Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes
Abstract
:1. Introduction
2. Results
2.1. The Cytoprotective Effect of Fisetin and Inhibitory Effect of Fisetin on UVA-Induced MMP-1 and MMP-3 Expression
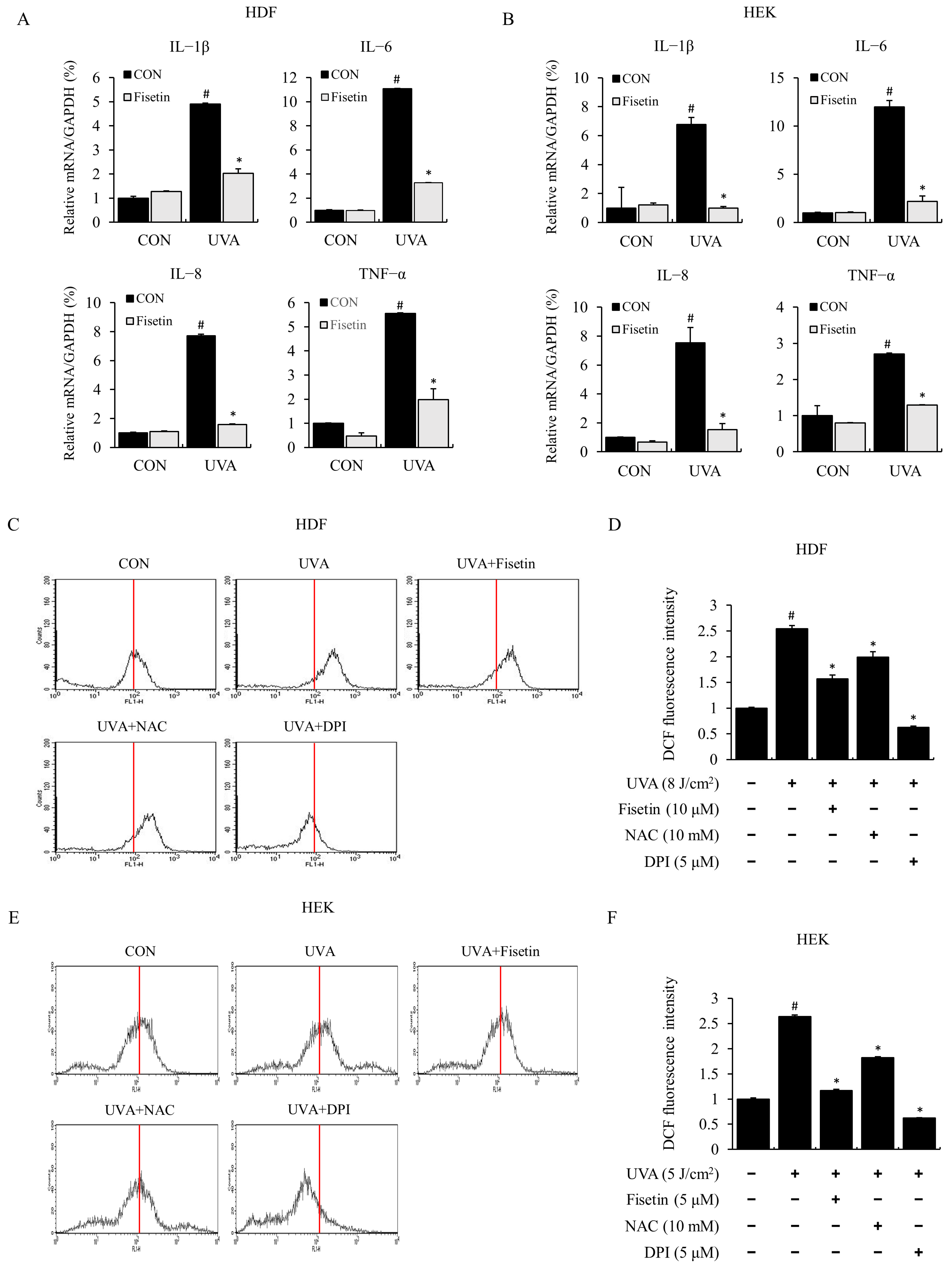
2.2. Fisetin Inhibited UVA-Induced Inflammatory Cytokine Expression and ROS
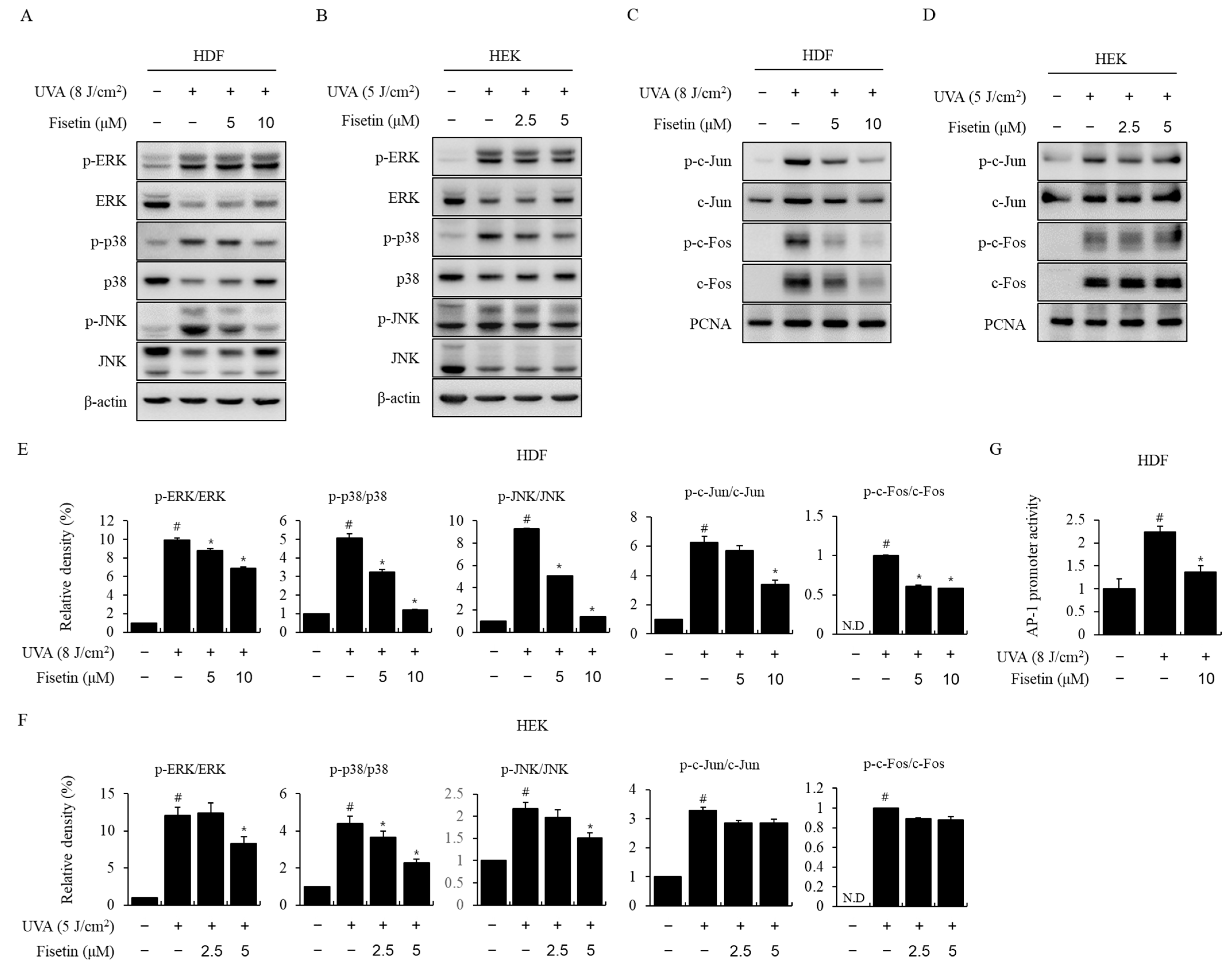
2.3. Fisetin Inhibited UVA-Induced MAPK/AP-1 Activation
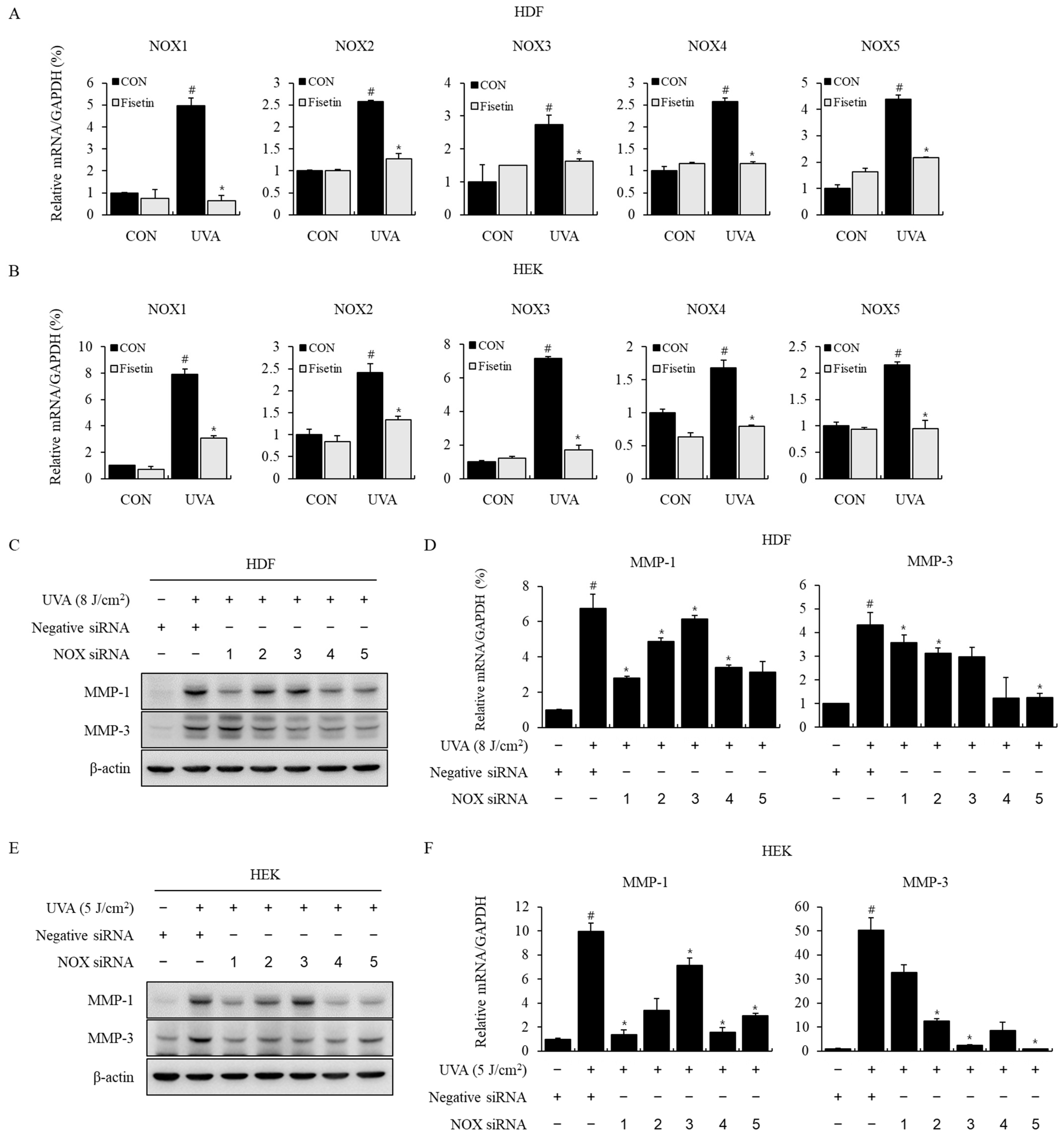
2.4. Effects of Fisetin on NOX Signaling Pathway and MMP-1, 3 Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. UV Irradiation
4.4. Cell Viability Assay
4.5. Preparation of Nuclear Extract and Western Blotting Analysis
4.6. Luciferase Assay
4.7. Quantification of Intracellular ROS
4.8. RNA Isolation and Quantitative Reverse Transcription PCR (RT-qPCR)
4.9. siRNA Transfection
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilchrest, B.A.; Yaar, M. Ageing and photoageing of the skin: Observations at the cellular and molecular level. Br. J. Dermatol. 1992, 127, 25–30. [Google Scholar] [CrossRef]
- Brozyna, A.; Zbytek, B.; Granese, J.; Carlson, J.A.; Ross, J.; Slominski, A. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev. Dermatol. 2007, 2, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Brożyna, A.A.; Janjetovic, Z.; Jeayeng, S.; Oak, A.S.W.; Kim, T.; Panich, U.; Reiter, R.J.; Slominski, A.T. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res. 2018, 65, e12501. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2014, 91, 140–155. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Lee, M.K.; Eun, H.C.; Lee, J.H.