Relation of Plasma Catecholamine Concentrations and Myocardial Mitochondrial Respiratory Activity in Anesthetized and Mechanically Ventilated, Cardiovascular Healthy Swine
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Anesthesia and Surgery
4.2. Experimental Protocol
4.3. Measurements and Calculations
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witkowska, M.; Halawa, B. Beta-adrenergic receptors and catecholamines in acute myocardial infarction. Mater. Med. Pol. 1989, 21, 195–198. [Google Scholar] [PubMed]
- Merlet, P.; Delforge, J.; Syrota, A.; Angevin, E.; Mazière, B.; Crouzel, C.; Valette, H.; Loisance, D.; Castaigne, A.; Randé, J.L. Positron Emission Tomography with 11C CGP-12177 to Assess β-Adrenergic Receptor Concentration in Idiopathic Dilated Cardiomyopathy. Circulation 1993, 87, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.M.; Chen, L.; Slamowitz, D.; Rao, P.S. Effects of Continuous Positive Airway Pressure on Cardiac Output and Plasma Norepinephrine in Sedated Pigs. J. Crit. Care 1996, 11, 57–64. [Google Scholar] [CrossRef]
- Kribbs, S.B.; Merritt, W.M.; Clair, M.J.; Krombach, R.S.; Houck, W.V.; Dodd, M.G.; Mukherjee, R.; Spinale, F.G. Amlodipine Monotherapy, Angiotensin-Converting Enzyme Inhibition, and Combination Therapy with Pacing-Induced Heart Failure. Hypertension 1998, 31, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; de Gasparo, M.; Whitebread, S.; Hebbar, L.; Clair, M.J.; Melton, D.M.; Krombach, R.S.; Mukherjee, R.; Iannini, J.P.; O, S.J. Modulation of the Renin-Angiotensin Pathway through Enzyme Inhibition and Specific Receptor Blockade in Pacing-Induced Heart Failure: I. Effects on Left Ventricular Performance and Neurohormonal Systems. Circulation 1997, 96, 2385–2396. [Google Scholar] [CrossRef]
- King, M.K.; Coker, M.L.; Goldberg, A.; McElmurray, J.H.; Gunasinghe, H.R.; Mukherjee, R.; Zile, M.R.; O’Neill, T.P.; Spinale, F.G. Selective Matrix Metalloproteinase Inhibition with Developing Heart Failure: Effects on Left Ventricular Function and Structure. Circ. Res. 2003, 92, 177–185. [Google Scholar] [CrossRef]
- New, R.B.; Sampson, A.C.; King, M.K.; Hendrick, J.W.; Clair, M.J.; McElmurray, J.H.; Mandel, J.; Mukherjee, R.; de Gasparo, M.; Spinale, F.G. Effects of Combined Angiotensin II and Endothelin Receptor Blockade with Developing Heart Failure: Effects on Left Ventricular Performance. Circulation 2000, 102, 1447–1453. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef]
