In-Host HEV Quasispecies Evolution Shows the Limits of Mutagenic Antiviral Treatments
Abstract
:1. Introduction
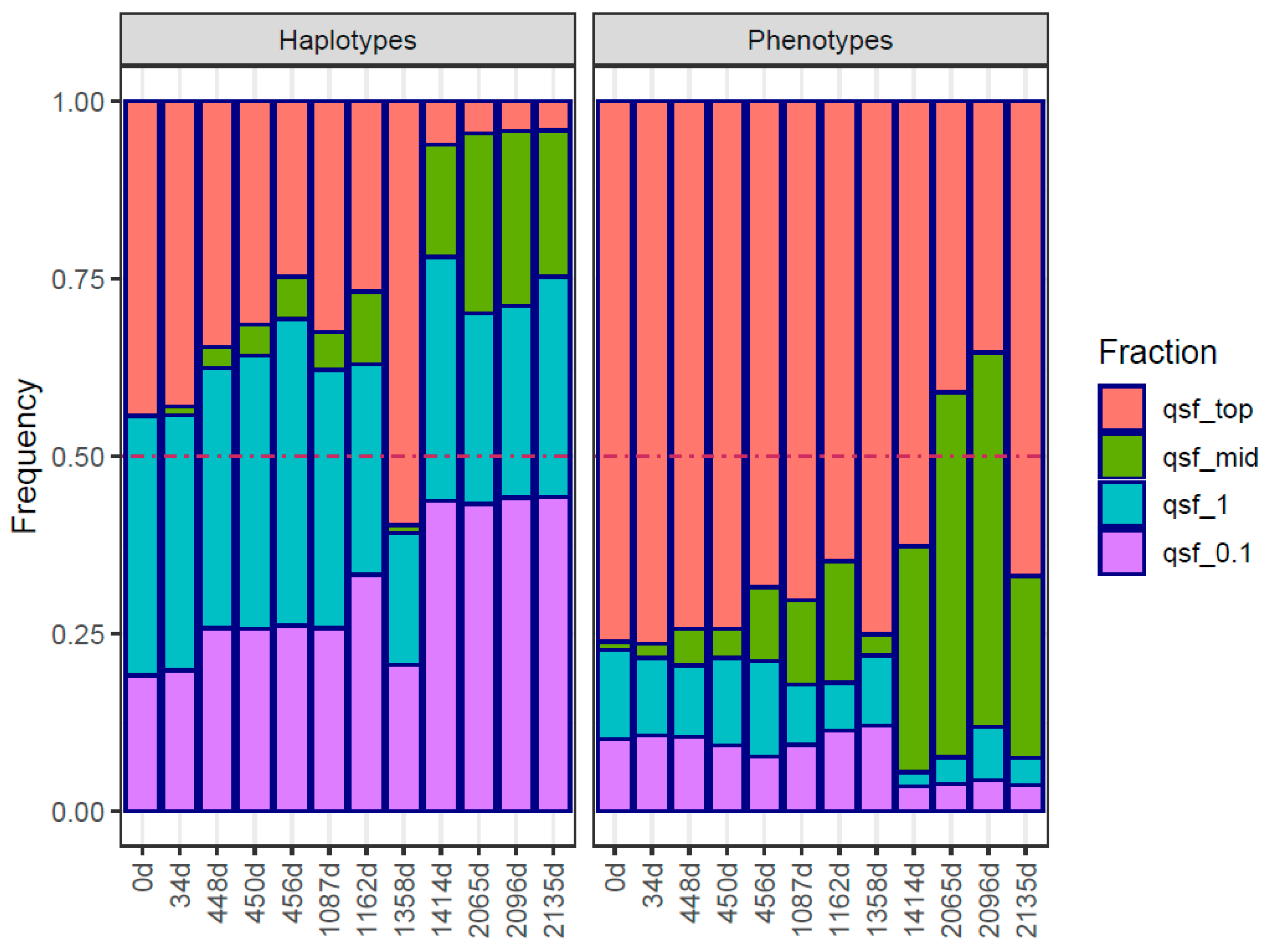
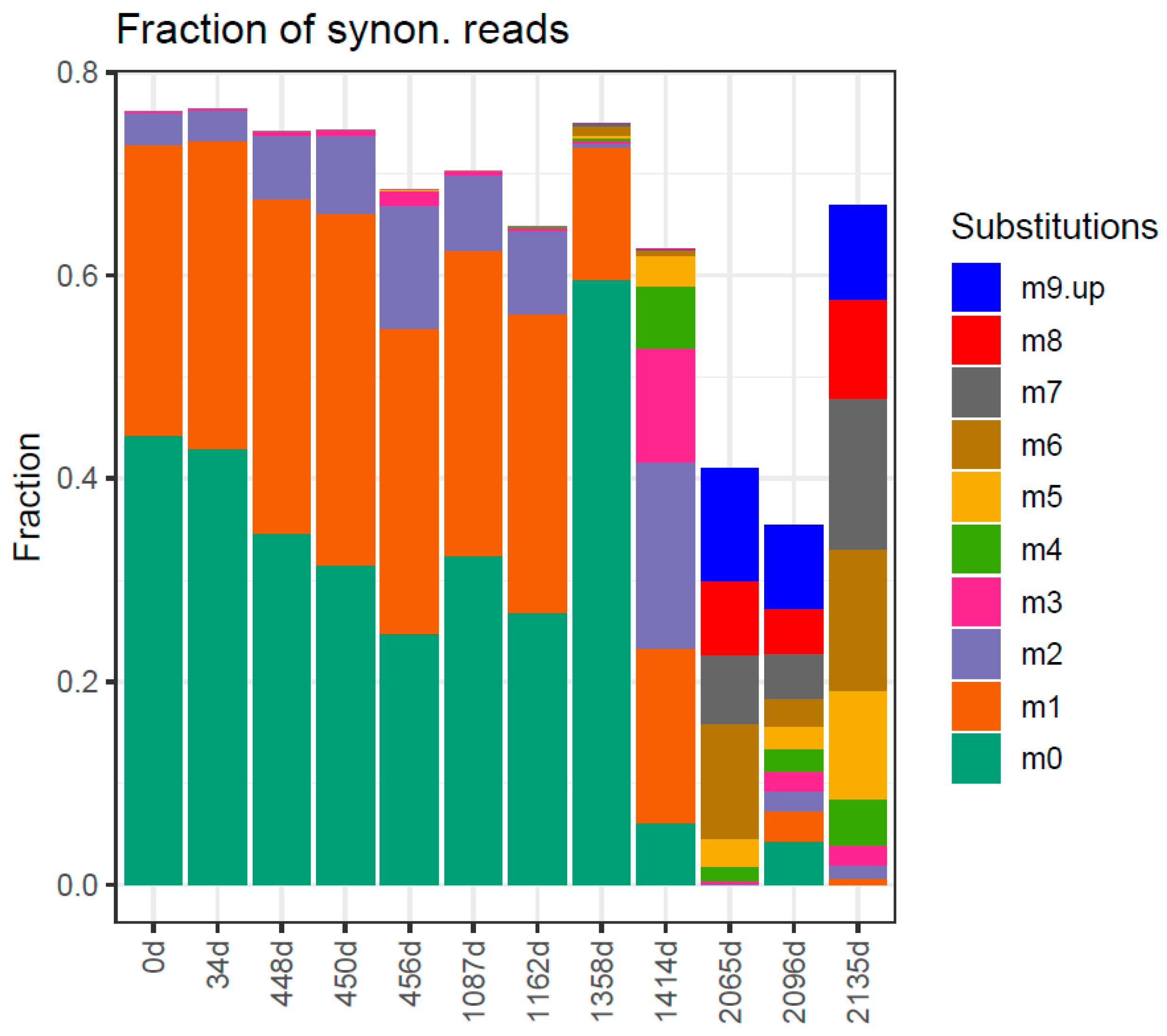
2. Results
Traits in Quasispecies Evolution
3. Discussion
4. Materials and Methods
4.1. Patient Data
4.2. RNA Extraction and Amplification
4.3. Library Preparation and Illumina Sequencing
4.4. Processing the Sequencing Data
- Obtain Fastq files with Illumina 2 × 300-bp paired-end reads;
- Recover full amplicon reads with FLASH [46] (minimum 20-bp overlap, maximum 10% mismatches). The 300-bp reads, when overlapped, result in reads covering complete ~400–500 bp amplicons;
- Remove full reads with 5% or more bases below a Phred score of Q30;
- Demultiplex and trim primers (max three differences accepted);
- Collapse reads (molecules) to haplotypes (amplicon-genomes) and their frequencies (read counts);
- Multiple alignment of all haplotypes in each sample/amplicon;
- Remove all haplotypes that are not common to both DNA strands and supported at least by 5 reads;
- Remove insertions in master haplotypes and repair single gaps in remaining haplotypes. Recollapse to haplotypes and vectors of frequencies;
- The haplotypes in each sample/amplicon are translated to phenotypes and recollapsed to obtain the set of phenotypes with corresponding frequencies (read counts).
