Evaluation of the Antitumor Activity of Quaternary Ammonium Surfactants
Abstract
:1. Introduction
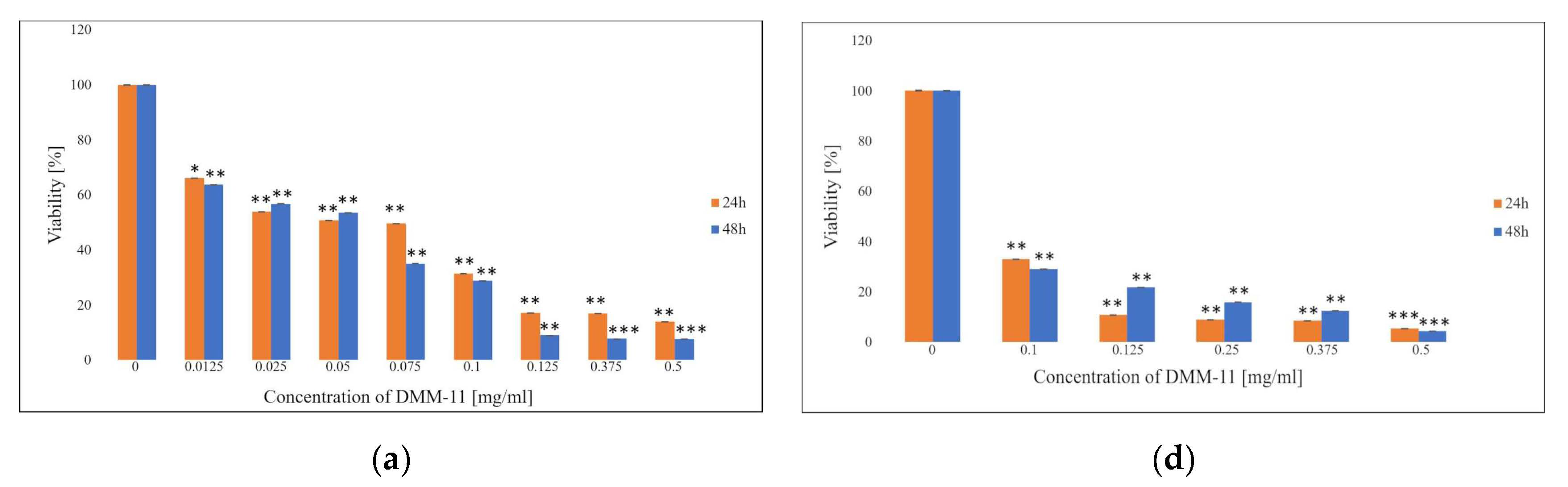
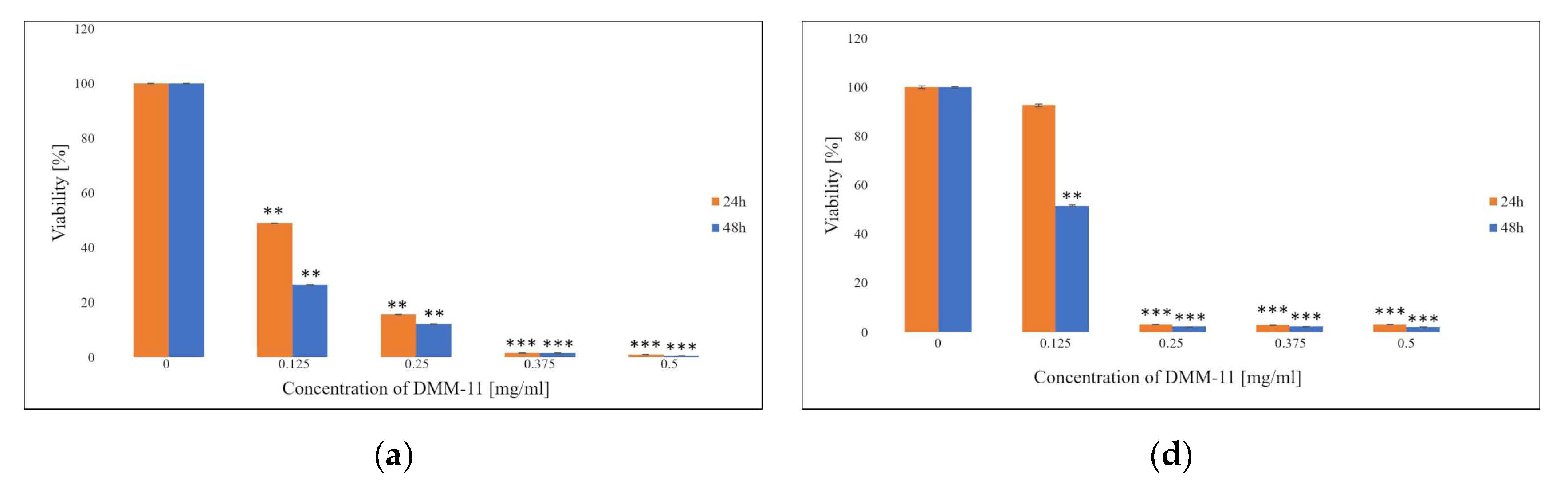
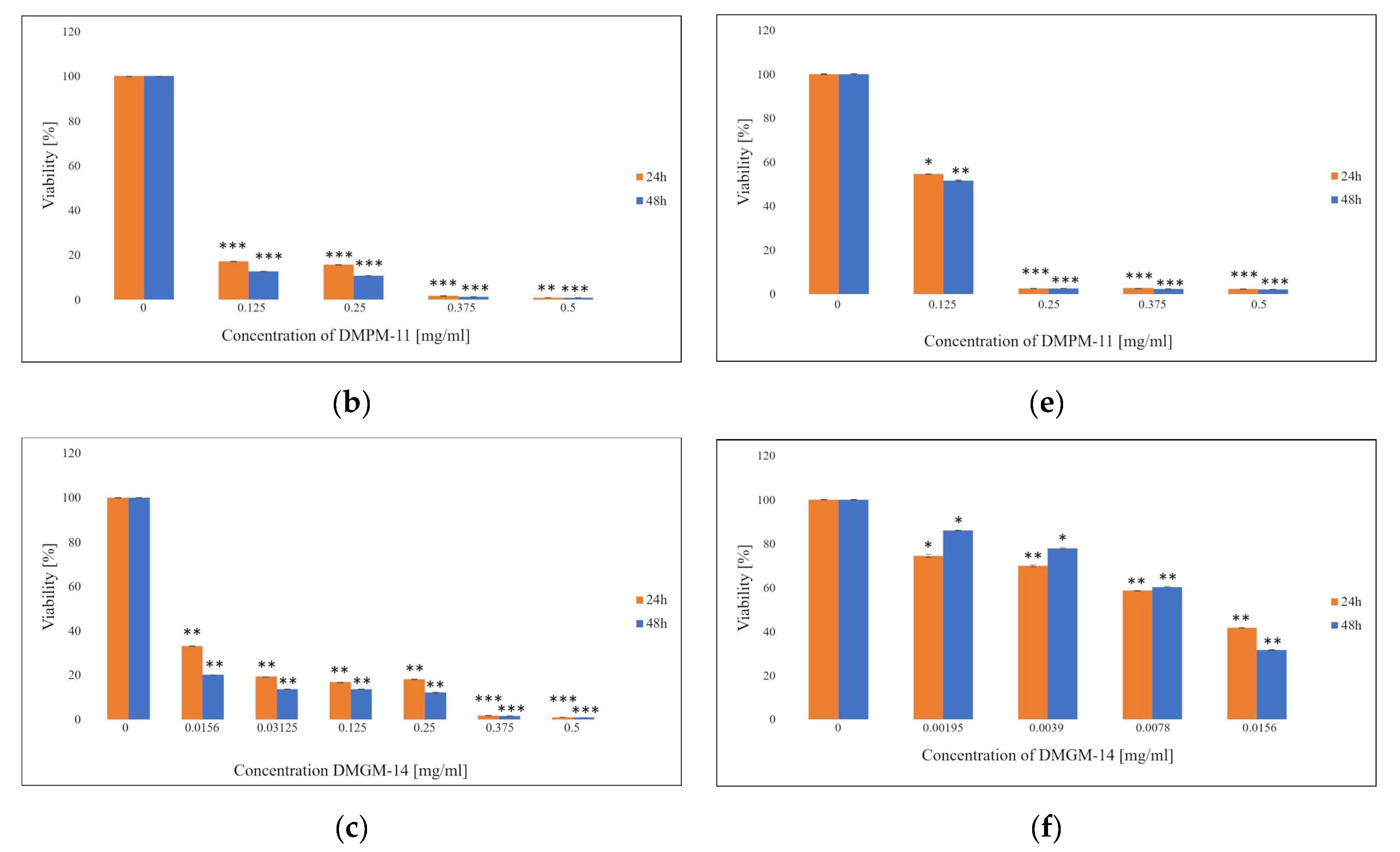
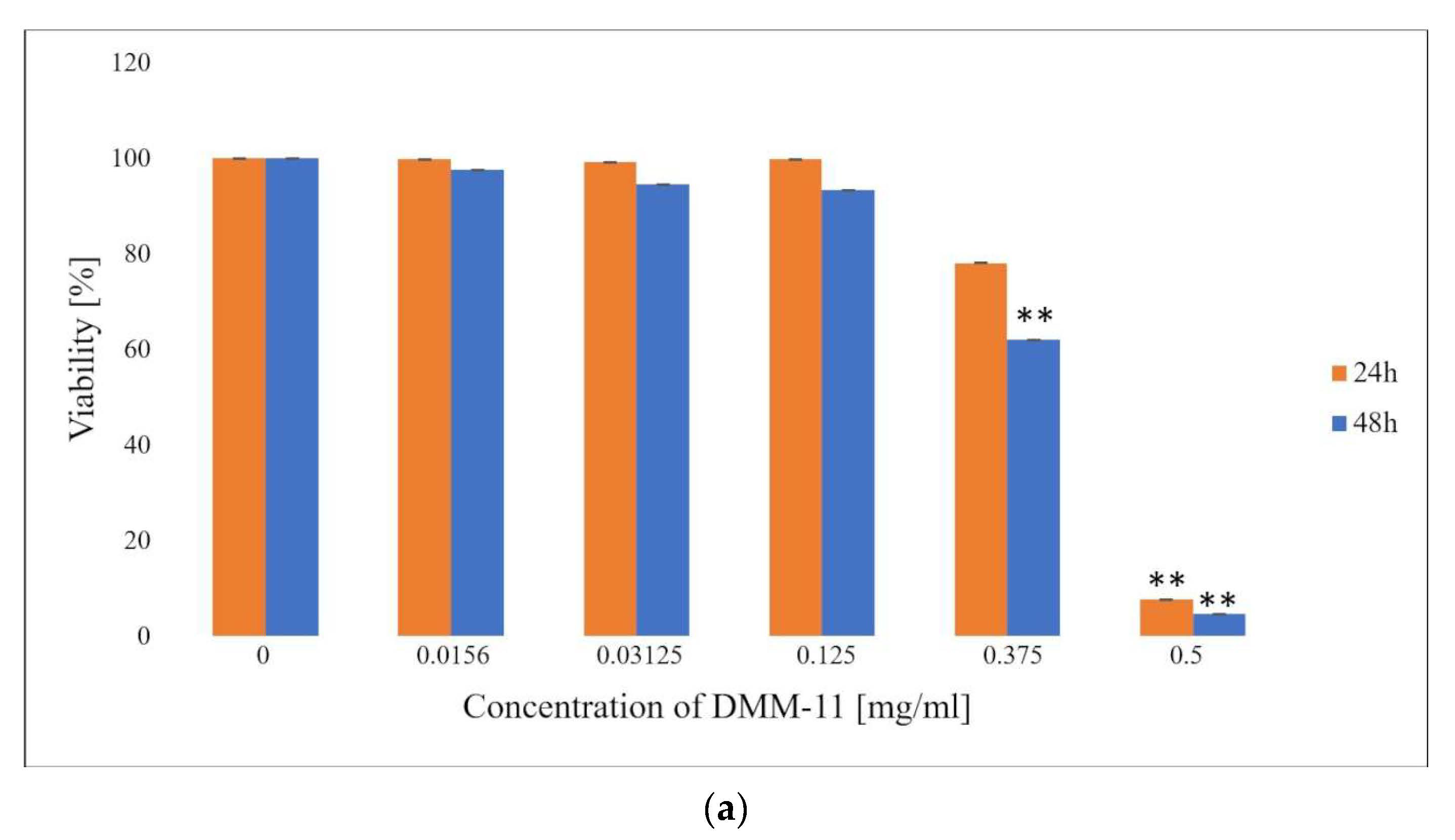
2. Results and Discussion
2.1. MTT Assay
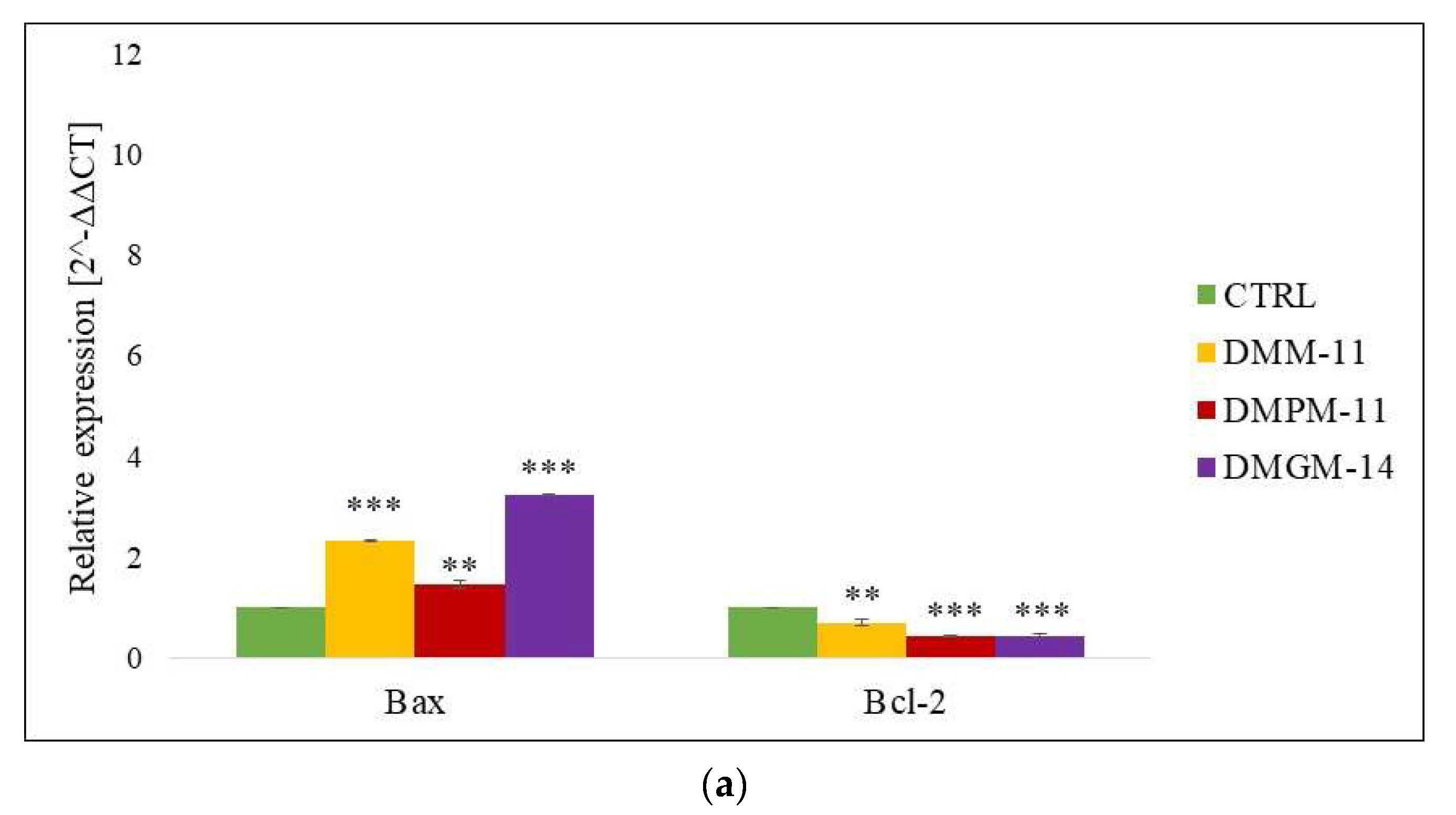
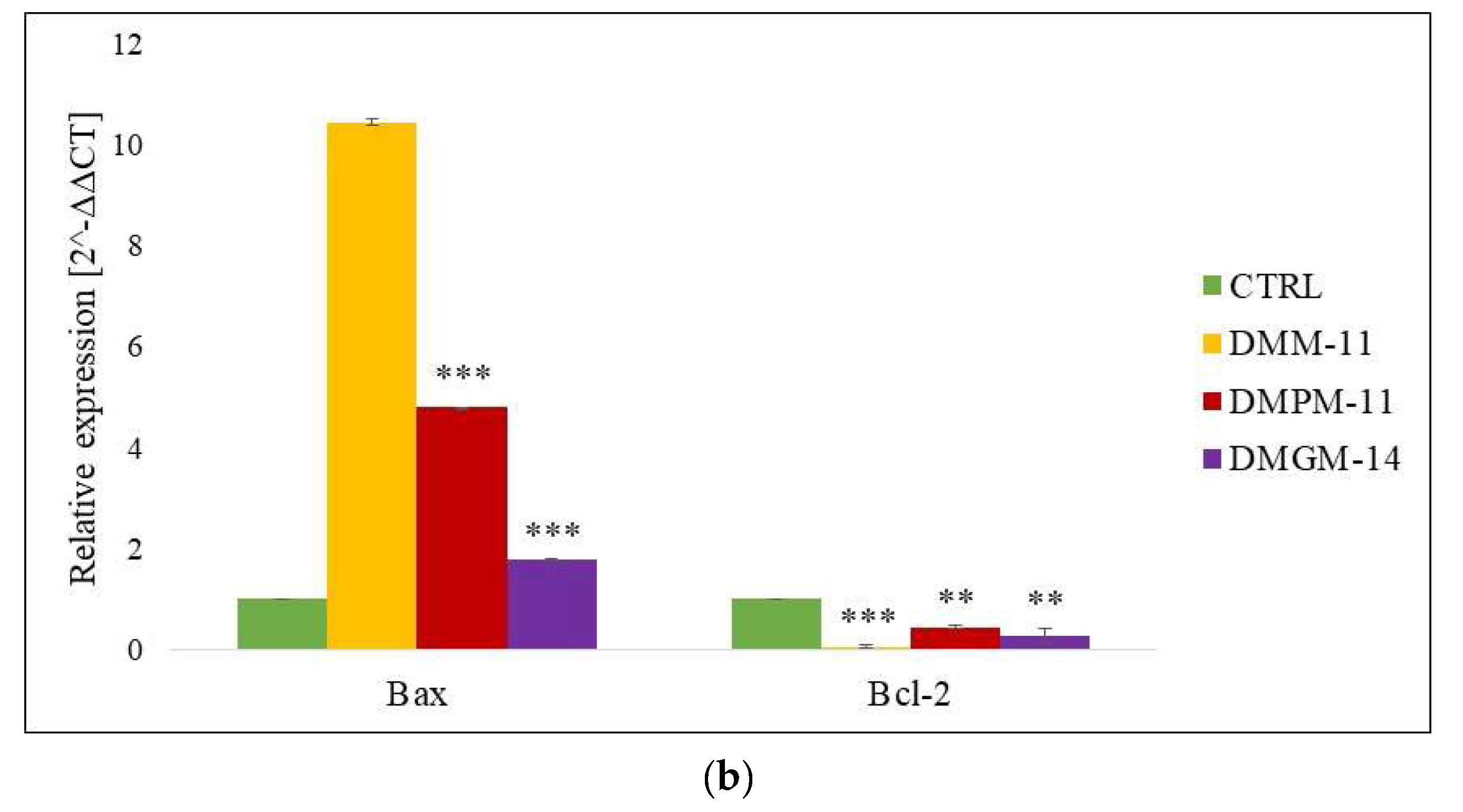
2.2. Gene Expression (RT-qPCR)
2.3. Migration—Scratch Assay
3. Materials and Methods
3.1. Cell Culture Conditions
3.2. Cell Treatment
3.3. Preparation of Liposomes
3.4. MTT Assay
3.5. RNA Isolation and Transcript Quantification
3.6. Migration—Scratch Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, M.-H.; Kim, Y.-T.; Park, S.J. Dieckol Inhibits Autophagic Flux and Induces Apoptotic Cell Death in A375 Human Melanoma Cells via Lysosomal Dysfunction and Mitochondrial Membrane Impairment. Int. J. Mol. Sci. 2022, 23, 14149. [Google Scholar] [CrossRef]
- Chang, C.-K.; Chu, S.-C.; Huang, J.-Y.; Chen, P.-N.; Hsieh, Y.-S. Terminalia Catappa Leaf Extracts Inhibited Metastasis of A2058 and A375 Melanoma Cells via Downregulating P-Src and β-Catenin Pathway in Vitro. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF Kinases for Cancer Therapy: BRAF-Mutated Melanoma and Beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, S.; Pi, D.; Wu, Y.; Zuo, Q.; Li, C.; Ouyang, M. Luteolin Induces Pyroptosis in HT-29 Cells by Activating the Caspase1/Gasdermin D Signalling Pathway. Front. Pharmacol. 2022, 13, 952587. [Google Scholar] [CrossRef]
