Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither?
Abstract
:1. Introduction
2. Well-Defined Drug Targets in COPD Therapeutics
2.1. Target 1: The β2-Adrenergic Receptor
2.2. Target 2: Muscarinic Receptors
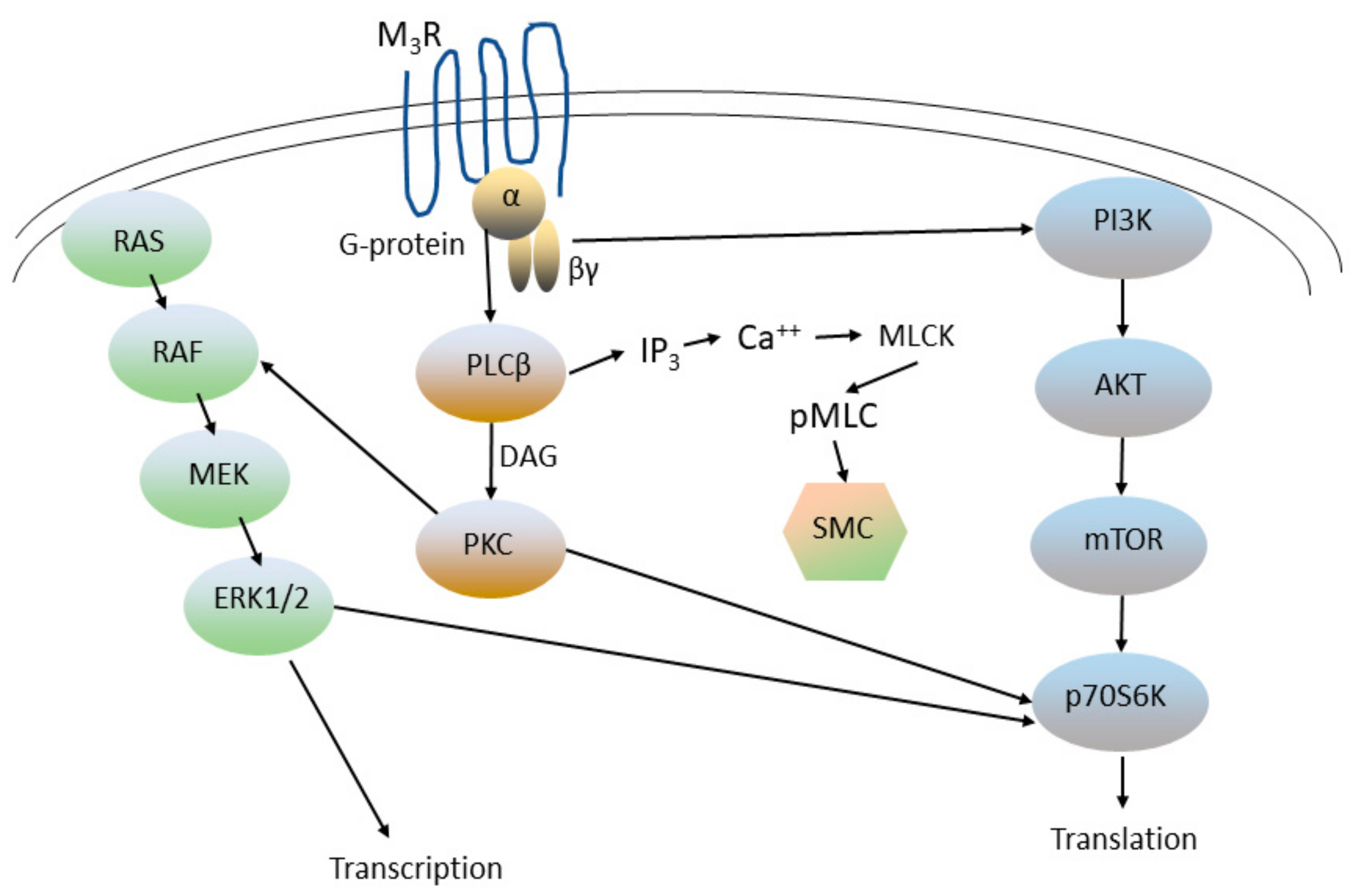
Regulation of Airway Smooth Muscle Tone by Other Molecular Targets
2.3. Target 3: The Glucocorticoid (Adrenal Corticosteroid) Receptor
2.4. Target 4: cAMP-Specific Cyclic Nucleotide Phosphodiesterases (PDE4s)
2.4.1. Roflumilast
2.4.2. Tanimilast
2.4.3. Ensifentrine
3. Emerging Targets in COPD Therapeutics
3.1. Target 5: The Interleukin-4/Interleukin-13 Receptor
Effects of IL-4 and IL-13 on Airway Mucus Production
3.2. Target 6: IL-33 Signaling
3.3. Target 7: Ion Channels and Mucociliary Clearance
3.3.1. Genetic Insights into COPD Pathogenesis
3.3.2. CF as a Severe Form of Bronchitis/COPD
3.3.3. Acquired CFTR Dysfunction in COPD
3.3.4. Regulation of CFTR by PKA Phosphorylation in CF and COPD
3.3.5. Direct Modulation of CFTR Cl− Ion Channel Activity in COPD
4. Implications for Clinical Care, Clinical Trials and Drug Discovery
- Defining a “precision medicine” approach to the care of patients with COPD. For this purpose, they can be classified into one of several distinct groups:
- ◦
- Patients with predominantly manifestations of bronchitis
- ◦
- Patients with eosinophilia (and potentially other biomarkers of Type 2 inflammation)
- ◦
- Patients without bronchitis
- Designing the next generation of clinical trials, to reflect emerging standards in COPD patient care and research:
- ◦
- Improved patient selection (inclusion/exclusion criteria and/or patient stratification), ideally reflecting the classification outlined above
- ◦
- The use of biomarkers as a patient selection tool, and as intermediate endpoints in early-phase clinical trials
- ◦
- Improved clinical trial design that controls for concomitant medications and ensures that patients are on stable doses of concomitant medications prior to initiation of protocol therapy
- ◦
- Active-comparator design
4.1. Implications for Clinical Care
4.1.1. Patients with Predominant Manifestations of Bronchitis
4.1.2. Patients with Eosinophilia
4.1.3. Patients without Bronchitis
4.2. Implications for Clinical Trial Design
