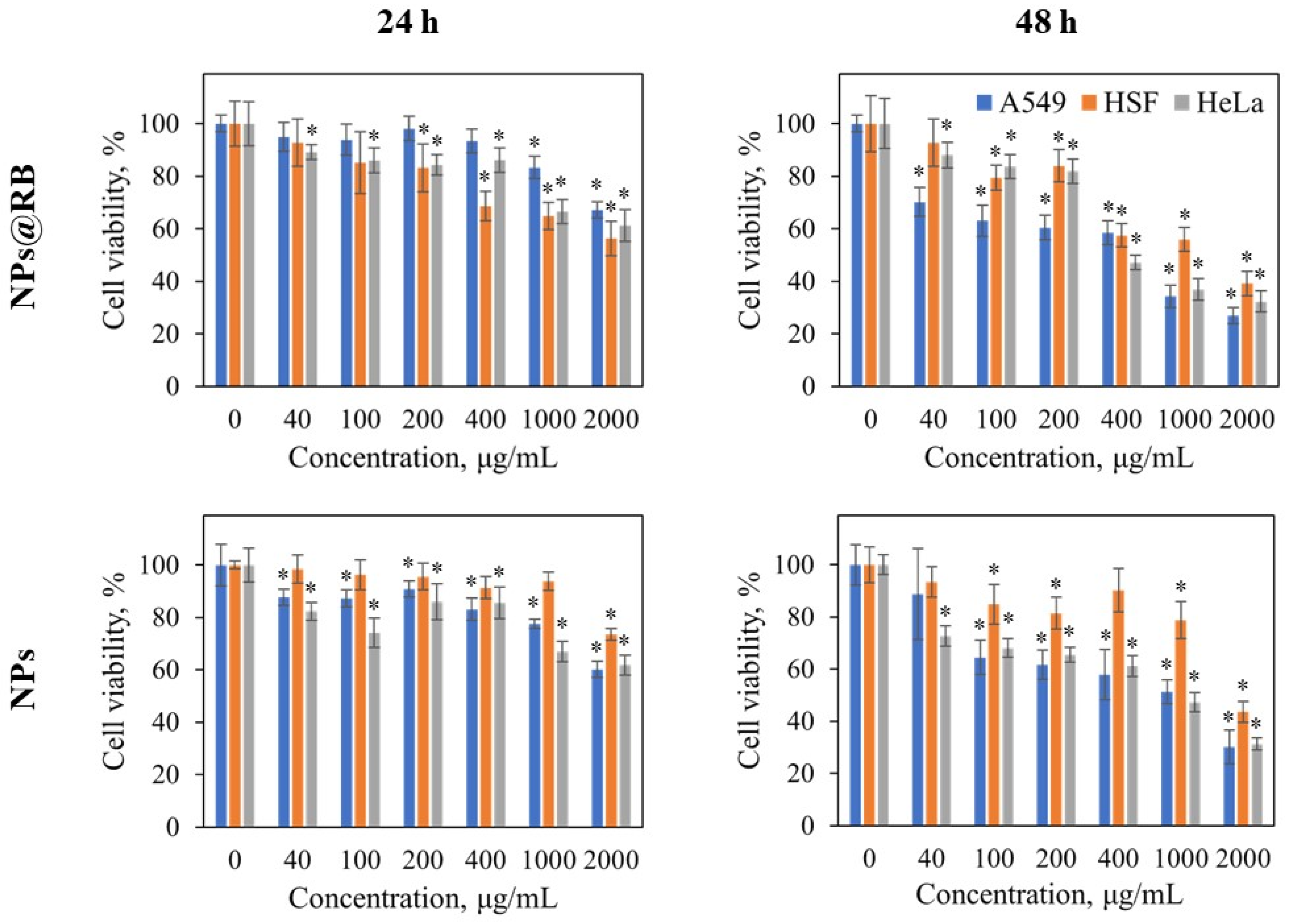
2.4. Cytotoxicity and Flow Cytometry Results
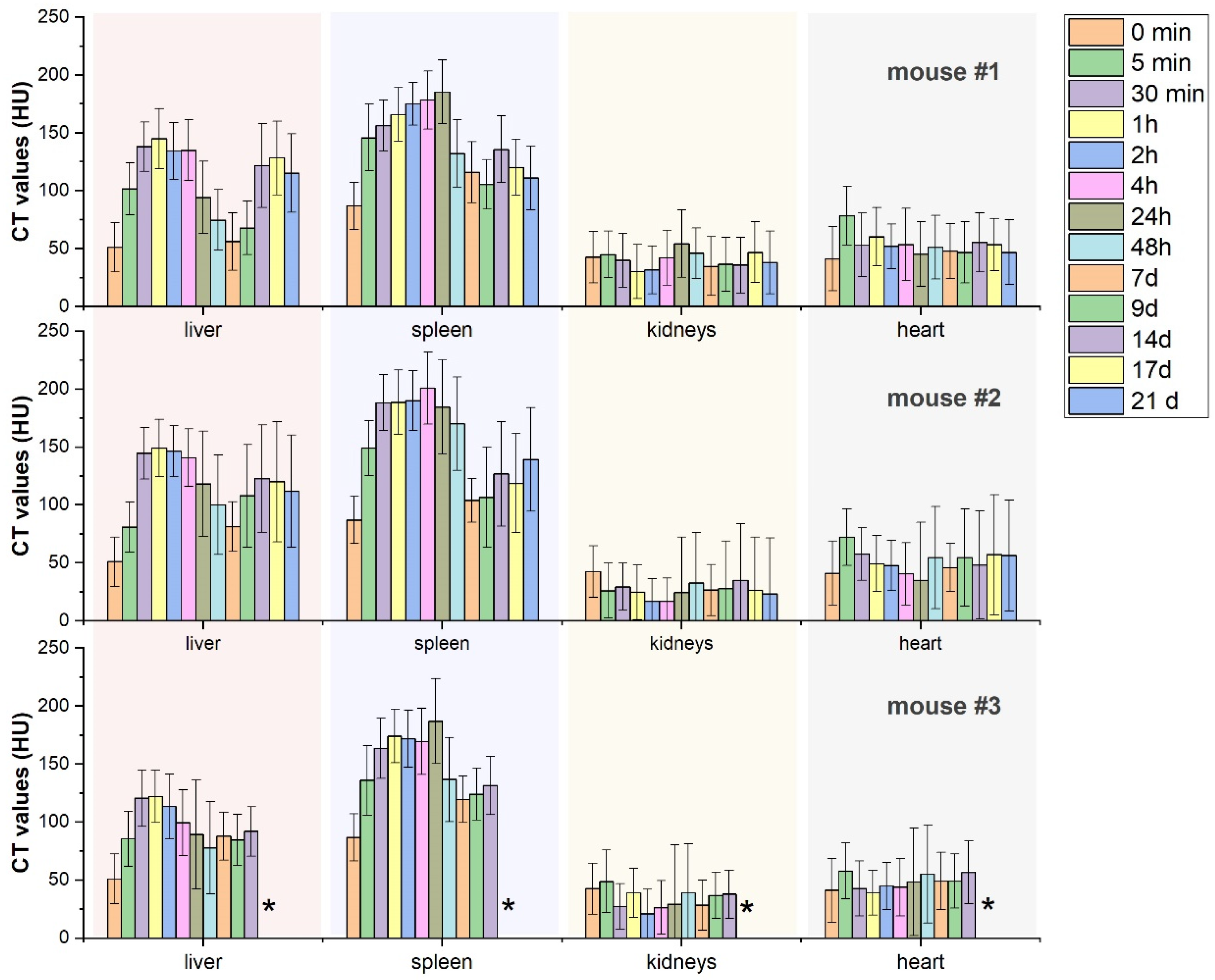
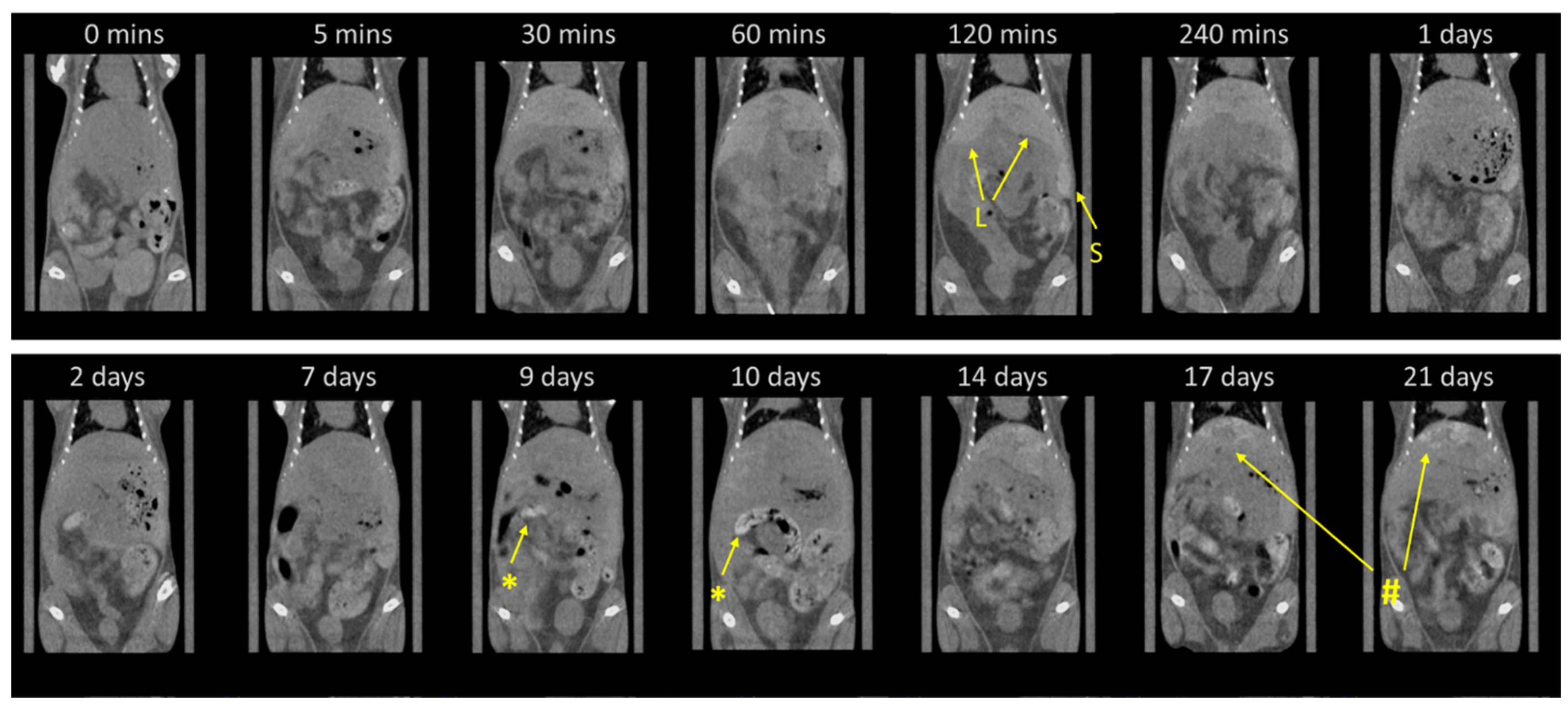
2.5. Long-Term Monitoring of Nanocomposite Biodistribution via Micro-CT
3. Materials and Methods
3.1. Microfluidic Setup and Microreactors
3.2. X-ray-Excited Optical Luminescence
3.3. Other Characterization Techniques
3.4. Cytotoxicity Test
3.5. Flow Cytometry
3.6. Statistical Analysis
3.7. Micro-CT In Vivo Diagnostics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Abrahamse, H.; Kruger, C.; Kadanyo, S.; Mishra, A. Nanoparticles for Advanced Photodynamic Therapy of Cancer. Photomed. Laser Surg. 2017, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Nguyen, H.; Chen, H.; Cox, P.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Naghavi, N.; Sazgarnia, A. A physicochemical model of X-ray induced photodynamic therapy (X-PDT) with an emphasis on tissue oxygen concentration and oxygenation. Sci. Rep. 2023, 13, 17882. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Liu, X.; Zhang, W.; Lu, H. X-ray excited luminescent nanoparticles for deep photodynamic therapy. RSC Adv. 2023, 13, 30133–30150. [Google Scholar] [CrossRef] [PubMed]
- Souris, J.S.; Leoni, L.; Zhang, H.J.; Pan, A.; Tanios, E.; Tsai, H.-M.; Balyasnikova, I.V.; Bissonnette, M.; Chen, C.-T. X-ray Activated Nanoplatforms for Deep Tissue Photodynamic Therapy. Nanomaterials 2023, 13, 673. [Google Scholar] [CrossRef]