- Sorescu, D.; Griendling, K.K. Reactive Oxygen Species, Mitochondria, and NAD(P)H Oxidases in the Development and Progression of Heart Failure. Congest. Heart Fail. 2002, 8, 132–140. [Google Scholar] [CrossRef]
- Lemieux, H.; Semsroth, S.; Antretter, H.; Höfer, D.; Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int. J. Biochem. Cell Biol. 2011, 43, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Sharov, V.; Riddle, J.M.; Kono, T.; Lesch, M.; Goldstein, S. Mitochondrial Abnormalities in Myocardium of Dogs with Chronic Heart Failure. J. Mol. Cell. Cardiol. 1992, 24, 1333–1347. [Google Scholar] [CrossRef]
- Lefroy, D.C.; de Silva, R.; Choudhury, L.; Uren, N.G.; Crake, T.; Rhodes, C.G.; Lammertsma, A.A.; Boyd, H.; Patsalos, P.N.; Nihoyannopoulos, P. Diffuse Reduction of Myocardial Beta-Adrenoceptors in Hypertrophic Cardiomyopathy: A Study with Positron Emission Tomography. J. Am. Coll. Cardiol. 1993, 22, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The Sympathetic Nervous System in Heart Failure: Physiology, Pathophysiology, and Clinical Implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.M. Targeting Adrenergic Receptors in Metabolic Therapies for Heart Failure. Int. J. Mol. Sci. 2021, 22, 5783. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S.; et al. β1- and β2-Adrenergic-Receptor Subpopulations in Nonfailing and Failing Human Ventricular Myocardium: Coupling of Both Receptor Subtypes to Muscle Contraction and Selective β1-Receptor Down-Regulation in Heart Failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E. Beta-arenoceptors in cardiac disease. Pharmacol. Ther. 1993, 60, 405–430. [Google Scholar] [CrossRef]
- Gengo, P.J.; Bowling, N.; Wyss, V.L.; Hayes, J.S. Effects of Prolonged Phenylephrine Infusion on Cardiac Adrenoceptors and Calcium Channels. J. Pharmacol. Exp. Ther. 1988, 244, 100–105. [Google Scholar]
- Neri, M.; Cerretani, D.; Fiaschi, A.I.; Laghi, P.F.; Lazzerini, P.E.; Maffione, A.B.; Micheli, L.; Bruni, G.; Nencini, C.; Giorgi, G.; et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J. Cell. Mol. Med. 2007, 11, 156–170. [Google Scholar] [CrossRef]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: Protective effect of curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef]