4.2.1. Patient Selection and Stratification
4.2.2. Increasing the Role of Biomarkers
4.2.3. Concurrent Therapy
- (a)
- Publications of placebo-controlled trials need to report all data on concurrent medications. In the recent dupilumab trial, all patients were receiving triple LABA/LAMA and inhaled glucocorticoid therapy (or had contra-indications to glucocorticoid therapy), but the proportion, if any, of patients who were taking roflumilast, or other COPD-modifying drugs, was not specified [47]. These data are essential to allow clinicians to determine the applicability of the trial to “real world” situations: i.e., to determine which patients in routine clinical care would be appropriate candidates for the therapy studied in the trial.
- (b)
- Future trials will need to use concurrent COPD medications as criteria for inclusion/exclusion, or as a stratification tool (i.e., as a pre-hoc factor in the randomization process).
- (c)
- Clinical trialists will also need to ensure that patients are on optimal therapy with generally approved COPD medications and that they are on stable doses of these medications before being entered in a clinical study. Otherwise, improvement in a disease variable after the initiation of the drug (or placebo) in the trial might actually be caused by recent changes in the concurrent medication, rather than the drug being studied.
4.2.4. Active-Comparator Trial Design
5. Implications for Future Drug Discovery Strategies
- Targets involved in the regulation of cAMP
- Targets on the CFTR Cl− ion channel
- Targets in the immune/inflammatory system
- Druggable targets essential for repair or regeneration of airway epithelium, airway smooth muscle and alveoli
5.1. Novel Agents That Modulate cAMP Levels
5.2. Novel CFTR Potentiators
5.3. Novel Anti-Inflammatory or Immunomodulatory Therapies
5.4. Novel Agents Targeting Airway Remodeling
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Mosnaim, G. Asthma in Adults. N. Engl. J. Med. 2023, 389, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pitre, P.J.; Sabbula, B.R.; Cascella, M. Restrictive Lung Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Drugs for COPD. Med. Lett. Drugs Ther. 2020, 62, 137–144.