- Naccache, R.; Yu, Q.; Capobianco, J. The Fluoride Host: Nucleation, Growth, and Upconversion of Lanthanide-Doped Nanoparticles. Adv. Opt. Mater. 2015, 3, 482–509. [Google Scholar] [CrossRef]
- Yi, T.; Zhiguang, F.; Xiaosheng, Z.; Chunjing, Z.; Jinwei, H.; Li, F.; Chaojun, S.; Qian, Y.; Mei, S.; Yu, W. Establishment of NaLuF4:15%Tb-based low dose X-PDT agent and its application on efficient antitumor therapy. Int. J. Miner. Metall. Mater. 2023. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Lei, T.; Zhang, X.; Lan, B.; Li, T.; Yu, J.; Lu, H.; Zhang, W. Growth phase diagram and X-ray excited luminescence properties of NaLuF4:Tb3+ nanoparticles. Arab. J. Chem. 2023, 16, 104672. [Google Scholar] [CrossRef]
- Fulbert, C.; Stelse-Masson, S.; Chaput, F.; Jacquet, T.; Nomezine, A.; Bohic, S.; Brueckner, D.; Garrevoet, J.; Moriscot, C.; Gallet, B.; et al. Using rare-earth based nanoscintillators for X-ray induced photodynamic therapy. Photodiagnosis Photodyn. Ther. 2023, 41, 103421. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, C.; Zhang, X.; Chen, Y.; Deng, X.; Liu, B.; Hou, Z.; Huang, S.; Jin, D.; Lin, J. Optimization of upconversion luminescence of Nd3+-sensitized BaGdF5-based nanostructures and their application in dual-modality imaging and drug delivery. Dalton Trans. 2016, 45, 1708–1716. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Eu3+/Tb3+ doped cubic BaGdF5 multifunctional nanophosphors: Multicolor tunable luminescence, energy transfer and magnetic properties. J. Lumin. 2017, 186, 6–15. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Dual-mode, tunable color, enhanced upconversion luminescence and magnetism of multifunctional BaGdF5:Ln3+ Ln = Yb/Er/Eu) nanophosphors. Phys. Chem. Chem. Phys. 2016, 18, 21518–21526. [Google Scholar] [CrossRef]