- Sone, T.; Miyazaki, Y.; Ogawa, K.; Satake, T. Effects of Excessive Noradrenaline on Cardiac Mitochondrial Calcium Transport and Oxidative Phosphorylation. Jpn. Circ. J. 1984, 48, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.L.; Baroldi, G.; Pieper, G.M.; Clayton, F.C.; Eliot, R.S. Experimental catecholamine-induced myocardial necrosis. II. Temporal development of isoproterenol-induced contraction band lesions correlated with ECG, hemodynamic and biochemical changes. J. Mol. Cell. Cardiol. 1985, 17, 647–656. [Google Scholar] [CrossRef]
- Boillot, A.; Massol, J.; Maupoil, V.; Grelier, R.; Capellier, G.; Berthelot, A.; Barale, F. Alterations of myocardial and vascular adrenergic receptor-mediated responses in Escherichia coli-induced septic shock in the rat. Crit. Care Med. 1996, 24, 1373–1380. [Google Scholar] [CrossRef]
- Mizumachi, K.; Yahagi, M.; Kawabata, H.; Tezuka, S.; Honda, T.; Okada, K. Decreased Beta-Adrenergic Receptor Density in Rat Myocardium during Hemorrhagic Shock. J. Anesth. 1991, 5, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kolseth, S.M.; Wahba, A.; Kirkeby-Garstad, I.; Aro, S.; Nordgaard, H.; Høydal, M.; Rognmo, Ø.; Nordhaug, D. A dose-response study of levosimendan in a porcine model of acute ischaemic heart failure. Eur. J. Cardiothorac. Surg. 2012, 41, 1377–1383. [Google Scholar] [CrossRef]
- Rosca, M.G.; Vazquez, E.J.; Kerner, J.; Parland, W.; Chandler, M.P.; Stanley, W.; Sabbah, H.N.; Hoppel, C.L. Cardiac mitochondria in heart failure: Decrease in respirasomes and oxidative phosphorylation. Cardiovasc. Res. 2008, 80, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Münz, F.; Wolfschmitt, E.-M.; Zink, F.; Abele, N.; Hogg, M.; Hoffmann, A.; Gröger, M.; Calzia, E.; Waller, C.; Radermacher, P.; et al. Porcine blood cell and brain tissue energy metabolism: Effects of “early life stress”. Front. Mol. Biosci. 2023, 10, 1113570. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, K.-Y. Effect of air cleaner on stress hormones of pig and pork quality. J. Anim. Sci. Technol. 2021, 63, 892–903. [Google Scholar] [CrossRef]
- Basu, S. F2-Isoprostanes in Human Health and Diseases: From Molecular Mechanisms to Clinical Implications. Antioxid. Redox Signal. 2008, 10, 1405–1434. [Google Scholar] [CrossRef]
- Gajski, G.; Langie, S.; Zhanataev, A. Recent applications of the Comet Assay: A report from the International Comet Assay Workshop 2019. Toxicol. Lett. 2020, 333, 1–3. [Google Scholar] [CrossRef]