- Gholipour, F.; Amini, M.; Baradaran, B.; Mokhtarzadeh, A.; Eskandani, M. Anticancer Properties of Curcumin-Treated Lactobacillus Plantarum against the HT-29 Colorectal Adenocarcinoma Cells. Sci. Rep. 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Villota, H.; Santa-González, G.A.; Uribe, D.; Henao, I.C.; Arroyave-Ospina, J.C.; Barrera-Causil, C.J.; Pedroza-Díaz, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Halaby, R. Influence of Lysosomal Sequestration on Multidrug Resistance in Cancer Cells. Cancer Drug Resist. 2019, 2, 31–42. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Toprak, M.; Noory, M.A.; Robertson, G.P. Effect of Lysosomotropic Molecules on Cellular Homeostasis. Pharmacol. Res. 2017, 117, 177–184. [Google Scholar] [CrossRef]
- Garcia, E.A.; Bhatti, I.; Henson, E.S.; Gibson, S.B. Prostate Cancer Cells Are Sensitive to Lysosomotropic Agent Siramesine through Generation Reactive Oxygen Species and in Combination with Tyrosine Kinase Inhibitors. Cancers 2022, 14, 5478. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Du, Y.; Fu, S.; Song, C.; Zhi, D.; Zhao, Y.; Chen, H.; Zhang, S.; Zhang, S. Novel Carbamate-Linked Quaternary Ammonium Lipids Containing Unsaturated Hydrophobic Chains for Gene Delivery. Bioorg. Med. Chem. 2018, 26, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Dan, W.; Gao, J.; Qi, X.; Wang, J.; Dai, J. Antibacterial Quaternary Ammonium Agents: Chemical Diversity and Biological Mechanism. Eur. J. Med. Chem. 2022, 243, 114765. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino Acid–Based Surfactants: New Antimicrobial Agents. Adv. Colloid. Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Hrubec, T.C.; Seguin, R.P.; Xu, L.; Cortopassi, G.A.; Datta, S.; Hanlon, A.L.; Lozano, A.J.; McDonald, V.A.; Healy, C.A.; Anderson, T.C.; et al. Altered Toxicological Endpoints in Humans from Common Quaternary Ammonium Compound Disinfectant Exposure. Toxicol. Rep. 2021, 8, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and Characterization of Novel Polymerizable Bis-Quaternary Ammonium Dimethacrylate Monomers with Antibacterial Activity as an Efficient Adhesive System for Dental Restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Markowski, A.; Zaremba-Czogalla, M.; Jaromin, A.; Olczak, E.; Zygmunt, A.; Etezadi, H.; Boyd, B.J.; Gubernator, J. Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation. Pharmaceutics 2023, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Lipka, D.; Gubernator, J.; Filipczak; Barnert, S.; Süss, R.; Kozubek, M.; Legut, M. Vitamin C-Driven Epirubicin Loading into Liposomes. Int. J. Nanomed. 2013, 2013, 3573–3585. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania Infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin Liposomes Ameliorate Streptozotocin-Induced Diabetic Nephropathy in Diabetic Rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [PubMed]