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Ultraviolet Modulation of Human Macrophage Metalloelastase in Human Skin In Vivo. J. Investig. Dermatol. 2002, 119, 507–512. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.Y.; Noh, E.; Song, H.; Lee, G.; Kwon, K.; Lee, Y. Reversine inhibits MMP-1 and MMP-3 expressions by suppressing of ROS/MAPK/AP-1 activation in UV-stimulated human keratinocytes and dermal fibroblasts. Exp. Dermatol. 2018, 27, 298–301. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, D.-R.; Kim, Y.-W.; Nam, T.-S.; Jang, K.Y.; Chung, H.T.; Kim, U.-H. The Essential Role of Ca2+ Signals in UVB–Induced IL-1β Secretion in Keratinocytes. J. Investig. Dermatol. 2019, 139, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chen, J.-H.; Chang, C.-N.; Lu, D.-Y.; Chang, P.-C.; Wang, S.-L.; Yeh, W.-L. Fisetin inhibits cell migration via inducing HO-1 and reducing MMPs expression in breast cancer cell lines. Food Chem. Toxicol. 2018, 120, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.I.; Yang, H.; Park, C.; Jin, Y.; Park, Y.S. Fisetin induces Nrf2-mediated HO-1 expression through PKC-δ and p38 in human umbilical vein endothelial cells. J. Cell. Biochem. 2011, 112, 2352–2360. [Google Scholar] [CrossRef]
- Noh, E.-M.; Park, Y.-J.; Kim, J.-M.; Kim, M.-S.; Kim, H.-R.; Song, H.-K.; Hong, O.-Y.; So, H.-S.; Yang, S.-H.; Kim, J.-S.; et al. Fisetin regulates TPA-induced breast cell invasion by suppressing matrix metalloproteinase-9 activation via the PKC/ROS/MAPK pathways. Eur. J. Pharmacol. 2015, 764, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Jeong, G.-S. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int. Immunopharmacol. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chan, S.-Y.; Chu, Y.; Wen, K.-C. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef]