- Milić, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Møller, P.; Teixeira, J.P.; et al. The hCOMET project: International database comparison of results with the comet assay in human biomonitoring. Baseline frequency of DNA damage and effect of main confounders. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108371. [Google Scholar] [CrossRef]
- Ewelina, G.; Krzysztof, S.; Marek, M.; Krzysztof, K. Blood free Radicals Concentration Determined by Electron Paramagnetic Resonance Spectroscopy and Delayed Cerebral Ischemia Occurrence in Patients with Aneurysmal Subarachnoid Hemorrhage. Cell Biochem. Biophys. 2017, 75, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, D.; Faes, E.; Gielis, J.; van Craenenbroeck, E.; Cos, P.; Spaanderman, M.; Gyselaers, W.; Cornette, J.; Jacquemyn, Y. Oxidative stress and endothelial function in normal pregnancy versus pre-eclampsia, a combined longitudinal and case control study. BMC Pregnancy Childbirth 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Sorop, O.; van Dalen, B.M.; Wüst, R.C.I.; van de Wouw, J.; de Beer, V.J.; Octavia, Y.; van Duin, R.W.B.; Hoogstrate, Y.; Blonden, L.; et al. Cellular, mitochondrial and molecular alterations associate with early left ventricular diastolic dysfunction in a porcine model of diabetic metabolic derangement. Sci. Rep. 2020, 10, 13173. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, D.; Markova, M.; Horak, J.; Nalos, L.; Benes, J.; Al-Obeidallah, M.; Tuma, Z.; Sviglerova, J.; Kuncova, J.; Matejovic, M.; et al. Cellular Mechanisms of Myocardial Depression in Porcine Septic Shock. Front. Physiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Strømholm, T.; Aadahl, P.; Saether, O.; Myking, O.; Myhre, H. Excessive Increase in Circulating Catecholamines during Cross-clamping of the Descending Thoracic Aorta in Pigs. Int. J. Angiol. 1999, 8, 91–94. [Google Scholar] [CrossRef]
- Dalin, A.M.; Magnusson, U.; Häggendal, J.; Nyberg, L. The Effect of Transport Stress on Plasma Levels of Catecholamines, Cortisol, Corticosteroid-Binding Globulin, Blood Cell Count, and Lymphocyte Proliferation in Pigs. Acta Vet. Scand. 1993, 34, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Feng, B.; Ma, X.; Sun, K.; Xu, G.; Zhou, Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2019, 18, 107. [Google Scholar] [CrossRef]
