Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation
Abstract
:1. Cancer Stem Cell Properties
1.1. Biological Characteristics of CSCs
1.2. CSC and Hypoxia
1.3. CSC Repopulation and Resistance to Therapy
2. Repopulation during Radiotherapy
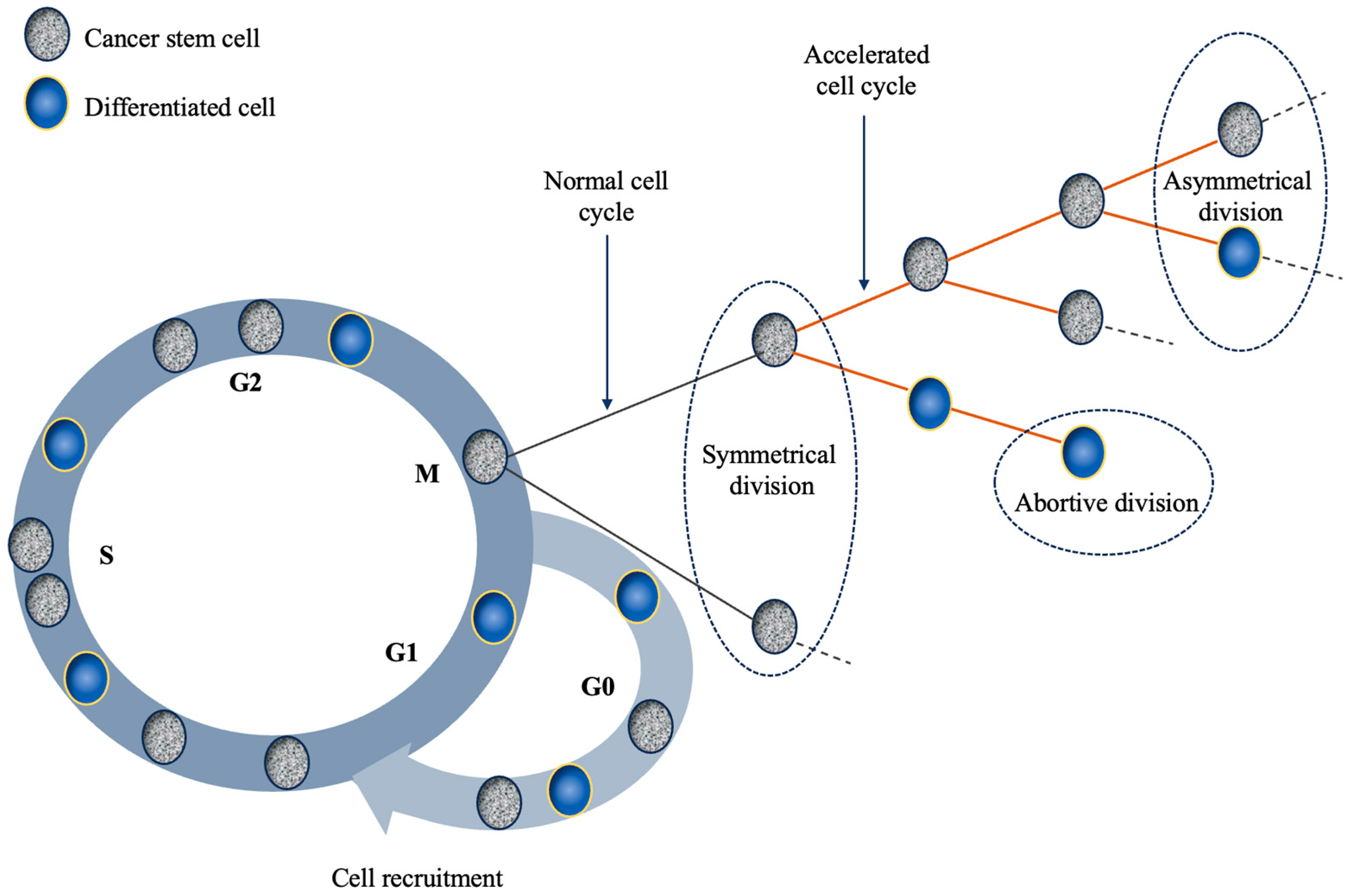
2.1. Cancer Stem Cells and Their Role in Repopulation during Radiotherapy
2.2. The Effect of Radiotherapy on CSCs
2.3. Strategies to Inhibit Tumour Regrowth after Radiotherapy and Future Perspectives
3. Repopulation during Chemotherapy
3.1. Cancer Stem Cells and Their Role in Repopulation during Chemotherapy
3.2. Clinical Evidence Concerning the Impact of CSC Repopulation on Treatment Outcome
3.3. CSC Sensitivity Assay-Guided Chemotherapy
3.4. CSC-Targeting Agents and Mechanisms
4. Future Avenues in CSC Management
4.1. Imaging
4.2. Theranostics
4.3. Nanotheranostics
4.4. Machine Learning
5. Final Thoughts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef]
- Reid, P.; Marcu, L.G.; Olver, I.; Moghaddasi, L.; Staudacher, A.H.; Bezak, E. Diversity of cancer stem cells in head and neck carcinomas: The role of HPV in cancer stem cell heterogeneity, plasticity and treatment response. Radiother. Oncol. 2019, 135, 1–12. [Google Scholar] [CrossRef]
- Gong, L.P.; Chen, J.N.; Dong, M.; Xiao, Z.D.; Feng, Z.Y.; Pan, Y.H.; Zhang, Y.; Du, Y.; Zhang, J.Y.; Bi, Y.H.; et al. Epstein-Barr virus-derived circular RNA LMP2A induces stemness in EBV-associated gastric cancer. EMBO Rep. 2020, 21, e49689. [Google Scholar] [CrossRef]
- Yasui, M.; Kunita, A.; Numakura, S.; Uozaki, H.; Ushiku, T.; Fukayama, M. Cancer stem cells in Epstein-Barr virus-associated gastric carcinoma. Cancer Sci. 2020, 111, 2598–2607. [Google Scholar] [CrossRef]
- Al Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Borovski, T.; De Sousa EMelo, F.; Vermeulen, L.; Medema, J.P. Cancer stem cell niche: The place to be. Cancer Res. 2011, 71, 634–639. [Google Scholar] [CrossRef]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef]
- Paul, R.; Dorsey, J.F.; Fan, Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol. Ther. 2022, 231, 107985. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Withers, H.R. Treatment-Induced Accelerated Human Tumour Growth. Semin. Radiat. Oncol. 1993, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Dörr, W. Three A’s of repopulation during fractionated irradiation of squamous epithelia: Asymmetry loss, acceleration of stem-cell divisions and abortive divisions. Int. J. Radiat. Biol. 1997, 72, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; van Doorn, T.; Olver, I. Modelling of post irradiation accelerated repopulation in squamous cell carcinomas. Phys. Med. Biol. 2004, 49, 3676–3779. [Google Scholar] [CrossRef]