4.5. Bioinformatic Procedures
4.6. Software and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 29 November 2023).
- Ministerio de Sanidad. 2o Estudio de Seroprevalencia En España. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/EstudioSeroprevalencia_EnfermedadesInmunoprevenibles.pdf (accessed on 29 November 2023).
- Sauleda, S.; Ong, E.; Bes, M.; Janssen, A.; Cory, R.; Babizki, M.; Shin, T.; Lindquist, A.; Hoang, A.; Vang, L.; et al. Seroprevalence of Hepatitis E Virus (HEV) and Detection of HEV RNA with a Transcription-Mediated Amplification Assay in Blood Donors from Catalonia (Spain). Transfusion 2015, 55, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B. ICTV Report Consortium ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Minguez, B.; Girones, R.; Rodriguez-Frias, F.; Quer, J.; Buti, M. Phylogenetic Demonstration of Hepatitis E Infection Transmitted by Pork Meat Ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Boxman, I.L.A.; Jansen, C.C.C.; Hägele, G.; Zwartkruis-Nahuis, A.; Tijsma, A.S.L.; Vennema, H. Monitoring of Pork Liver and Meat Products on the Dutch Market for the Presence of HEV RNA. Int. J. Food Microbiol. 2019, 296, 58–64. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis E. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef]
- Dalton, H.R.; Kamar, N.; Baylis, S.A.; Moradpour, D.; Wedemeyer, H.; Negro, F.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Hepatitis E Virus Infection. J. Hepatol. 2018, 68, 1256–1271. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute Liver Failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Zhou, X.; de Man, R.A.; de Knegt, R.J.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Epidemiology and Management of Chronic Hepatitis E Infection in Solid Organ Transplantation: A Comprehensive Literature Review. Rev. Med. Virol. 2013, 23, 295–304. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E Virus Infection. Nat. Rev. Dis. Primers 2017, 3, 17086. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Vallejo, N.; Lopez-Lopez, P.; Díaz-Mareque, A.I.; Frias, M.; Vallejo, A.; Caballero-Gómez, J.; Rodríguez-Velasco, M.; Molina, E.; Aguilera, A. Ribavirin as a First Treatment Approach for Hepatitis E Virus Infection in Transplant Recipient Patients. Microorganisms 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Emerson, S.U.; Wang, Y.; Pan, Q.; Balzarini, J.; Dallmeier, K.; Neyts, J. Ribavirin Inhibits in Vitro Hepatitis E Virus Replication through Depletion of Cellular GTP Pools and Is Moderately Synergistic with Alpha Interferon. Antimicrob. Agents Chemother. 2014, 58, 267–273. [Google Scholar] [CrossRef]
- Gregori, J.; Colomer-Castell, S.; Campos, C.; Ibañez-Lligoña, M.; Garcia-Cehic, D.; Rando-Segura, A.; Adombi, C.M.; Pintó, R.; Guix, S.; Bosch, A.; et al. Quasispecies Fitness Partition to Characterize the Molecular Status of a Viral Population. Negative Effect of Early Ribavirin Discontinuation in a Chronically Infected HEV Patient. Int. J. Mol. Sci. 2022, 23, 14654. [Google Scholar] [CrossRef]
- Perales, C.; Gallego, I.; de Ávila, A.I.; Soria, M.E.; Gregori, J.; Quer, J.; Domingo, E. The Increasing Impact of Lethal Mutagenesis of Viruses. Future Med. Chem. 2019, 11, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Hassine, I.H.; Ben M’hadheb, M.; Menéndez-Arias, L. Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity. Viruses 2022, 14, 841. [Google Scholar] [CrossRef]