- Liu, D.; Jing, Y.; Wang, K.; Wang, Y.; Luo, G. Reaction study of α-phase NaYF4:Yb,Er generation via a tubular microreactor: Discovery of an efficient synthesis strategy. Nanoscale 2019, 11, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, Y.; Liu, J.; Zhou, Y.; Chen, Y.; Li, C.; Ma, P.a.; Lin, J. Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes. Biomaterials 2014, 35, 2011–2023. [Google Scholar] [CrossRef]
- Zeng, S.; Tsang, M.-K.; Chan, C.-F.; Wong, K.-L.; Fei, B.; Hao, J. Dual-modal fluorescent/magnetic bioprobes based on small sized upconversion nanoparticles of amine-functionalized BaGdF5:Yb/Er. Nanoscale 2012, 4, 5118–5124. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Polyakov, V.; Pankin, I.; Butova, V.; Kirsanova, D.; Soldatov, M.; Khodakova, D.; Goncharova, A.; Mukhanova, E.; Belanova, A.; et al. BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 13040. [Google Scholar] [CrossRef]
- Kirsanova, D.; Polyakov, V.; Butova, V.; Zolotukhin, P.; Belanova, A.; Gadzhimagomedova, Z.; Soldatov, M.; Pankin, I.; Soldatov, A. The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy. Nanomaterials 2021, 11, 3212. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Wang, Y.; Zhang, J.; Dong, P. Crystal structure and up- and down-conversion properties of Yb3+, Ho3+ codoped BaGdF5 solid-solution with different morphologies. CrystEngComm 2012, 14, 3131–3141. [Google Scholar] [CrossRef]