- Harriss-Phillips, W.M.; Bezak, E.; Yeoh, E.K. Monte Carlo radiotherapy simulations of accelerated repopulation and reoxygenation for hypoxic head and neck cancer. Br. J. Radiol. 2011, 84, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Levy, D. Role of symmetric and asymmetric division of stem cells in developing drug resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 16766–16771. [Google Scholar] [CrossRef]
- Withers, H.R.; Taylor, J.M.; Maciejewski, B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988, 27, 131–146. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Alhiyari, Y.; Phillips, T.M.; Bochkur Dratver, M.; Pajonk, F. Radiation-induced Notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 609–618. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, S.H.; Yang, J.H.; Yang, K.; Yi, J.M. Stem cell-like gene expression signature identified in ionizing radiation-treated cancer cells. Gene 2015, 572, 285–291. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Cleton-Jansen, A.M.; Gomes, C.M.F. Therapy-induced enrichment of cancer stem-like cells in solid human tumors: Where do we stand? Pharmacol. Res. 2018, 137, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells-Key Players in Tumour Relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Franken, P.; Fodde, R. Tumour-stroma interactions in colorectal cancer: Converging on beta-catenin activation and cancer stemness. Br. J. Cancer 2008, 98, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Urbano, M.A.; Grinan-Lison, C.; Marchal, J.A.; Nunez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Meng, Y.; Dekmezian, C.; Kim, K.; Pajonk, F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010, 12, R13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Luo, J.; Zhou, H. Radiotherapy targeting cancer stem cells “awakens” them to induce tumour relapse and metastasis in oral cancer. Int. J. Oral Sci. 2020, 12, 19. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef]
- Gao, X.; Sishc, B.J.; Nelson, C.B.; Hahnfeldt, P.; Bailey, S.M.; Hlatky, L. Radiation-Induced Reprogramming of Pre-Senescent Mammary Epithelial Cells Enriches Putative CD44(+)/CD24(-/low) Stem Cell Phenotype. Front. Oncol. 2016, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Dekmezian, C.; Pajonk, F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 2012, 30, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogenet 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Kurth, I.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Discovery of the cancer stem cell related determinants of radioresistance. Radiother. Oncol. 2013, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casal, R.; Bhattacharya, C.; Ganesh, N.; Bailey, L.; Basse, P.; Gibson, M.; Epperly, M.; Levina, V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 2013, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, X.A. Impact of tumor repopulation on radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 220–227. [Google Scholar] [CrossRef]