- Inácio, Â.S.; Costa, G.N.; Domingues, N.S.; Santos, M.S.; Moreno, A.J.M.; Vaz, W.L.C.; Vieira, O.V. Mitochondrial Dysfunction Is the Focus of Quaternary Ammonium Surfactant Toxicity to Mammalian Epithelial Cells. Antimicrob. Agents Chemother. 2013, 57, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, R.; Suzuki, C.; Ohno, M.; Ohasi, T.; Futagami, R.; Ishikawa, K.; Komae, M.; Nishino, T.; Konishi, Y.; Lee, E. Cationic Surfactants Induce Apoptosis in Normal and Cancer Cells. Ann. N. Y Acad. Sci. 2007, 1095, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schonewolf, C.A.; Mehta, M.; Schiff, D.; Wu, H.; Haffty, B.G.; Karantza, V.; Jabbour, S.K. Autophagy Inhibition by Chloroquine Sensitizes HT-29 Colorectal Cancer Cells to Concurrent Chemoradiation. World J. Gastrointest. Oncol. 2014, 6, 74. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Y.; Na, K.; Wang, Y.; Wang, L.; Li, Z.; Guo, C.; Guo, D.; Wang, X. Autophagic Flux Disruption Contributes to Ganoderma Lucidum Polysaccharide-Induced Apoptosis in Human Colorectal Cancer Cells via MAPK/ERK Activation. Cell Death Dis. 2019, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chiu, C.; Chen, C.; Wang, H.D. 7-Hydroxydehydronuciferine Induces Human Melanoma Death via Triggering Autophagy and Apoptosis. Exp. Dermatol. 2015, 24, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Jurek, I.; Szuplewska, A.; Chudy, M.; Wojciechowski, K. Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models. Molecules 2021, 26, 5628. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active Methods of Drug Loading into Liposomes: Recent Strategies for Stable Drug Entrapment and Increased in Vivo Activity. Expert. Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef]