- Spinale, F.G.; Mukherjee, R.; Krombach, R.S.; Clair, M.J.; Hendrick, J.W.; Houck, W.V.; Hebbar, L.; Kribbs, S.B.; Zellner, J.L.; Dodd, M.G. Chronic Amlodipine Treatment during the Development of Heart Failure. Circulation 1998, 98, 1666–1674. [Google Scholar] [CrossRef]
- Klemcke, H.G.; Nienaber, J.A.; Hahn, G.L. Plasma Adrenocorticotropic Hormone and Cortisol in Pigs: Effects of Time of Day on Basal and Stressor-Altered Concentrations. Proc. Soc. Exp. Biol. Med. 1989, 190, 42–53. [Google Scholar] [CrossRef]
- Waller, C.; Rhee, D.-S.; Gröger, M.; Rappel, M.; Maier, T.; Müller, M.; Rottler, E.; Nerz, K.; Nerz, C.; Brill, S.; et al. Social Stress-Induced Oxidative DNA Damage Is Related to Prospective Cardiovascular Risk. J. Clin. Med. 2020, 9, 3783. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Ind, P.W.; Brown, M.J. Plasma Histamine and Catecholamines in Stable Asthmatic Subjects. Clin. Sci. 1982, 62, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Gillberg, M.; Hjemdahl, P.; Sigurdson, K.; Gustavsson, I.; Daleskog, M.; Pollare, T. Comparison of urinary and plasma catecholamine responses to mental stress. Acta Physiol. Scand. 1983, 117, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, H.; Saito, I.; Hasegawa, C.; Nagano, S.; Saruta, T. Circulatory and Plasma Catecholamine Responses to Mental Stress in Young Subjects with Two Different Types of Hypertension. Angiology 1994, 45, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Boeck, C.; Koenig, A.M.; Schury, K.; Geiger, M.L.; Karabatsiakis, A.; Wilker, S.; Waller, C.; Gündel, H.; Fegert, J.M.; Calzia, E.; et al. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 2016, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Boeck, C.; Krause, S.; Karabatsiakis, A.; Schury, K.; Gündel, H.; Waller, C.; Kolassa, I.-T. History of child maltreatment and telomere length in immune cell subsets: Associations with stress- and attachment-related hormones. Dev. Psychopathol. 2018, 30, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Gumpp, A.M.; Behnke, A.; Ramo-Fernández, L.; Radermacher, P.; Gündel, H.; Ziegenhain, U.; Karabatsiakis, A.; Kolassa, I.-T. Investigating mitochondrial bioenergetics in peripheral blood mononuclear cells of women with childhood maltreatment from post-parturition period to one-year follow-up. Psychol. Med. 2023, 53, 3793–3804. [Google Scholar] [CrossRef]