- Comparison table: Inhaled short-acting bronchodilators for treatment of COPD. Med. Lett. Drugs Ther. 2020, 62, e144-e5.
- Comparison table: Inhaled long-acting bronchodilators for treatment of COPD. Med. Lett. Drugs Ther. 2020, 62, e146-e7.
- Rasmussen, S.G.; Devree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Klein, A.M. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef]
- Gloerich, M.; Bos, J.L. Epac: Defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 355–375. [Google Scholar] [CrossRef]
- Baldwin, T.A.; Li, Y.; Marsden, A.N.; Rinne, S.; Garza-Carbajal, A.; Schindler, R.F.R.; Zhang, M.; Garcia, M.A.; Venna, V.R.; Decher, N.; et al. POPDC1 scaffolds a complex of adenylyl cyclase 9 and the potassium channel TREK-1 in heart. EMBO Rep. 2022, 23, e55208. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Al-Sawalha, N.A.; Parra, S.; Pokkunuri, I.; Omoluabi, O.; Okulate, A.A.; Li, E.W.; Hazen, M.; Gonzalez-Granado, J.M.; Daly, C.J.; et al. β2-Adrenoceptor signaling in airway epithelial cells promotes eosinophilic inflammation, mucous metaplasia, and airway contractility. Proc. Natl. Acad. Sci. USA 2017, 114, E9163–E9171. [Google Scholar] [CrossRef]
- Gosens, R.; Zaagsma, J.; Meurs, H.; Halayko, A.J. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir. Res. 2006, 7, 73. [Google Scholar] [CrossRef]
- Disse, B.; Speck, G.A.; Rominger, K.L.; Witek, T.J., Jr.; Hammer, R. Tiotropium (Spiriva): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999, 64, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Qu, Q.; Robertson, M.J.; Skiniotis, G.; Kobilka, B.K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 2019, 364, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Ring, A.M.; Manglik, A.; Hu, J.; Hu, K.; Eitel, K.; Hübner, H.; Pardon, E.; Valant, C.; Sexton, P.M.; et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 2013, 504, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S. Emerging concepts in smooth muscle contributions to airway structure and function: Implications for health and disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1113–L1140. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, K.R.; Reznikov, L.R. Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis. Respir. Res. 2018, 19, 149. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Bolger, G.B. The multienzyme PDE4 cyclic AMP-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and anti-depressant actions. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 44, pp. 225–342. [Google Scholar]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.B. The PDE-Opathies: Diverse Phenotypes Produced by a Functionally Related Multigene Family. Trends Genet. 2021, 37, 669–681. [Google Scholar] [CrossRef]
- Hatzelmann, A.; Morcillo, E.J.; Lungarella, G.; Adnot, S.; Sanjar, S.; Beume, R.; Schudt, C.; Tenor, H. The preclinical pharmacology of roflumilast—A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010, 23, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Dent, G.; White, S.R.; Tenor, H.; Bodtke, K.; Schudt, C.; Leff, A.R.; Magnussen, H.; Rabe, K.F. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: Characterization of isoenzymes and functional effects of PDE inhibitors. Pulm. Pharmacol. Ther. 1998, 11, 47–56. [Google Scholar] [CrossRef]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Calverley, P.M.; Izquierdo-Alonso, J.L.; Bundschuh, D.S.; Brose, M.; Martinez, F.J.; Rabe, K.F. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: Two randomised clinical trials. Lancet 2009, 374, 695–703. [Google Scholar] [CrossRef]
- Janjua, S.; Fortescue, R.; Poole, P. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 5, CD002309. [Google Scholar] [CrossRef]
- Martinez, F.J.; Calverley, P.M.; Goehring, U.M.; Brose, M.; Fabbri, L.M.; Rabe, K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicentre randomised controlled trial. Lancet 2015, 385, 857–866. [Google Scholar] [CrossRef]