- Chiblak, S.; Tang, Z.; Campos, B.; Gal, Z.; Unterberg, A.; Debus, J.; Herold-Mende, C.; Abdollahi, A. Radiosensitivity of Patient-Derived Glioma Stem Cell 3-Dimensional Cultures to Photon, Proton, and Carbon Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 112–119. [Google Scholar] [CrossRef]
- Davis, A.J.; Tannock, J.F. Repopulation of tumour cells between cycles of chemotherapy: A neglected factor. Lancet Oncol. 2000, 1, 86–93. [Google Scholar] [CrossRef]
- Marcu, L.; Bezak, E. Modelling of tumour repopulation after chemotherapy. Australas. Phys. Eng. Sci. Med. 2010, 33, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G. Tumour repopulation and the role of abortive division in squamous cell carcinomas during chemotherapy. Cell Prolif. 2014, 47, 318–325. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Olver, I.; van Doorn, T. Tumour resistance to cisplatin: A modelling approach. Phys. Med. Biol. 2005, 50, 93–102. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumour microenvironment enriches the stemness features: The architectural event of therapy resistance and metastasis. Mol. Cancer 2022, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Fujii, M.; Takahashi, S.; Takano, A.; Nanki, K.; Matano, M.; Hanyu, H.; Saito, M.; Shimokawa, M.; Nishikori, S.; et al. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature 2022, 608, 784–794. [Google Scholar] [CrossRef]
- Xie, X.P.; Laks, D.R.; Sun, D.; Ganbold, M.; Wang, Z.; Pedraza, A.M.; Bale, T.; Tabar, V.; Brennan, C.; Zhou, X.; et al. Quiescent human glioblastoma cancer stem cells drive tumor initiation, expansion, and recurrence following chemotherapy. Dev. Cell 2022, 10, 32–46.e8. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.E.; Ren, X.C.; Wang, N.; Chen, X.J.; Wang, D.Y.; Zong, J.; Peng, Y.; Guo, Z.J.; Hu, J. Dose escalation of accelerated hypofractionated three-dimensional conformal radiotherapy (at 3 Gy/fraction) with concurrent vinorelbine and carboplatin chemotherapy in unresectable stage III non-small-cell lung cancer: A phase I trial. Radiat. Oncol. 2013, 8, 201. [Google Scholar] [CrossRef]
- Maguire, J.; Khan, I.; McMenemin, R.; O'Rourke, N.; McNee, S.; Kelly, V.; Peedell, C.; Snee, M. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur. J. Cancer. 2014, 50, 2939–2949. [Google Scholar] [CrossRef]
- Bütof, R.; Simon, M.; Löck, S.; Troost, E.G.C.; Appold, S.; Krause, M.; Baumann, M. PORTAF—Postoperative radiotherapy of non-small cell lung cancer: Accelerated versus conventional fractionation—Study protocol for a randomized controlled trial. Trials 2017, 18, 608. [Google Scholar] [CrossRef]