- Herrling, T.; Jung, K.; Fuchs, J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 840–845. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef]
- Yao, K.; Zhang, Y.; Ye, P.; Zhang, P.; Zhu, N. The flavonoid, fisetin, inhibits UV radiation-induced oxidative stress and the activation of NF-kappaB and MAPK signaling in human lens epithelial cells. Mol. Vis. 2008, 14, 1865–1871. [Google Scholar] [PubMed]
- Pal, H.C.; Diamond, A.C.; Strickland, L.R.; Kappes, J.C.; Katiyar, S.K.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget 2015, 7, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Lee, S.; Oh, J.-M.; Lee, M.-S.; Yoon, K.-H.; Park, B.H.; Kim, J.W.; Song, H.; Kim, S.-H. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol. Res. 2007, 55, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wätjen, W.; Michels, G.; Steffan, B.; Niering, P.; Chovolou, Y.; Kampkötter, A.; Tran-Thi, Q.-H.; Proksch, P.; Kahl, R. Low Concentrations of Flavonoids Are Protective in Rat H4IIE Cells Whereas High Concentrations Cause DNA Damage and Apoptosis. J. Nutr. 2005, 135, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Moon, Y.J.; Seo, J.-E.; Lee, Y.; Kim, K.H.; Chung, J.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic. Biol. Med. 2008, 44, 635–645. [Google Scholar] [CrossRef]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y.M. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.; Cornelissen, C.; Lüscher, B.; Baron, J. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Kawahara, T.; Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007, 43, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX Activation by Subunit Interaction and Underlying Mechanisms in Disease. Front. Cell. Neurosci. 2017, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Raad, H.; Serrano-Sanchez, M.; Harfouche, G.; Mahfouf, W.; Bortolotto, D.; Bergeron, V.; Kasraian, Z.; Dousset, L.; Hosseini, M.; Taieb, A.; et al. NADPH Oxidase-1 Plays a Key Role in Keratinocyte Responses to UV Radiation and UVB-Induced Skin Carcinogenesis. J. Investig. Dermatol. 2017, 137, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Glady, A.; Tanaka, M.; Moniaga, C.S.; Yasui, M.; Hara-Chikuma, M. Involvement of NADPH oxidase 1 in UVB-induced cell signaling and cytotoxicity in human keratinocytes. Biochem. Biophys. Rep. 2018, 14, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Park, S.R.; Choi, Y.H.; Park, E.K.; Jin, C.-Y.; Yu, H.; Jo, W.S.; Lee, K.T.; Kim, G.-Y. GSK-3β-Targeting Fisetin Promotes Melanogenesis in B16F10 Melanoma Cells and Zebrafish Larvae through β-Catenin Activation. Int. J. Mol. Sci. 2020, 21, 312. [Google Scholar] [CrossRef]
- Panjapa, K.; Bungorn, S. Protective effects of Lagerstroemia speciose extract against UV-A damage on skin cells. Ind. Crops Prod. 2018, 124, 9–19. [Google Scholar] [CrossRef]
- Jang, H.Y.; Hong, O.Y.; Youn, H.J.; Jung, J.U.; Chung, E.Y.; Jung, S.H.; Kim, J.S. CDDO, a PPAR-γ ligand, inhibits TPA-induced cell migration and invasion through a PPAR-γ-independent mechanism. Oncol. Lett. 2022, 24, 354. [Google Scholar] [CrossRef]


| Gene | Primer Sequences | Accession No. |
|---|---|---|