- Rennard, S.I.; Calverley, P.M.; Goehring, U.M.; Bredenbroker, D.; Martinez, F.J. Reduction of exacerbations by the PDE4 inhibitor roflumilast—The importance of defining different subsets of patients with COPD. Respir. Res. 2011, 12, 18. [Google Scholar] [CrossRef]
- Facchinetti, F.; Civelli, M.; Singh, D.; Papi, A.; Emirova, A.; Govoni, M. Tanimilast, A Novel Inhaled Pde4 Inhibitor for the Treatment of Asthma and Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2021, 12, 740803. [Google Scholar] [CrossRef]
- Singh, D.; Emirova, A.; Francisco, C.; Santoro, D.; Govoni, M.; Nandeuil, M.A. Efficacy and safety of CHF6001, a novel inhaled PDE4 inhibitor in COPD: The PIONEER study. Respir. Res. 2020, 21, 246. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Oxford, A.W.; Comer, M.B.; Seeds, E.A.; Page, C.P. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6, 7-tetrahydro-2H-pyrimido [6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquino lin-4-one]. J. Pharmacol. Exp. Ther. 2006, 318, 840–848. [Google Scholar] [CrossRef]
- Carzaniga, L.; Amari, G.; Rizzi, A.; Capaldi, C.; De Fanti, R.; Ghidini, E.; Villetti, G.; Carnini, C.; Moretto, N.; Facchinetti, F.; et al. Discovery and Optimization of Thiazolidinyl and Pyrrolidinyl Derivatives as Inhaled PDE4 Inhibitors for Respiratory Diseases. J. Med. Chem. 2017, 60, 10026–10046. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Dauletbaev, N.; Lands, L.C.; Hanrahan, J.W. The Phosphodiesterase Inhibitor Ensifentrine Reduces Production of Proinflammatory Mediators in Well Differentiated Bronchial Epithelial Cells by Inhibiting PDE4. J. Pharmacol. Exp. Ther. 2020, 375, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Abbott-Banner, K.; Bengtsson, T.; Newman, K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur. Respir. J. 2018, 52, 1801074. [Google Scholar] [CrossRef] [PubMed]
- Anzueto, A.; Barjaktarevic, I.Z.; Siler, T.M.; Rheault, T.; Bengtsson, T.; Rickard, K.; Sciurba, F. Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am. J. Respir. Crit. Care Med. 2023, 208, 406–416. [Google Scholar] [CrossRef]
- Drugs for rheumatoid arthritis. Med. Lett. Drugs Ther. 2021, 63, 177–184.
- Drugs for inflammatory bowel disease. Med. Lett. Drugs Ther. 2023, 65, 105–112. [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Hurdayal, R.; Brombacher, F. Interleukin-4 Receptor Alpha: From Innate to Adaptive Immunity in Murine Models of Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Ralpha antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, E.; Pizzichini, M.M.; Gibson, P.; Parameswaran, K.; Gleich, G.J.; Berman, L.; Dolovich, J.; Hargreave, F.E. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am. J. Respir. Crit. Care Med. 1998, 158, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Siva, R.; Green, R.H.; Brightling, C.E.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.J.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Martinez, F.J.; Papi, A.; Pavord, I.D.; Wedzicha, J.A.; Vogelmeier, C.F.; Halpin, D.M.G. Blood Eosinophils and Chronic Obstructive Pulmonary Disease: A Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 Review. Am. J. Respir. Crit. Care Med. 2022, 206, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef]
- Dabbagh, K.; Takeyama, K.; Lee, H.M.; Ueki, I.F.; Lausier, J.A.; Nadel, J.A. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999, 162, 6233–6237. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Fujimoto, K.; Yasuo, M.; Tsushima, K.; Yoshida, K.; Ise, H.; Yamaya, M. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. Clin. Exp. Allergy 2008, 38, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Johansson, K.; Joo, A.; Bonser, L.R.; Koh, K.D.; Le Tonqueze, O.; Bolourchi, S.; Bautista, R.A.; Zlock, L.; Roth, T.L.; et al. Epithelial miR-141 regulates IL-13-induced airway mucus production. JCI Insight 2021, 6, e139019. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Yousuf, A.J.; Mohammed, S.; Carr, L.; Yavari Ramsheh, M.; Micieli, C.; Mistry, V.; Haldar, K.; Wright, A.; Novotny, P.; Parker, S.; et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): A phase 2a, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 469–477. [Google Scholar] [CrossRef]