- Panteliadou, M.; Giatromanolaki, A.; Touloupidis, S.; Destouni, E.; Tsoutsou, P.G.; Pantelis, P.; Abatzoglou, I.; Sismanidou, K.; Koukourakis, M.I. Treatment of invasive bladder cancer with conformal hypofractionated accelerated radiotherapy and amifostine (HypoARC). Urol. Oncol. 2012, 30, 813–820. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, M.; Todaro, M.; Salvini, J.; Benfante, A.; Colorito, M.L.; D'Incecco, A.; Landi, L.; Apuzzo, T.; Rossi, E.; Sani, S.; et al. Cancer Stem Cells Sensitivity Assay (STELLA) in Patients with Advanced Lung and Colorectal Cancer: A Feasibility Study. PLoS ONE 2015, 10, e0125037. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Valluri, J.; Alberico, A.; Julien, T.; Mazagri, R.; Marsh, R.; Alastair, H.; Cortese, A.; Griswold, M.; Wang, W.; et al. Analysis of Chemopredictive Assay for Targeting Cancer Stem Cells in Glioblastoma Patients. Transl. Oncol. 2017, 10, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, T.; Howard, C.M.; Yu, A.; Xu, L.; Aziz, K.; Jho, D.; Leonardo, J.; Hameed, M.A.; Karlovits, S.M.; Wegner, R.E.; et al. Cancer Stem Cell Chemotherapeutics Assay for Prospective Treatment of Recurrent Glioblastoma and Progressive Anaplastic Glioma: A Single-Institution Case Series. Transl. Oncol. 2020, 13, 100755. [Google Scholar] [CrossRef]
- Ranjan, T.; Sengupta, S.; Glantz, M.J.; Green, R.M.; Yu, A.; Aregawi, D.; Chaudhary, R.; Chen, R.; Zuccarello, M.; Lu-Emerson, C.; et al. Cancer stem cell assay-guided chemotherapy improves survival of patients with recurrent glioblastoma in a randomized trial. Cell Rep. Med. 2023, 4, 101025. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Bush S 2nd Zgheib, N.B.; Lirette, S.T.; Cortese, A.; Mollo, A.; Valluri, J.; Claudio, P.P. Cancer Stem Cell Assay for the Treatment of Platinum-Resistant Recurrent Ovarian Cancer. HSOA J. Stem Cells Res. Dev. Ther. 2021, 7, 076. [Google Scholar] [PubMed]
- Yoon, C.; Park, D.J.; Schmidt, B.; Thomas, N.J.; Lee, H.J.; Kim, T.S.; Janjigian, Y.Y.; Cohen, D.J.; Yoon, S.S. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014, 20, 3974–3988. [Google Scholar] [CrossRef]
- Yoon, C.; Cho, S.J.; Aksoy, B.A.; Park, D.J.; Schultz, N.; Ryeom, S.W.; Yoon, S.S. Chemotherapy Resistance in Diffuse-Type Gastric Adenocarcinoma Is Mediated by RhoA Activation in Cancer Stem-Like Cells. Clin. Cancer Res. 2016, 22, 971–983. [Google Scholar] [CrossRef]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Cannot Target What Cannot Be Seen: Molecular Imaging of Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 1524. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Park, S.J.; Park, J.J.; Cheon, J.H.; Kim, W.H.; Kim, T.I. Metformin Suppresses Cancer Stem Cells through AMPK Activation and Inhibition of Protein Prenylation of the Mevalonate Pathway in Colorectal Cancer. Cancers 2020, 12, 2554. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.clinicaltrials.gov (accessed on 12 October 2023).