| MMP-1 | For: AGTGACTGGGAAACCGATGCTGA Rev: GCTCTTGGCAAATCTGGCCTGTAA | NM_001145938 |
| MMP-3 | For: ATTCCATGGAGCCAGGCTTTC Rev: CATTTGGGTCAAACTCCAACTGTG | NM_002422 |
| IL-1β | For: TCCTGCGTGTTGAAAGATGATAA Rev: CAAATCGCTTTTCCATCTTCTTC | NM_000576 |
| IL-6 | For: TACCCCCAGGAGAAGATTCC Rev: GCCATCTTTGGAAGGTTCAG | NM_000600 |
| IL-8 | For: AGACAGCAGAGCACACAAGC Rev: ATGGTTCCTTCCGGTGGT | NM_000584 |
| TNF-α | For: CTGCTGCACTTTGGAGTGAT Rev: AGATGATCTGACTGCCTGGG | NM_000594 |
| GAPDH | For: ATGGAAATCCC ATCACCATCTT Rev: CGCCCCACTTGA TTTTGG | NM_002046 |
| Gene | Company | Catalog No. | Accession No. |
| NOX1 | QIAGEN | PPH06068A | NM_007052 |
| NOX2 | QIAGEN | PPH10407A | NM_000397 |
| NOX3 | QIAGEN | PPH06074A | NM_015718 |
| NOX4 | QIAGEN | PPH06078A | NM_016931 |
| NOX5 | QIAGEN | PPH17569A | NM_024505 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-Y.; Kim, G.-B.; Kim, J.-M.; Kang, S.Y.; Youn, H.-J.; Park, J.; Ro, S.Y.; Chung, E.-Y.; Park, K.-H.; Kim, J.-S. Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes. Int. J. Mol. Sci. 2023, 24, 17358. https://doi.org/10.3390/ijms242417358
Jang H-Y, Kim G-B, Kim J-M, Kang SY, Youn H-J, Park J, Ro SY, Chung E-Y, Park K-H, Kim J-S. Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes. International Journal of Molecular Sciences. 2023; 24(24):17358. https://doi.org/10.3390/ijms242417358
Chicago/Turabian StyleJang, Hye-Yeon, Gi-Beum Kim, Jeong-Mi Kim, Sang Yull Kang, Hyun-Jo Youn, Jinny Park, Su Yeon Ro, Eun-Yong Chung, Kwang-Hyun Park, and Jong-Suk Kim. 2023. "Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes" International Journal of Molecular Sciences 24, no. 24: 17358. https://doi.org/10.3390/ijms242417358