- England, E.; Rees, D.G.; Scott, I.C.; Carmen, S.; Chan, D.T.Y.; Chaillan Huntington, C.E.; Houslay, K.F.; Erngren, T.; Penney, M.; Majithiya, J.B.; et al. Tozorakimab (MEDI3506): An anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. Sci. Rep. 2023, 13, 9825. [Google Scholar] [CrossRef]
- Wilkinson, T.; De Soyza, A.; Carroll, M.; Chalmers, J.D.; Crooks, M.G.; Griffiths, G.; Shankar-Hari, M.; Ho, L.P.; Horsley, A.; Kell, C.; et al. A randomised phase 2a study to investigate the effects of blocking interleukin-33 with tozorakimab in patients hospitalised with COVID-19: ACCORD-2. ERJ Open Res. 2023, 9, 00249–02023. [Google Scholar] [CrossRef]
- Stoltz, D.A.; Meyerholz, D.K.; Welsh, M.J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 2015, 372, 351–362. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Levring, J.; Terry, D.S.; Kilic, Z.; Fitzgerald, G.; Blanchard, S.C.; Chen, J. CFTR function, pathology and pharmacology at single-molecule resolution. Nature 2023, 616, 606–614. [Google Scholar] [CrossRef]
- Pilewski, J.M.; Frizzell, R.A. Role of CFTR in airway disease. Physiol. Rev. 1999, 79 (Suppl. 1), S215–S255. [Google Scholar] [CrossRef]
- Grasemann, H. CFTR Modulator Therapy for Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2085–2088. [Google Scholar] [CrossRef]
- Cooper, J.L.; Quinton, P.M.; Ballard, S.T. Mucociliary transport in porcine trachea: Differential effects of inhibiting chloride and bicarbonate secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L184–L190. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hanrahan, J.W.; Bilodeau, G.; Ellis, L.; Dupuis, A.; Liao, J.; Zielenski, J.; Durie, P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care Med. 2006, 173, 1139–1144. [Google Scholar] [CrossRef]
- Clunes, L.A.; Davies, C.M.; Coakley, R.D.; Aleksandrov, A.A.; Henderson, A.G.; Zeman, K.L.; Worthington, E.N.; Gentzsch, M.; Kreda, S.M.; Cholon, D.; et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Wilhelm, A.M.; Flanagan, B.; Courville, C.; Tidwell, S.L.; Raju, S.V.; Gaggar, A.; Steele, C.; Tang, L.P.; Liu, B.; et al. Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction in the Lower Airways in COPD. Chest 2013, 144, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Rab, A.; Rowe, S.M.; Raju, S.V.; Bebok, Z.; Matalon, S.; Collawn, J.F. Cigarette smoke and CFTR: Implications in the pathogenesis of COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L530–L541. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.V.; Jackson, P.L.; Courville, C.A.; McNicholas, C.M.; Sloane, P.A.; Sabbatini, G.; Tidwell, S.; Tang, L.P.; Liu, B.; Fortenberry, J.A.; et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am. J. Respir. Crit. Care Med. 2013, 188, 1321–1330. [Google Scholar] [CrossRef]
- Kume, H.; Yamada, R.; Sato, Y.; Togawa, R. Airway Smooth Muscle Regulated by Oxidative Stress in COPD. Antioxidants 2023, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; McCann, J.D.; Liedtke, C.M.; Nairn, A.C.; Greengard, P.; Welsh, M.J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature 1988, 331, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.P.; Rich, D.P.; Gregory, R.J.; Smith, A.E.; Welsh, M.J. Generation of cAMP-activated chloride currents by expression of CFTR. Science 1991, 251, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Rich, D.P.; Gregory, R.J.; Anderson, M.P.; Manavalan, P.; Smith, A.E.; Welsh, M.J. Effect of deleting the R domain on CFTR-generated chloride channels. Science 1991, 253, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Rich, D.P.; Marshall, J.; Gregory, R.J.; Welsh, M.J.; Smith, A.E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 1991, 66, 1027–1036. [Google Scholar] [CrossRef]