- Schulz, M.; Papp, C.P.; Bock, C.-T.; Hofmann, J.; Gerlach, U.A.; Maurer, M.M.; Eurich, D.; Mueller, T. Combination Therapy of Sofosbuvir and Ribavirin Fails to Clear Chronic Hepatitis E Infection in a Multivisceral Transplanted Patient. J. Hepatol. 2019, 71, 225–227. [Google Scholar] [CrossRef]
- Sastre, L.; García-López, M.; Pérez-Del-Pulgar, S.; Lens, S.; Costa, J.; Navasa, M.; Forns, X. The Challenge of Chronic Hepatitis E in Liver Transplant Recipients: Failure of Sofosbuvir plus Ribavirin Therapy. Gastroenterol. Hepatol. 2020, 43, 136–137. [Google Scholar] [CrossRef]
- Takahashi, K.; Toyota, J.; Karino, Y.; Kang, J.H.; Maekubo, H.; Abe, N.; Mishiro, S. Estimation of the Mutation Rate of Hepatitis E Virus Based on a Set of Closely Related 7.5-Year-Apart Isolates from Sapporo, Japan. Hepatol. Res. 2004, 29, 212–215. [Google Scholar] [CrossRef]
- Holland, J.J.; Spindler, K.; Horodyski, F.; Grabau, E.; Nichol, S.; VandePol, S. Rapid Evolution of RNA Genomes. Science 1982, 215, 1577–1585. [Google Scholar] [CrossRef]
- Vignuzzi, M.; Stone, J.K.; Arnold, J.J.; Cameron, C.E.; Andino, R. Quasispecies Diversity Determines Pathogenesis through Cooperative Interactions in a Viral Population. Nature 2006, 439, 344–348. [Google Scholar] [CrossRef]
- Domingo, E. Virus as Populations. Composition, Complexity, Dynamics and Biological Implications. In Virus as Populations, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2016; pp. 1–412. [Google Scholar]
- Wilke, C.O.; Wang, J.L.; Ofria, C.; Lenski, R.E.; Adami, C. Evolution of Digital Organisms at High Mutation Rates Leads to Survival of the Flattest. Nature 2001, 412, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Schuster, P.; Swetina, J. Stationary Mutant Distributions and Evolutionary Optimization. Bull. Math. Biol. 1988, 50, 635–660. [Google Scholar] [CrossRef] [PubMed]
- Tejero, H.; Montero, F.; Nuño, J.C. Theories of Lethal Mutagenesis: From Error Catastrophe to Lethal Defection. Curr. Top. Microbiol. Immunol. 2016, 392, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.O. Quasispecies Theory in the Context of Population Genetics. BMC Evol. Biol. 2005, 5, 44. [Google Scholar] [CrossRef]
- Lauring, A.S.; Andino, R. Quasispecies Theory and the Behavior of RNA Viruses. PLoS Pathog. 2010, 6, e1001005. [Google Scholar] [CrossRef]
- Beaucourt, S.; Vignuzzi, M. Ribavirin: A Drug Active against Many Viruses with Multiple Effects on Virus Replication and Propagation. Molecular Basis of Ribavirin Resistance. Curr. Opin. Virol. 2014, 8, 10–15. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Schuster, P. Quasispecies on Fitness Landscapes. Curr. Top. Microbiol. Immunol. 2016, 392, 61–120. [Google Scholar] [CrossRef]
- Swetina, J.; Schuster, P. Self-Replication with Errors. A Model for Polynucleotide Replication. Biophys. Chem. 1982, 16, 329–345. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle. Naturwissenschaften Sci. Nat. 1977, 64, 541–565. [Google Scholar] [CrossRef]
- Orgel, L.E. The Maintenance of the Accuracy of Protein Synthesis and Its Relevance to Ageing. Proc. Natl. Acad. Sci. USA 1963, 49, 517–521. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The Hypercycle. A Principle of Natural Self-Organization; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Ortega-Prieto, A.M.; Sheldon, J.; Grande-Perez, A.; Tejero, H.; Gregori, J.; Quer, J.; Esteban, J.I.; Domingo, E.; Perales, C. Extinction of Hepatitis C Virus by Ribavirin in Hepatoma Cells Involves Lethal Mutagenesis. PLoS ONE 2013, 8, e71039. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Dávila, M.; Lowenstein, P.R.; Domingo, E. Response of Foot-and-Mouth Disease Virus to Increased Mutagenesis: Influence of Viral Load and Fitness in Loss of Infectivity. J. Virol. 2000, 74, 8316–8323. [Google Scholar] [CrossRef] [PubMed]