- Rojewska, M.; Prochaska, K.; Olejnik, A.; Rychlik, J. Adsorption Properties of Biologically Active Derivatives of Quaternary Ammonium Surfactants and Their Mixtures at Aqueous/Air Interface II. Dynamics of Adsorption, Micelles Dissociation and Cytotoxicity of QDLS. Colloids Surf. B Biointerfaces 2014, 119, 154–161. [Google Scholar] [CrossRef]
- Salajkova, S.; Sramek, M.; Malinak, D.; Havel, F.; Musilek, K.; Benkova, M.; Soukup, O.; Vasicova, P.; Prchal, L.; Dolezal, R.; et al. Highly Hydrophilic Cationic Gold Nanorods Stabilized by Novel Quaternary Ammonium Surfactant with Negligible Cytotoxicity. J. Biophotonics 2019, 12, e201900024. [Google Scholar] [CrossRef]
- Khowdairy, M.M.; Mohamed, M.Z.; Mohamed, A.S. Surface and Biological Activity of Some Prepared Quaternary Ammonium Surfactants. J. Dispers. Sci. Technol. 2011, 32, 760–769. [Google Scholar] [CrossRef]
- Rao, R.; Balusu, R.; Fiskus, W.; Mudunuru, U.; Venkannagari, S.; Chauhan, L.; Smith, J.E.; Hembruff, S.L.; Ha, K.; Atadja, P.; et al. Combination of Pan-Histone Deacetylase Inhibitor and Autophagy Inhibitor Exerts Superior Efficacy against Triple-Negative Human Breast Cancer Cells. Mol. Cancer Ther. 2012, 11, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nozawa, H.; Hiyoshi, M.; Tada, N.; Murono, K.; Nirei, T.; Emoto, S.; Kishikawa, J.; Iida, Y.; Sunami, E.; et al. Temsirolimus and Chloroquine Cooperatively Exhibit a Potent Antitumor Effect against Colorectal Cancer Cells. J. Cancer Res. Clin. Oncol. 