- Guan, H.; Song, Y.; Zheng, K.; Sheng, Y.; Zou, H. BaGdF5:Dy3+,Tb3+,Eu3+ multifunctional nanospheres: Paramagnetic, luminescence, energy transfer, and tunable color. Phys. Chem. Chem. Phys. 2016, 18, 13861–13873. [Google Scholar] [CrossRef] [PubMed]
- Becerro, A.I.; González-Mancebo, D.; Cantelar, E.; Cussó, F.; Stepien, G.; de la Fuente, J.M.; Ocaña, M. Ligand-Free Synthesis of Tunable Size Ln:BaGdF5 (Ln = Eu3+ and Nd3+) Nanoparticles: Luminescence, Magnetic Properties, and Biocompatibility. Langmuir 2016, 32, 411–420. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Q.; Zou, H.; Zhang, X.; Xiao, X.; Shi, Z.; Song, Y. Ionic liquid/H2O two-phase synthesis and luminescence properties of BaGdF5:RE3+ (RE = Ce/Dy/Eu/Yb/Er) octahedra. New J. Chem. 2021, 45, 742–750. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Liu, X.; Bai, T.; Dong, W.; Zhang, X.; Shi, Z.; Feng, S. Microwave-assisted synthesis and up–down conversion luminescent properties of multicolor hydrophilic LaF3:Ln3+ nanocrystals. Dalton Trans. 2013, 42, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Alakshin, E.M.; Klochkov, A.V.; Kondratyeva, E.I.; Korableva, S.L.; Kiiamov, A.G.; Nuzhina, D.S.; Stanislavovas, A.A.; Tagirov, M.S.; Zakharov, M.Y.; Kodjikian, S. Microwave-Assisted Hydrothermal Synthesis and Annealing of DyF3 Nanoparticles. J. Nanomater. 2016, 2016, 7148307. [Google Scholar] [CrossRef]