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Nacarelli, T.; Fukumoto, T.; Zundell, J.A.; Fatkhutdinov, N.; Jean, S.; Cadungog, M.G.; Borowsky, M.E.; Zhang, R. NAMPT Inhibition Suppresses Cancer Stem-like Cells Associated with Therapy-Induced Senescence in Ovarian Cancer. Cancer Res. 2020, 80, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Yoshida, Y.; Hara, E.; Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2023, 290, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef]
- Mahajan, A.; Goh, V.; Basu, S.; Vaish, R.; Weeks, A.J.; Thakur, M.H.; Cook, G.J. Bench to bedside molecular functional imaging in translational cancer medicine: To image or to imagine? Clin. Radiol. 2015, 70, 1060–1082. [Google Scholar] [CrossRef]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Imaging of Tumour Characteristics and Molecular Pathways With PET: Developments Over the Last Decade Toward Personalized Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1165–1182. [Google Scholar] [CrossRef]
- Hu, K.; Ma, X.; Xie, L.; Zhang, Y.; Hanyu, M.; Obata, H.; Zhang, L.; Nagatsu, K.; Suzuki, H.; Shi, R.; et al. Development of a Stable Peptide-Based PET Tracer for Detecting CD133-Expressing Cancer Cells. ACS Omega 2021, 7, 334–341. [Google Scholar] [CrossRef]
- Jin, Z.H.; Sogawa, C.; Furukawa, T.; Saito, Y.; Aung, W.; Fujibayashi, Y.; Saga, T. Basic studies on radioimmunotargeting of CD133-positive HCT116 cancer stem cells. Mol. Imaging 2012, 11, 445–450. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Wang, C.; Wang, M.; Wang, Y.; Ye, M.; Liu, Y. Peptide-based 68Ga-PET radiotracer for imaging CD133 expression in colorectal cancer. Nucl. Med. Commun. 2021, 42, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, F.; Liu, A.; Wang, L.; Wang, J.C.; Ren, L.; Liu, W.; Tu, Q.; Li, L.; Wang, J. Cancer stem cell labeling using poly(L-lysine)-modified iron oxide nanoparticles. Biomaterials 2012, 33, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kaol, G.D.; Tsourkas, A.; et al. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Oriuchi, N.; Aoki, M.; Ukon, N.; Washiyama, K.; Tan, C.; Shimoyama, S.; Nishijima, K.I.; Takahashi, K.; Ito, H.; Ikezoe, T.; et al. Possibility of cancer-stem-cell-targeted radioimmunotherapy for acute myelogenous leukemia using 211At-CXCR4 monoclonal antibody. Sci. Rep. 2020, 10, 6810. [Google Scholar] [CrossRef] [PubMed]
- Al-Ejeh, F.; Shi, W.; Miranda, M.; Simpson, P.T.; Vargas, A.C.; Song, S.; Wiegmans, A.P.; Swarbrick, A.; Welm, A.L.; Brown, M.P.; et al. Treatment of triple-negative breast cancer using anti-EGFR-directed radioimmunotherapy combined with radiosensitizing chemotherapy and PARP inhibitor. J. Nucl. Med. 2013, 54, 913–921. [Google Scholar] [CrossRef]
- Leyton, J.V.; Gao, C.; Williams, B.; Keating, A.; Minden, M.; Reilly, R.M. A radiolabeled antibody targeting CD123(+) leukemia stem cells—Initial radioimmunotherapy studies in NOD/SCID mice engrafted with primary human AML. Leuk. Res. Rep. 2015, 4, 55–59. [Google Scholar] [CrossRef]
- Jandl, T.; Revskaya, E.; Jiang, Z.; Harris, M.; Dorokhova, O.; Tsukrov, D.; Casadevall, A.; Dadachova, E. Melanoma stem cells in experimental melanoma are killed by radioimmunotherapy. Nucl. Med. Biol. 2013, 40, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Jin, X.; Qin, S.; Lan, X.; Chen, C.; Sun, X.; She, X.; Dong, C.; An, R. Radioimmunotherapy for CD133(+) colonic cancer stem cells inhibits tumor development in nude mice. Oncotarget 2017, 8, 44004–44014. [Google Scholar]