- Karrasch, S.; Matits, L.; Bongartz, W.; Mavioğlu, R.N.; Gumpp, A.M.; Mack, M.; Tumani, V.; Behnke, A.; Steinacker, J.M.; Kolassa, I.-T. An exploratory study of hypnosis-induced blood count changes in chronically stressed individuals. Biol. Psychol. 2023, 178, 108527. [Google Scholar] [CrossRef]
- Karrasch, S.; Mavioğlu, R.N.; Matits, L.; Gumpp, A.M.; Mack, M.; Behnke, A.; Tumani, V.; Karabatsiakis, A.; Bongartz, W.; Kolassa, I.-T. Randomized controlled trial investigating potential effects of relaxation on mitochondrial function in immune cells: A pilot experiment. Biol. Psychol. 2023, 183, 108656. [Google Scholar] [CrossRef]
- Berthon, D.; Herpin, P.; Bertin, R.; de Marco, F.; le Dividich, J. Metabolic Changes Associated with Sustained 48-Hr Shivering Thermogenesis in the Newborn Pig. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 327–335. [Google Scholar] [CrossRef]
- Hiemstra, J.A.; Gutiérrez-Aguilar, M.; Marshall, K.D.; McCommis, K.S.; Zgoda, P.J.; Cruz-Rivera, N.; Jenkins, N.T.; Krenz, M.; Domeier, T.L.; Baines, C.P.; et al. A new twist on an old idea part 2: Cyclosporine preserves normal mitochondrial but not cardiomyocyte function in mini-swine with compensated heart failure. Physiol. Rep. 2014, 2, e12050. [Google Scholar] [CrossRef]
- Communal, C.; Ribuot, C.; Durand, A.; Demenge, P. Myocardial β-adrenergic reactivity in volume overload-induced cardiac hypertrophy in the rat. Fundam. Clin. Pharmacol. 1998, 12, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Naik, G.O.; Larosa, G. Myocardial β-adrenoceptors in pacing-induced heart failure: Regulation by enalapril? Can. J. Physiol. Pharmacol. 1994, 72, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.J.; Miller, M.; Peters, V.B.M.; Singer, M. The role of hormones in sepsis: An integrated overview with a focus on mitochondrial and immune cell dysfunction. Clin. Sci. (Lond) 2023, 137, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Kotarska, K.; Aboul-Enein, B.H. Physical Exercise and Catecholamines Response: Benefits and Health Risk—Possible Mechanisms. Free Radic. Res. 2020, 54, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.F.E.; Schierholz, J.; Klaus, W. Studies on the Cardiotoxicity of Noradrenaline in Isolated Rabbit Hearts. Arzneimittelforschung 2002, 52, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.F.; Klaus, W. Evidence for norepinephrine cardiotoxicity mediated by superoxide anion radicals in isolated rabbit hearts. Naunyn Schmiedeberg’s Arch. Pharmacol. 