- Nizamutdinov, A.; Lukinova, E.; Shamsutdinov, N.; Zelenikhin, P.; Khusainova, A.; Gafurov, M.; Zinchenko, S.; Safin, D.; Pudovkin, M. CeF3-YF3-TbF3 Nanoparticle-Polymer—“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity. J. Compos. Sci. 2023, 7, 255. [Google Scholar] [CrossRef]
- Lei, Y.; Pang, M.; Fan, W.; Feng, J.; Song, S.; Dang, S.; Zhang, H. Microwave-assisted synthesis of hydrophilic BaYF5:Tb/Ce,Tb green fluorescent colloid nanocrystals. Dalton Trans. 2011, 40, 142–145. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Q.; Li, Y.; Wang, H. Redispersible and water-soluble LaF3:Ce,Tb nanocrystalsvia a microfluidic reactor with temperature steps. J. Mater. Chem. 2008, 18, 5060–5062. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Q.; Li, Y.; Wang, H. Facile crystallization control of LaF3/LaPO4:Ce, Tb nanocrystals in a microfluidic reactor using microwave irradiation. J. Mater. Chem. 2010, 20, 1766–1771. [Google Scholar] [CrossRef]
- Ma, J.; Yi, C.; Li, C.-W. Facile synthesis and functionalization of color-tunable Ln3+-doped KGdF4 nanoparticles on a microfluidic platform. Mater. Sci. Eng. C 2020, 108, 110381. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Liu, J.-H.; Liu, Y.; Yuan, B.-Y.; Gong, X.; Yuan, Q.-H.; Gong, T.-T.; Wang, L. Synthesis of PEGylated BaGdF5 Nanoparticles as Efficient CT/MRI Dual-modal Contrast Agents for Gastrointestinal Tract Imaging. Chin. J. Anal. Chem. 2020, 48, 1004–1011. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Wang, J.; Yang, Y.; Wu, D.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Yang, S. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials 2015, 42, 66–77. [Google Scholar] [CrossRef]