- Tilly, B.C.; Winter, M.C.; Ostedgaard, L.S.; O’Riordan, C.; Smith, A.E.; Welsh, M.J. Cyclic AMP-dependent protein kinase activation of cystic fibrosis transmembrane conductance regulator chloride channels in planar lipid bilayers. J. Biol. Chem. 1992, 267, 9470–9473. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Hug, M.J.; Bradbury, N.A.; Frizzell, R.A. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J. Biol. Chem. 2000, 275, 14360–14366. [Google Scholar] [CrossRef]
- Huang, P.; Trotter, K.; Boucher, R.C.; Milgram, S.L.; Stutts, M.J. PKA holoenzyme is functionally coupled to CFTR by AKAPs. Am. J. Physiol. Cell Physiol. 2000, 278, C417–C422. [Google Scholar] [CrossRef]
- Csanady, L.; Seto-Young, D.; Chan, K.W.; Cenciarelli, C.; Angel, B.B.; Qin, J.; McLachlin, D.T.; Krutchinsky, A.N.; Chait, B.T.; Nairn, A.C.; et al. Preferential phosphorylation of R-domain Serine 768 dampens activation of CFTR channels by PKA. J. Gen. Physiol. 2005, 125, 171–186. [Google Scholar] [CrossRef]
- Kanelis, V.; Hudson, R.P.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. NMR evidence for differential phosphorylation-dependent interactions in WT and DeltaF508 CFTR. EMBO J. 2010, 29, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Hudson, R.P.; Kanelis, V.; Choy, W.Y.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat. Struct. Mol. Biol. 2007, 14, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, L.A.; Fay, J.F.; Riordan, J.R. R-Domain Phosphorylation by Protein Kinase A Stimulates Dissociation of Unhydrolyzed ATP from the First Nucleotide-Binding Site of the Cystic Fibrosis Transmembrane Conductance Regulator. Biochemistry 2018, 57, 5073–5075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H. Protein kinase A phosphorylation potentiates cystic fibrosis transmembrane conductance regulator gating by relieving autoinhibition on the stimulatory C terminus of the regulatory domain. J. Biol. Chem. 2020, 295, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.P.; Livera, G.; Huang, P.; Sun, C.; O’Neal, W.K.; Conti, M.; Stutts, M.J.; Milgram, S.L. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J. Biol. Chem. 2005, 280, 7997–8003. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Veilleux, A.; Zhang, L.; Young, A.; Kwok, E.; Laliberte, F.; Chung, C.; Tota, M.R.; Dube, D.; Friesen, R.W.; et al. Dynamic activation of cystic fibrosis transmembrane conductance regulator by type 3 and type 4D phosphodiesterase inhibitors. J. Pharmacol. Exp. Ther. 2005, 314, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.A.; Raju, S.V.; Tang, L.P.; McNicholas, C.M.; Li, Y.; Courville, C.A.; Farris, R.F.; Coricor, G.E.; Smoot, L.H.; Mazur, M.M.; et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 549–558. [Google Scholar] [CrossRef]
- Blanchard, E.; Zlock, L.; Lao, A.; Mika, D.; Namkung, W.; Xie, M.; Scheitrum, C.; Gruenert, D.C.; Verkman, A.S.; Finkbeiner, W.E.; et al. Anchored PDE4 regulates chloride conductance in wild-type and DeltaF508-CFTR human airway epithelia. FASEB J. 2014, 28, 791–801. [Google Scholar] [CrossRef]
- Milara, J.; Armengot, M.; Banuls, P.; Tenor, H.; Beume, R.; Artigues, E.; Cortijo, J. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br. J. Pharmacol. 2012, 166, 2243–2262. [Google Scholar] [CrossRef]
- Barber, R.; Baillie, G.S.; Bergmann, R.; Shepherd, M.C.; Sepper, R.; Houslay, M.D.; Heeke, G.V. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L332–L343. [Google Scholar] [CrossRef]
- Zuo, H.; Han, B.; Poppinga, W.J.; Ringnalda, L.; Kistemaker, L.E.M.; Halayko, A.J.; Gosens, R.; Nikolaev, V.O.; Schmidt, M. Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells. Br. J. Pharmacol. 2018, 175, 2988–3006. [Google Scholar] [CrossRef]