1994, 349, 295–300. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- McLamb, B.L.; Gibson, A.J.; Overman, E.L.; Stahl, C.; Moeser, A.J. Early Weaning Stress in Pigs Impairs Innate Mucosal Immune Responses to Enterotoxigenic E. coli Challenge and Exacerbates Intestinal Injury and Clinical Disease. PLoS ONE 2013, 8, e59838. [Google Scholar] [CrossRef]
- Moeser, A.J.; Ryan, K.A.; Nighot, P.K.; Blikslager, A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G413–G421. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [PubMed]
- Zink, F.; Vogt, J.; Wachter, U.; Hartert, J.; Horchler, M.; Zhang, X.; Hezel, F.; Kapapa, T.; Datzmann, T.; Hoffmann, A.; et al. Effects of acute subdural hematoma-induced brain injury on energy metabolism in peripheral blood mononuclear cells. Shock 2021, 55, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Ost, M.; Doerrier, C.; Gama-Perez, P.; Moreno-Gomez, S. Analysis of mitochondrial respiratory function in tissue biopsies and blood cells. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 336–342. [Google Scholar] [CrossRef]
- Speit, G.; Hartmann, A. The comet assay: A Sensitive Genotoxicity Test for the Detection of DNA Damage and Repair. Methods Mol. Biol. 2006, 314, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Michael, S.L.; Ault, S.G.; Pumford, N.R. Western Blot Analysis for Nitrotyrosine Protein Adducts in Livers of Saline-Treated and Acetaminophen-Treated Mice. Toxicol. Sci. 2000, 53, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Datzmann, T.; Kapapa, T.; Scheuerle, A.; McCook, O.; Merz, T.; Unmuth, S.; Hoffmann, A.; Mathieu, R.; Mayer, S.; Mauer, U.M.; et al. In-depth characterization of a long-term, resuscitated model of acute subdural hematoma-induced brain injury. J. Neurosurg. 2019, 134, 223–234. [Google Scholar] [CrossRef]
- Hartmann, C.; Loconte, M.; Antonucci, E.; Holzhauser, M.; Hölle, T.; Katzsch, D.; Merz, T.; McCook, O.; Wachter, U.; Vogt, J.A.; et al. Effects of Hyperoxia during Resuscitation from Hemorrhagic Shock in Swine with Preexisting Coronary Artery Disease. Crit. Care Med. 2017, 45, e1270–e1279. [Google Scholar] [CrossRef]