- Zhang, P.; He, Y.; Liu, J.; Feng, J.; Sun, Z.; Lei, P.; Yuan, Q.; Zhang, H. Core–shell BaYbF5:Tm@BaGdF5:Yb,Tm nanocrystals for in vivo trimodal UCL/CT/MR imaging. RSC Adv. 2016, 6, 14283–14289. [Google Scholar] [CrossRef]
- Polyakov, V.; Gadzhimagomedova, Z.; Kirsanova, D.; Soldatov, A. Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size. Materials 2022, 15, 8559. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Isikawa, M.; Guidelli, E. Microfluidic Synthesis of Theranostic Nanoparticles with Near-Infrared Scintillation: Toward Next-Generation Dosimetry in X-ray-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Jochums, A.; Friehs, E.; Sambale, F.; Lavrentieva, A.; Bahnemann, D.; Scheper, T. Revelation of Different Nanoparticle-Uptake Behavior in Two Standard Cell Lines NIH/3T3 and A549 by Flow Cytometry and Time-Lapse Imaging. Toxics 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.M.; Massaro, E.J.; Sanders, K.M.; Degn, L.L.; Boyes, W.K. Detection of TiO2 nanoparticles in cells by flow cytometry. Cytom. Part A 2010, 7, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jia, G.; Cheng, C.; Wang, Q.; Li, X.; Liu, Y.; He, C.; Chen, L.; Sun, G.; Zuo, C. Active targeted dual-modal CT/MR imaging of VX2 tumors using PEGylated BaGdF5 nanoparticles conjugated with RGD. New J. Chem. 2018, 42, 11565–11572. [Google Scholar] [CrossRef]
- Hubbel, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NIST Stand. Ref. Database; National Inst. of Standards and Technology—PL: Gaithersburg, MD, USA, 1995; p. 126.