- Ghaderi, F.; Jokar, N.; Gholamrezanezhad, A.; Assadi, M.; Ahmadzadehfar, H. Toward radiotheranostics in cancer stem cells: A promising initial step for tumour eradication. Clin. Translat. Imag. 2021, 9, 561–578. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Seno, H.; Fukuoka, A.; Ueo, T.; Yamaga, Y.; Maruno, T.; Nakanishi, N.; Kanda, K.; Komekado, H.; Kawada, M.; et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet. 2013, 45, 98–103. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Zhao, H.; Qiao, G.; Liu, M.; Zhang, C.; Cui, D.; Ma, L. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018, 25, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Hadebe, B.; Sathekge, M.M.; Aldous, C.; Vorster, M. Current Status of 68Ga-Pentixafor in Solid Tumours. Diagnostics 2022, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [177Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Mancini, A.; Colapietro, A.; Vitale, F.; Vetuschi, A.; Pompili, S.; Rossi, G.; Marampon, F.; Richardson, P.J.; Patient, L.; et al. The novel CXCR4 antagonist, PRX177561, reduces tumor cell proliferation and accelerates cancer stem cell differentiation in glioblastoma preclinical models. Tumour Biol. 2017, 39, 1010428317695528. [Google Scholar] [CrossRef]
- Ladju, R.B.; Ulhaq, Z.S.; Soraya, G.V. Nanotheranostics: A powerful next-generation solution to tackle hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 176–187. [Google Scholar] [CrossRef]
- Kola, P.; Nagesh, P.K.B.; Roy, P.K.; Deepak, K.; Reis, R.L.; Kundu, S.C.; Mandal, M. Innovative nanotheranostics: Smart nanoparticles based approach to overcome breast cancer stem cells mediated chemo- and radioresistances. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1876. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Zhang, Z.; Tang, B.; Zhou, Z.; Chen, H. Nanoparticle-Based RNAi Therapeutics Targeting Cancer Stem Cells: Update and Prospective. Pharmaceutics 2021, 13, 2116. [Google Scholar] [CrossRef]
- Smiley, S.B.; Yun, Y.; Ayyagari, P.; Shannon, H.E.; Pollok, K.E.; Vannier, M.W.; Das, S.K.; Veronesi, M.C. Development of CD133 Targeting Multi-Drug Polymer Micellar Nanoparticles for Glioblastoma—In Vitro Evaluation in Glioblastoma Stem Cells. Pharm. Res. 2021, 38, 1067–1079. [Google Scholar] [CrossRef]
- Koh, D.M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E., Jr.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial intelligence and machine learning in cancer imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef]
- Zhang, Z.; Ishihata, H.; Maruyama, R.; Kasai, T.; Kameda, H.; Sugiyama, T. Deep Learning of Phase-Contrast Images of Cancer Stem Cells Using a Selected Dataset of High Accuracy Value Using Conditional Generative Adversarial Networks. Int. J. Mol. Sci. 2023, 24, 5323. [Google Scholar] [CrossRef]
- Yang, F.; Wan, Y.; Xu, L.; Wu, Y.; Shen, X.; Wang, J.; Lu, D.; Shao, C.; Zheng, S.; Niu, T.; et al. MRI-Radiomics Prediction for Cytokeratin 19-Positive Hepatocellular Carcinoma: A Multicenter Study. Front. Oncol. 2021, 11, 672126. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Chen, M.; Deng, W.; Bie, L.; Ma, Y.; Zhang, C.; Liu, K.; Shen, W.; Wang, S.; Yang, C.; et al. Characterization of gastric cancer stem-like molecular features, immune and pharmacogenomic landscapes. Brief. Bioinform. 2022, 23, bbab386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Man, Q.; Li, X.; Xie, Y.; Hou, X.; Wang, H.; Yan, J.; Wei, X.; Bai, W.; Liu, Z.; et al. Artificial intelligence-based comprehensive analysis of immune-stemness-tumor budding profile to predict survival of patients with pancreatic adenocarcinoma. Cancer Biol. Med. 2023, 20, 196–217. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcu, L.G.; Dell’Oro, M.; Bezak, E. Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation. Int. J. Mol. Sci. 2023, 24, 17258. https://doi.org/10.3390/ijms242417258
Marcu LG, Dell’Oro M, Bezak E. Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation. International Journal of Molecular Sciences. 2023; 24(24):17258. https://doi.org/10.3390/ijms242417258
Chicago/Turabian StyleMarcu, Loredana G., Mikaela Dell’Oro, and Eva Bezak. 2023. "Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation" International Journal of Molecular Sciences 24, no. 24: 17258. https://doi.org/10.3390/ijms242417258