- Merz, T.; Lukaschewski, B.; Wigger, D.; Rupprecht, A.; Wepler, M.; Gröger, M.; Hartmann, C.; Whiteman, M.; Szabo, C.; Wang, R.; et al. Interaction of the hydrogen sulfide system with the oxytocin system in the injured mouse heart. Intensive Care Med. Exp. 2018, 6, 41. [Google Scholar] [CrossRef]
- Merz, T.; Denoix, N.; Wigger, D.; Waller, C.; Wepler, M.; Vettorazzi, S.; Tuckermann, J.; Radermacher, P.; McCook, O. The Role of Glucocorticoid Receptor and Oxytocin Receptor in the Septic Heart in a Clinically Relevant, Resuscitated Porcine Model with Underlying Atherosclerosis. Front. Endocrinol. (Lausanne) 2020, 11, 299. [Google Scholar] [CrossRef]
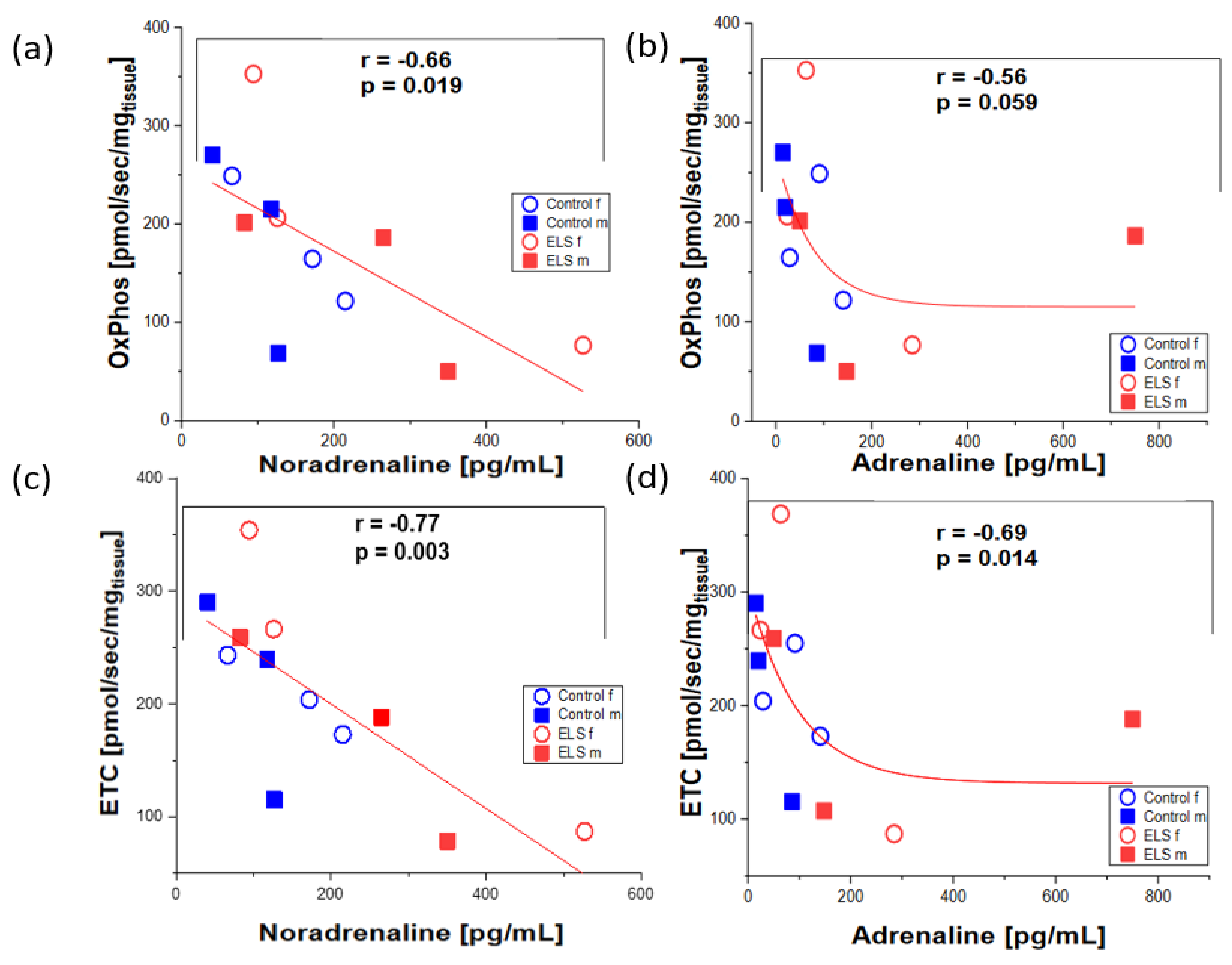
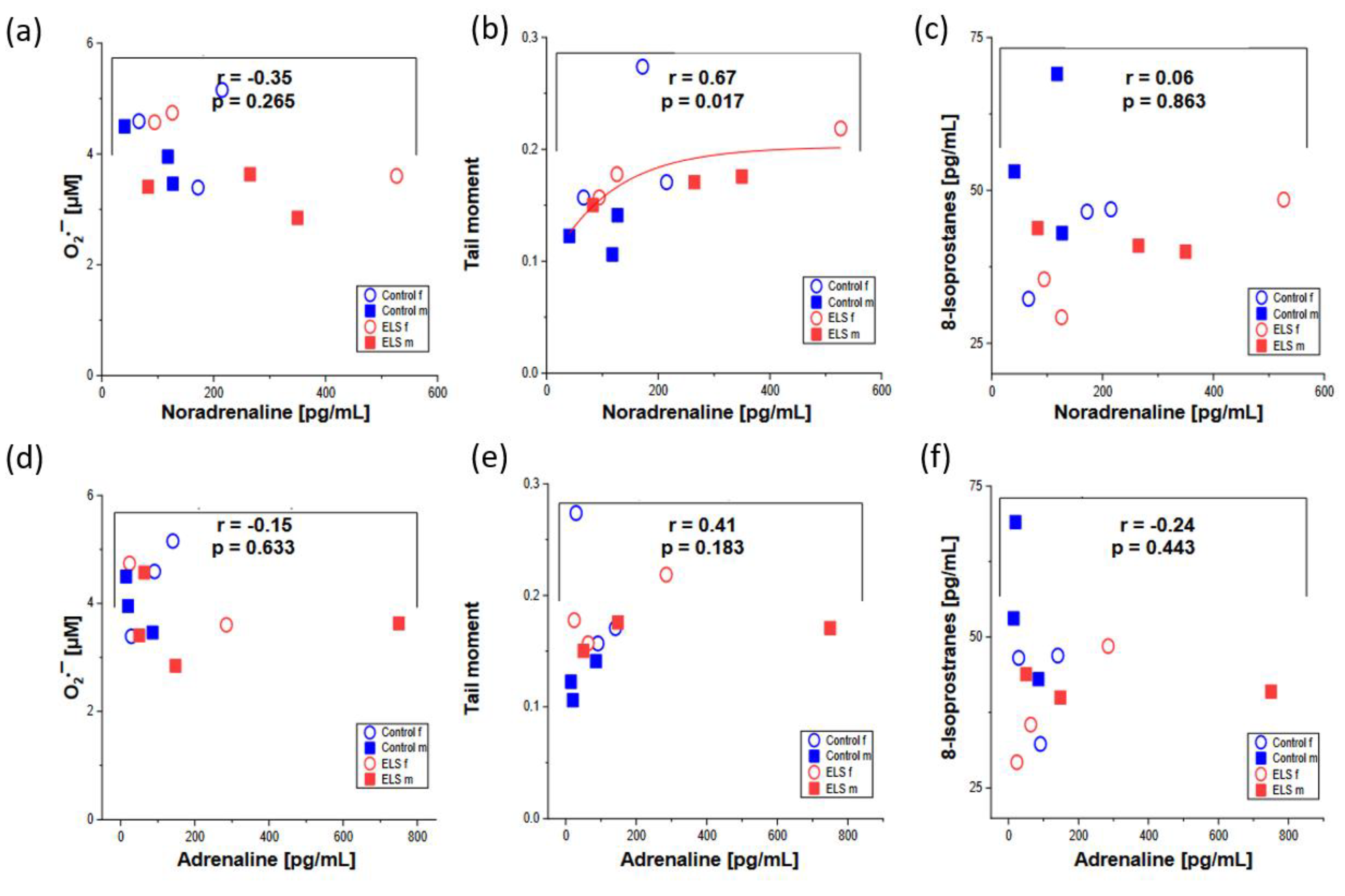
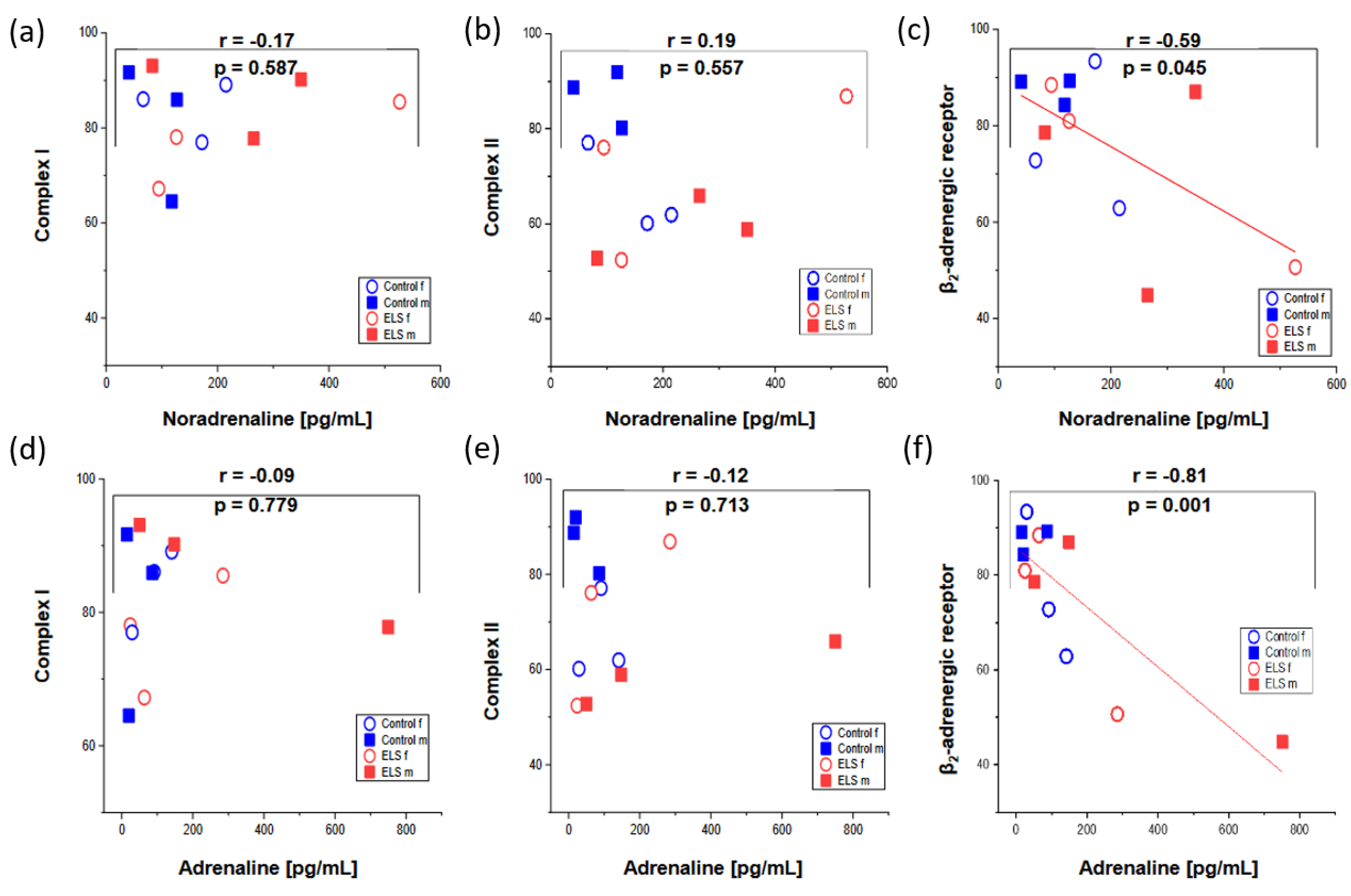
| Adrenaline [pg/mL] | Noradrenaline [pg/mL] | Isoprostane [pg/mL] | O2•− [μmol/L] | Tail Moment |
|---|---|---|---|---|
| 75 (28; 143) | 127 (92; 228) | 43 (39; 47) | 3.8 (3.5; 4.6) | 0.16 (0.15; 0.18) |
| JO2 -OxPhos [pmol/s/mgtissue] | JO2-ETC [pmol/s/mgtissue] | Complex I [%] | Complex II [%] | β2-Adrenoreceptor [%] |
|---|---|---|---|---|
| 194 (110; 223) | 222 (158; 261) | 86 (78; 89) | 71 (60; 82) | 83 (70; 89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abele, N.; Münz, F.; Zink, F.; Gröger, M.; Hoffmann, A.; Wolfschmitt, E.-M.; Hogg, M.; Calzia, E.; Waller, C.; Radermacher, P.; et al. Relation of Plasma Catecholamine Concentrations and Myocardial Mitochondrial Respiratory Activity in Anesthetized and Mechanically Ventilated, Cardiovascular Healthy Swine. Int. J. Mol. Sci. 2023, 24, 17293. https://doi.org/10.3390/ijms242417293
Abele N, Münz F, Zink F, Gröger M, Hoffmann A, Wolfschmitt E-M, Hogg M, Calzia E, Waller C, Radermacher P, et al. Relation of Plasma Catecholamine Concentrations and Myocardial Mitochondrial Respiratory Activity in Anesthetized and Mechanically Ventilated, Cardiovascular Healthy Swine. International Journal of Molecular Sciences. 2023; 24(24):17293. https://doi.org/10.3390/ijms242417293
Chicago/Turabian StyleAbele, Nadja, Franziska Münz, Fabian Zink, Michael Gröger, Andrea Hoffmann, Eva-Maria Wolfschmitt, Melanie Hogg, Enrico Calzia, Christiane Waller, Peter Radermacher, and et al. 2023. "Relation of Plasma Catecholamine Concentrations and Myocardial Mitochondrial Respiratory Activity in Anesthetized and Mechanically Ventilated, Cardiovascular Healthy Swine" International Journal of Molecular Sciences 24, no. 24: 17293. https://doi.org/10.3390/ijms242417293