- Kelley, T.J.; Al Nakkash, L.; Drumm, M.L. CFTR-mediated chloride permeability is regulated by type III phosphodiesterases in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1995, 13, 657–664. [Google Scholar] [CrossRef]
- Penmatsa, H.; Zhang, W.; Yarlagadda, S.; Li, C.; Conoley, V.G.; Yue, J.; Bahouth, S.W.; Buddington, R.K.; Zhang, G.; Nelson, D.J.; et al. Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol. Biol. Cell 2010, 21, 1097–1110. [Google Scholar] [CrossRef]
- Turner, M.J.; Matthes, E.; Billet, A.; Ferguson, A.J.; Thomas, D.Y.; Randell, S.H.; Ostrowski, L.E.; Abbott-Banner, K.; Hanrahan, J.W. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L59–L70. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Luo, Y.; Thomas, D.Y.; Hanrahan, J.W. The dual phosphodiesterase 3/4 inhibitor RPL554 stimulates rare class III and IV CFTR mutants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L908–L920. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [PubMed]
- Accurso, F.J.; Rowe, S.M.; Clancy, J.P.; Boyle, M.P.; Dunitz, J.M.; Durie, P.R.; Sagel, S.D.; Hornick, D.B.; Konstan, M.W.; Donaldson, S.H.; et al. Effect of VX-770 in Persons with Cystic Fibrosis and the G551D-CFTR Mutation. N. Engl. J. Med. 2010, 363, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Eckford, P.D.; Li, C.; Ramjeesingh, M.; Bear, C.E. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J. Biol. Chem. 2012, 287, 36639–36649. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Burton, B.; Huang, C.J.; Worley, J.; Cao, D.; Johnson, J.P., Jr.; Urrutia, A.; Joubran, J.; Seepersaud, S.; Sussky, K.; et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J. Cyst. Fibros. 2012, 11, 237–245. [Google Scholar] [CrossRef]
- Van Goor, F.; Yu, H.; Burton, B.; Hoffman, B.J. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J. Cyst. Fibros. 2014, 13, 29–36. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Levit, A.; Levring, J.; Touhara, K.K.; Shoichet, B.K.; Chen, J. Structural identification of a hotspot on CFTR for potentiation. Science 2019, 364, 1184–1188. [Google Scholar] [CrossRef]
- Fiedorczuk, K.; Chen, J. Molecular structures reveal synergistic rescue of Δ508 CFTR by Trikafta modulators. Science 2022, 378, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jih, K.Y.; Hwang, T.C. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Pyle, L.C.; Ehrhardt, A.; Mitchell, L.H.; Fan, L.; Ren, A.; Naren, A.P.; Li, Y.; Clancy, J.P.; Bolger, G.B.; Sorscher, E.J.; et al. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L587–L597. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.V.; Lin, V.Y.; Liu, L.; McNicholas, C.M.; Karki, S.; Sloane, P.A.; Tang, L.; Jackson, P.L.; Wang, W.; Wilson, L.; et al. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2017, 56, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.A.; Shastry, S.; Wilhelm, A.; Courville, C.; Tang, L.P.; Backer, K.; Levin, E.; Raju, S.V.; Li, Y.; Mazur, M.; et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE 2012, 7, e39809. [Google Scholar] [CrossRef] [PubMed]
- Solomon, G.M.; Hathorne, H.; Liu, B.; Raju, S.V.; Reeves, G.; Acosta, E.P.; Dransfield, M.T.; Rowe, S.M. Pilot evaluation of ivacaftor for chronic bronchitis. Lancet Respir. Med. 2016, 4, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- Grand, D.L.; Gosling, M.; Baettig, U.; Bahra, P.; Bala, K.; Brocklehurst, C.; Budd, E.; Butler, R.; Cheung, A.K.; Choudhury, H.; et al. Discovery of Icenticaftor (QBW251), a Cystic Fibrosis Transmembrane Conductance Regulator Potentiator with Clinical Efficacy in Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. J. Med. Chem. 2021, 64, 7241–7260. [Google Scholar] [CrossRef]
- Rowe, S.M.; Jones, I.; Dransfield, M.T.; Haque, N.; Gleason, S.; Hayes, K.A.; Kulmatycki, K.; Yates, D.P.; Danahay, H.; Gosling, M.; et al. Efficacy and Safety of the CFTR Potentiator Icenticaftor (QBW251) in COPD: Results from a Phase 2 Randomized Trial. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2399–2409. [Google Scholar] [CrossRef]