- Tapia, N.; Fernàndez, G.; Parera, M.; Gómez-Mariano, G.; Clotet, B.; Quiñones-Mateu, M.; Domingo, E.; Martínez, M.A. Combination of a Mutagenic Agent with a Reverse Transcriptase Inhibitor Results in Systematic Inhibition of HIV-1 Infection. Virology 2005, 338, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pariente, N.; Sierra, S.; Lowenstein, P.R.; Domingo, E. Efficient Virus Extinction by Combinations of a Mutagen and Antiviral Inhibitors. J. Virol. 2001, 75, 9723–9730. [Google Scholar] [CrossRef] [PubMed]
- Perales, C.; Agudo, R.; Tejero, H.; Manrubia, S.C.; Domingo, E. Potential Benefits of Sequential Inhibitor-Mutagen Treatments of RNA Virus Infections. PLoS Pathog. 2009, 5, e1000658. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.; Grande-Pérez, A.; Domingo, E.; Martín, V. Arenaviruses and Lethal Mutagenesis. Prospects for New Ribavirin-Based Interventions. Viruses 2012, 4, 2786–2805. [Google Scholar] [CrossRef]
- Huang, A.S.; Baltimore, D. Defective Viral Particles and Viral Disease Processes. Nature 1970, 226, 325–327. [Google Scholar] [CrossRef]
- Gallego, I.; Gregori, J.; Soria, M.E.; Garcia-Crespo, C.; Garcia-Alvarez, M.; Gomez-Gonzalez, A.; Valiergue, R.; Gomez, J.; Esteban, J.I.; Quer, J.; et al. Resistance of High Fitness Hepatitis C Virus to Lethal Mutagenesis. Virology 2018, 523, 100–109. [Google Scholar] [CrossRef]
- Adombi, C.M.; Bosch, A.; Buti, M.; Campos, C.; Colomer-Castel, S.; Cortese, M.F.; Domingo, E.; Esteban, J.I.; Gallego, I.; Garcia-Cehic, D.; et al. Viral Quasispecies Diversity and Evolution: A Bioinformatics Molecular Approach; Gregori, J., Rodriguez-Frias, F., Quer, J., Eds.; Il Pensiero Scientifico Editore: Rome, Italy, 2023; ISBN 9788849007589. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pages, H.; Aboyoun, P.; Gentleman, R.; DebRoy, S. Biostrings: String Objects Representing Biological Sequences, and Matching Algorithms, R Package 2.38.4. 2023. Available online: https://www.bioconductor.org/packages//2.7/bioc/html/Biostrings.html (accessed on 4 December 2023).
- Morgan, M.; Anders, S.; Lawrence, M.; Aboyoun, P.; Pages, H.; Gentleman, R. ShortRead: A Bioconductor Package for Input, Quality Assessment and Exploration of High-Throughput Sequence Data. Bioinformatics 2009, 25, 2607–2608. [Google Scholar] [CrossRef]
- Guerrero-Murillo, M.; Gregori, J. QSutils: Quasispecies Diversity. R Package Version 1.0.0. Available online: https://bioconductor.org/packages/release/bioc/html/QSutils.html (accessed on 2 December 2023).
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open Software Development for Computational Biology and Bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Pelé, J.; Bécu, J.-M.; Abdi, H.; Chabbert, M. Bios2mds: An R Package for Comparing Orthologous Protein Families by Metric Multidimensional Scaling. BMC Bioinform. 2012, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Welcome to Master the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Valero-Mora, P.M. ggplot2: Elegant graphics for data analysis. J. Stat. Softw. 2010, 35, 1–3. [Google Scholar] [CrossRef]
- Gregori, J.; Soria, M.E.; Gallego, I.; Guerrero-Murillo, M.; Esteban, J.I.; Quer, J.; Perales, C.; Domingo, E. Rare Haplotype Load as Marker for Lethal Mutagenesis. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; ISBN 9780231886710. [Google Scholar]