- Medvedev, P.V.; Pankin, I.A.; Soldatov, M.A.; Polozhentsev, O.E.; Soldatov, A.V. Investigation of Specific Features of Recording the Optical Response of X-ray Phosphors for X-ray Photodynamic Therapy. Opt. Spectrosc. 2022, 130, 409–417. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; p. 246. [Google Scholar] [CrossRef]



| Solvent Composition | |
| X% of EG | Particle concentration, mg/mL |
| 100 EG | 1.5–2 |
| 75 EG | 10–12 |
| 50 EG | chip clogging |
| 25 EG | chip clogging |
| 0 EG | chip clogging |
| Synthesis time (total flow rates) | |
| Time | Particle concentration, mg/mL |
| 2 h | chip clogging |
| 1 h | 10–12 |
| 30 min | 10–12 |
| 12 min | 10–12 |
| 6 min | 10–12 |
| Metals chloride (precursors) concentration * | |
| Concentration, mol/L | Particle concentration, mg/mL |
| 0.1 | 10–12 |
| 0.2 | 17–19 |
| 0.3 | 25–30 |
| 0.4 | chip clogging |
| 0.5 | chip clogging |
| Sample | Precursor Flow Rates, μL/s | |||
|---|---|---|---|---|
| Ba2+ | Gd3+ | Tb3+ | F− | |
| 5Tb | 10.28 | 9.77 | 0.51 | 10.28 |
| 10Tb | 9.25 | 1.03 | ||
| 15Tb | 8.74 | 1.54 | ||
| 20Tb | 8.22 | 2.06 | ||
| 25Tb | 7.71 | 2.57 | ||
| Sample | Expected Tb Content, at.% | Actual Tb Content, at.% | Cell Parameters, Å | Cell Volume, Å3 | Goodness of Fit (GOF) | R-Factor |
|---|---|---|---|---|---|---|
| 5Tb | 0.71 | 0.66 | 5.9388 (14) | 209.46 (8) | 1.06 | 0.1506 |
| 10Tb | 1.43 | 1.29 | 5.9408 (14) | 209.67 (9) | 1.06 | 0.1449 |
| 15Tb | 2.13 | 2.11 | 5.9355 (12) | 209.11 (7) | 1.03 | 0.1275 |
| 20Tb | 2.86 | 2.86 | 5.9352 (16) | 209.08 (10) | 1.08 | 0.18 |
| 25Tb | 3.57 | 3.63 | 5.9337 (11) | 208.92 (7) | 1.01 | 0.1217 |
| Sample | A549 | HSF | HeLa |
|---|---|---|---|
| Control | 28.156 ± 8.823 | 32.261 ± 12.467 | 31.060 ± 10.525 |
| NPs@RB | 40.398 ± 13.794 * | 52.504 ± 31.848 * | 32.343 ± 19.897 |
| NPs | 45.946 ± 13.134 * | 60.513 ± 30.340 * | 37.061 ± 15.484 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadzhimagomedova, Z.; Pankin, I.; Polyakov, V.; Khodakova, D.; Medvedev, P.; Zelenikhin, P.; Shamsutdinov, N.; Chapek, S.; Goncharova, A.; Soldatov, A. Single-Stage Microfluidic Synthesis Route for BaGdF5:Tb3+-Based Nanocomposite Materials: Synthesis, Characterization and Biodistribution. Int. J. Mol. Sci. 2023, 24, 17159. https://doi.org/10.3390/ijms242417159
Gadzhimagomedova Z, Pankin I, Polyakov V, Khodakova D, Medvedev P, Zelenikhin P, Shamsutdinov N, Chapek S, Goncharova A, Soldatov A. Single-Stage Microfluidic Synthesis Route for BaGdF5:Tb3+-Based Nanocomposite Materials: Synthesis, Characterization and Biodistribution. International Journal of Molecular Sciences. 2023; 24(24):17159. https://doi.org/10.3390/ijms242417159
Chicago/Turabian StyleGadzhimagomedova, Zaira, Ilia Pankin, Vladimir Polyakov, Darya Khodakova, Pavel Medvedev, Pavel Zelenikhin, Nail Shamsutdinov, Sergey Chapek, Anna Goncharova, and Alexander Soldatov. 2023. "Single-Stage Microfluidic Synthesis Route for BaGdF5:Tb3+-Based Nanocomposite Materials: Synthesis, Characterization and Biodistribution" International Journal of Molecular Sciences 24, no. 24: 17159. https://doi.org/10.3390/ijms242417159