- Martinez, F.J.; Criner, G.J.; Gessner, C.; Jandl, M.; Scherbovsky, F.; Shinkai, M.; Siler, T.M.; Vogelmeier, C.F.; Voves, R.; Wedzicha, J.A.; et al. Icenticaftor, a CFTR Potentiator, in COPD: A Multicenter, Parallel-Group, Double-Blind Clinical Trial. Am. J. Respir. Crit. Care Med. 2023, 208, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Ye, W.; Wang, D.; White, E.; Arjomandi, M.; Barjaktarevic, I.Z.; Brown, S.A.; Buhr, R.G.; Comellas, A.P.; Cooper, C.B.; et al. Bronchodilators in Tobacco-Exposed Persons with Symptoms and Preserved Lung Function. N. Engl. J. Med. 2022, 387, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Mih, J.D.; George, S.C. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 97–104. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St Rose, E.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhong, N.S.; Li, X.; Chen, S.; Zheng, J.; Zhao, D.; Yao, W.; Zhi, R.; Wei, L.; He, B.; et al. Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 923–935. [Google Scholar] [CrossRef]
- Varani, K.; Caramori, G.; Vincenzi, F.; Adcock, I.; Casolari, P.; Leung, E.; Maclennan, S.; Gessi, S.; Morello, S.; Barnes, P.J.; et al. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 398–406. [Google Scholar] [CrossRef]
- Sun, C.X.; Zhong, H.; Mohsenin, A.; Morschl, E.; Chunn, J.L.; Molina, J.G.; Belardinelli, L.; Zeng, D.; Blackburn, M.R. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Investig. 2006, 116, 2173–2182. [Google Scholar] [CrossRef]
- Polosa, R.; Blackburn, M.R. Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol. Sci. 2009, 30, 528–535. [Google Scholar] [CrossRef]
- Polosa, R. Adenosine-receptor subtypes: Their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur. Respir. J. 2002, 20, 488–496. [Google Scholar] [CrossRef]
- Caruso, M.; Varani, K.; Tringali, G.; Polosa, R. Adenosine and adenosine receptors: Their contribution to airway inflammation and therapeutic potential in asthma. Curr. Med. Chem. 2009, 16, 3875–3885. [Google Scholar] [CrossRef]
- Alqarni, A.A.; Aldhahir, A.M.; Alghamdi, S.A.; Alqahtani, J.S.; Siraj, R.A.; Alwafi, H.; AlGarni, A.A.; Majrshi, M.S.; Alshehri, S.M.; Pang, L. Role of prostanoids, nitric oxide and endothelin pathways in pulmonary hypertension due to COPD. Front. Med. 2023, 10, 1275684. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Smith, K.H.; Koziol-White, C.J.; Li, F.; Kazarian, A.G.; Corpuz, M.L.; Shumyatcher, M.; Ehlert, F.J.; Himes, B.E.; Panettieri, R.A., Jr.; et al. PDE8 Is Expressed in Human Airway Smooth Muscle and Selectively Regulates cAMP Signaling by beta2-Adrenergic Receptors and Adenylyl Cyclase 6. Am. J. Respir. Cell Mol. Biol. 2018, 58, 530–541. [Google Scholar] [CrossRef]
- Turner, M.J.; Sato, Y.; Thomas, D.Y.; Abbott-Banner, K.; Hanrahan, J.W. Phosphodiesterase 8A Regulates CFTR Activity in Airway Epithelial Cells. Cell Physiol. Biochem. 2021, 55, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef]
- Isa, N.; Mudhafar, D.; Ju, C.; Man, K.K.; Lau, W.C.; Cheng, L.Y.; Wei, L. Effects of Phosphodiesterase-5 Inhibitors in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. COPD 2022, 19, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaci, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.Y.; Liu, Z.; Gontijo Lima, R.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolger, G.B. Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither? Int. J. Mol. Sci. 2023, 24, 17363. https://doi.org/10.3390/ijms242417363
Bolger GB. Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither? International Journal of Molecular Sciences. 2023; 24(24):17363. https://doi.org/10.3390/ijms242417363
Chicago/Turabian StyleBolger, Graeme B. 2023. "Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither?" International Journal of Molecular Sciences 24, no. 24: 17363. https://doi.org/10.3390/ijms242417363