2014, 140, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Tokay, E. Determination of Cytotoxic Effect and Expression Analyses of Apoptotic and Autophagic Related Genes in Thymoquinone-Treated Colon Cancer Cells. Sakarya Üniversitesi Fen. Bilimleri Enstitüsü Dergisi 2020, 24, 189–196. [Google Scholar] [CrossRef]
- Tajmohammadi, I.; Mohammadian, J.; Sabzichi, M.; Mahmuodi, S.; Ramezani, M.; Aghajani, M.; Ramezani, F. Identification of Nrf2/STAT3 Axis in Induction of Apoptosis through Sub-G 1 Cell Cycle Arrest Mechanism in HT-29 Colon Cancer Cells. J. Cell Biochem. 2019, 120, 14035–14043. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Chiang, Y.-C.; Vudhya Gowrisankar, Y.; Lin, K.-Y.; Huang, S.-T.; Shrestha, S.; Chang, G.-R.; Yang, H.-L. The In Vitro and In Vivo Anticancer Properties of Chalcone Flavokawain B through Induction of ROS-Mediated Apoptotic and Autophagic Cell Death in Human Melanoma Cells. Cancers 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, J.; Zhang, J.; Liu, N.; Yan, B.; Tang, L.; Chen, X.; Peng, C. Novel Chloroquine Derivative Suppresses Melanoma Cell Growth by DNA Damage through Increasing ROS Levels. J. Cell Mol. Med. 2022, 26, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Khodavirdipour, A.; Zarean, R.; Safaralizadeh, R. Evaluation