- Grishin, V.N.; Grishin, N. V Euclidian Space and Grouping of Biological Objects. Bioinformatics 2002, 18, 1523–1534. [Google Scholar] [CrossRef]
- Gregori, J.; Ibañez-Lligoña, M.; Quer, J. Quantifying In-Host Quasispecies Evolution. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Fitch, W.M. An Improved Method of Testing for Evolutionary Homology. J. Mol. Biol. 1966, 16, 9–16. [Google Scholar] [CrossRef]
- Grantham, R. Amino Acid Difference Formula to Help Explain Protein Evolution. Science 1974, 185, 862–864. [Google Scholar] [CrossRef]





| Intervention * | ID a | Date | Days b | Weeks c | CumDays d | Viral Load |
|---|---|---|---|---|---|---|
| Infection | October 2012 | |||||
| 1st evidence | S01 | 13 November 2012 | 0 | 0.0 | 0 | 5.04 × 106 |
| S02 | 15 December 2012 | 34 | 4.9 | 34 | 6.84 × 106 | |
| RBV 200 mg | 3 February 2014 | 413 | 59.0 | 447 | ||
| S03 | 4 February 2014 | 1 | 0.1 | 448 | 4.00 × 106 | |
| S04 | 6 February 2014 | 2 | 0.3 | 450 | 2.78 × 106 | |
| S05 | 12 February 2014 | 6 | 0.9 | 456 | 1.17 × 106 | |
| EOT | 3 May 2014 | 80 | 11.4 | 536 | RNA− | |
| Relapse | 1 January 2015 | 243 | 34.7 | 779 | RNA+ | |
| S06 | 5 November 2015 | 308 | 44.0 | 1087 | 3.56 × 106 | |
| RBV 200/400 mg | S07 | 19 January 2016 | 75 | 10.7 | 1162 | 1.30 × 107 |
| EOT | 5 July 2016 | 168 | 19.7 | 1300 | RNA+ | |
| S08 | 2 August 2016 | 28 | 8.3 | 1358 | 2.00 × 104 | |
| S09 | 27 September 2016 | 56 | 8.0 | 1414 | 6.93 × 105 | |
| RBV 400 mg | 9 April 2018 | 559 | 79.9 | 1973 | 1.27 × 107 | |
| S13 | 10 July 2018 | 92 | 13.1 | 2065 | 1.51 × 105 | |
| S15 | 10 August 2018 | 31 | 4.4 | 2096 | 8.22 × 104 | |
| S18 | 18 September 2018 | 39 | 5.6 | 2135 | 7.50 × 104 | |
| EOT | 27 November 2018 | 70 | 10.0 | 2205 | 8.20 × 104 | |
| 2 March 2019 | 95 | 13.6 | 2300 | 7.00 × 106 |
| WT | Var | N | Fitch | Grantham | qGrantham |
|---|---|---|---|---|---|
| T | N | 27 | 1 | 65 | 0.2579 |
| T | A | 20 | 1 | 58 | 0.2105 |
| G | S | 3 | 1 | 56 | 0.2000 |
| V | I | 3 | 1 | 29 | 0.0789 |
| A | T | 2 | 1 | 58 | 0.2105 |
| E | K | 2 | 1 | 56 | 0.2000 |
| I | M | 2 | 1 | 10 | 0.0105 |
| L | F | 2 | 1 | 22 | 0.0368 |
| A | V | 1 | 1 | 64 | 0.2421 |
| F | Y | 1 | 1 | 22 | 0.0368 |
| I | V | 1 | 1 | 29 | 0.0789 |
| S | A | 1 | 1 | 99 | 0.5368 |
| S | P | 1 | 1 | 74 | 0.2947 |
| V | A | 1 | 1 | 64 | 0.2421 |
| Y | H | 1 | 1 | 83 | 0.3421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomer-Castell, S.; Gregori, J.; Garcia-Cehic, D.; Riveiro-Barciela, M.; Buti, M.; Rando-Segura, A.; Vico-Romero, J.; Campos, C.; Ibañez-Lligoña, M.; Adombi, C.M.; et al. In-Host HEV Quasispecies Evolution Shows the Limits of Mutagenic Antiviral Treatments. Int. J. Mol. Sci. 2023, 24, 17185. https://doi.org/10.3390/ijms242417185
Colomer-Castell S, Gregori J, Garcia-Cehic D, Riveiro-Barciela M, Buti M, Rando-Segura A, Vico-Romero J, Campos C, Ibañez-Lligoña M, Adombi CM, et al. In-Host HEV Quasispecies Evolution Shows the Limits of Mutagenic Antiviral Treatments. International Journal of Molecular Sciences. 2023; 24(24):17185. https://doi.org/10.3390/ijms242417185
Chicago/Turabian StyleColomer-Castell, Sergi, Josep Gregori, Damir Garcia-Cehic, Mar Riveiro-Barciela, Maria Buti, Ariadna Rando-Segura, Judit Vico-Romero, Carolina Campos, Marta Ibañez-Lligoña, Caroline Melanie Adombi, and et al. 2023. "In-Host HEV Quasispecies Evolution Shows the Limits of Mutagenic Antiviral Treatments" International Journal of Molecular Sciences 24, no. 24: 17185. https://doi.org/10.3390/ijms242417185