of the Anti-Cancer Effect of Syzygium Cumini Ethanolic Extract on HT-29 Colorectal Cell Line. J. Gastrointest. Cancer 2021, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, Z.; Zuo, C.; Fang, M. Promotion Effects of Mono-2-ethyhexyl Phthalate (MEHP) on Migration and Invasion of Human Melanoma Cells via Activation of TGF-β Signals. Cell Biochem. Funct. 2020, 38, 38–46. [Google Scholar] [CrossRef]
- Aldaghi, S.A.; Jalal, R. Concentration-Dependent Dual Effects of Ciprofloxacin on SB-590885-Resistant BRAF V600E A375 Melanoma Cells. Chem. Res. Toxicol. 2019, 32, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Czyżnikowska, Ż.; Łuczyński, J.; Gudiña, E.J.; Rodrigues, L.R.; Gałęzowska, J. Physicochemical Study of Biomolecular Interactions between Lysosomotropic Surfactants and Bovine Serum Albumin. Colloids Surf. B Biointerfaces 2017, 159, 750–758. [Google Scholar] [CrossRef]
- Janek, T.; Sałek, K.; Burger, J.; Czyżnikowska, Ż.; Euston, S.R. Investigating the Biomolecular Interactions between Model Proteins and Glycine Betaine Surfactant with Reference to the Stabilization of Emulsions and Antimicrobial Properties. Colloids Surf. B Biointerfaces 2020, 194, 111226. [Google Scholar] [CrossRef] [PubMed]







| Size [nm] | Average Size [nm] | SD | |||
|---|---|---|---|---|---|
| DMM-11 | 206.8 | 196.7 | 183.2 | 195.56 | 11.84 |
| DMPM-11 | 176.8 | 165.7 | 163.2 | 168.56 | 7.23 |
| DMGM-14 | 95.10 | 94.27 | 93.63 | 94.33 | 0.73 |
| PDI | Average Value | SD | |||
|---|---|---|---|---|---|
| DMM-11 | 0.2512 | 0.2244 | 0.2456 | 0.2404 | 0.0115 |
| DMPM-11 | 0.2127 | 0.2081 | 0.2080 | 0.2096 | 0.0021 |
| DMGM-14 | 0.1091 | 0.07784 | 0.1057 | 0.0975 | 0.0140 |
| HT-29 | ||
| β-actin | Forward | 5′CTGTCTGGCGGCACCACCAT3′ |
| Reverse | 5′GCAACTAAGTCATAGTCCGC3′ | |
| Bax | Forward | 5′AAGCTGAGCGAGTGTCTCAAGCGC3′ |
| Reverse | 5′TCCCGCCACAAAGATGGTCACG3′ | |
| Bcl-2 | Forward | 5′ATGGCAGCAGTAAAGCAAGCGC3′ |
| Reverse | 5′TTCTCCTGGTGGCAATGGCG3′ | |
| A375 | ||
| GAPDH | Forward | 5′CAAGGTCATCCATGACAACTTTG3′ |
| Reverse | 5′GTCCACCACCCTGTTGCTGTAG3′ | |
| Bax | Forward | 5′CAGAACTGGACAGTAACATGGAG3′ |
| Reverse | 5′CAGTTTGCTGGCAAAGTAGAAAAG3′ | |
| Bcl-2 | Forward | 5′ATGTGTGTGGAGAGCGTCAA3′ |
| Reverse | 5′GAGACAGCCAGGACAAATCAA3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyla, K.; Jama, D.; Grzywacz, A.; Janek, T. Evaluation of the Antitumor Activity of Quaternary Ammonium Surfactants. Int. J. Mol. Sci. 2023, 24, 17237. https://doi.org/10.3390/ijms242417237
Hyla K, Jama D, Grzywacz A, Janek T. Evaluation of the Antitumor Activity of Quaternary Ammonium Surfactants. International Journal of Molecular Sciences. 2023; 24(24):17237. https://doi.org/10.3390/ijms242417237
Chicago/Turabian StyleHyla, Kinga, Dominika Jama, Aleksandra Grzywacz, and Tomasz Janek. 2023. "Evaluation of the Antitumor Activity of Quaternary Ammonium Surfactants" International Journal of Molecular Sciences 24, no. 24: 17237. https://doi.org/10.3390/ijms242417237






