Patients with Chronic Spinal Cord Injury Display a Progressive Alteration over the Years of the Activation Stages of the T Lymphocyte Compartment
Abstract
:1. Introduction
2. Results
2.1. Demographic Profile of Chronic SCI Patients
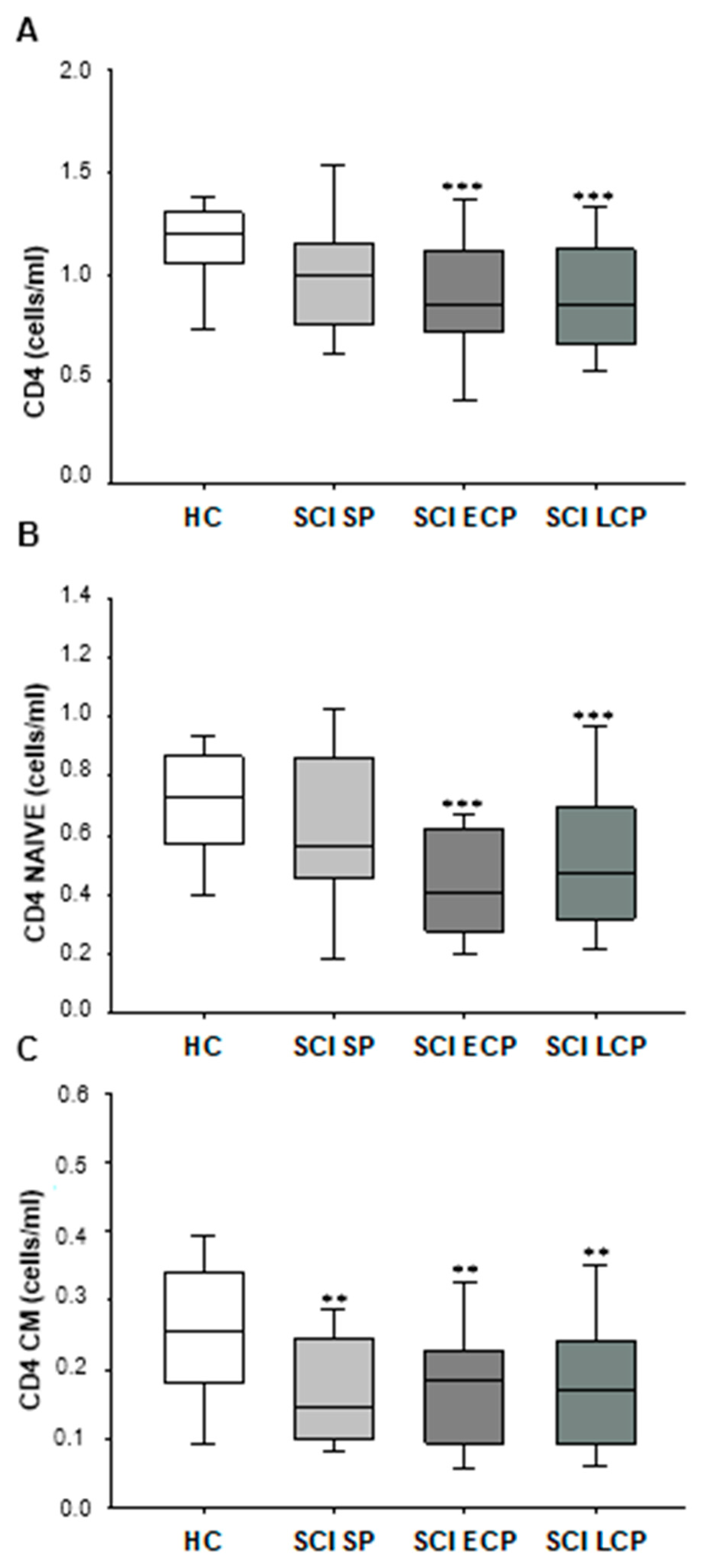
2.2. Patients with Chronic SCI Show a Long-Term Decrease in Circulating CD4+ T Lymphocytes, Explained by a Reduction in the Naïve and CM Subsets
2.3. Patients with Chronic SCI Have a Long-Term Diminution of Circulating CD8+ T Lymphocytes with Early Reduction of the Naïve Subset
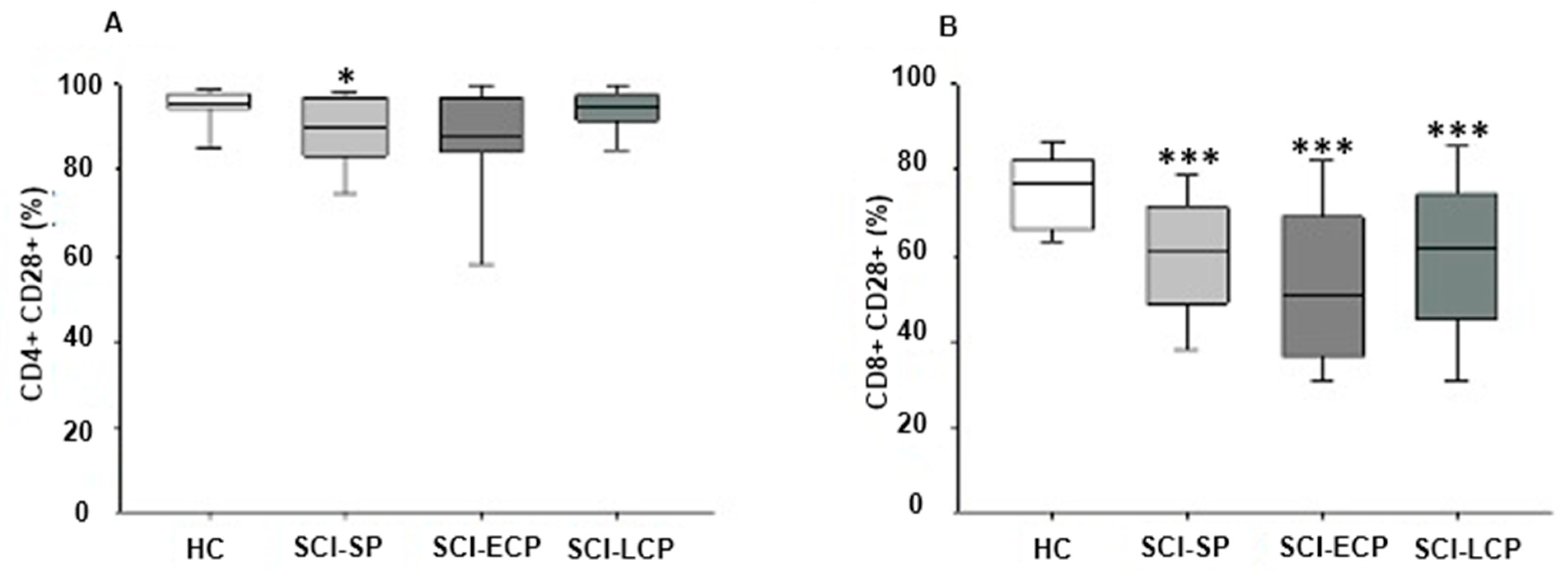
2.4. Chronic SCI Patients Show Maintained Reduced Expression of CD28+ on CD8+ T Lymphocytes and on CD4+ T Lymphocytes Selectively in Those with Long-Term Evolution
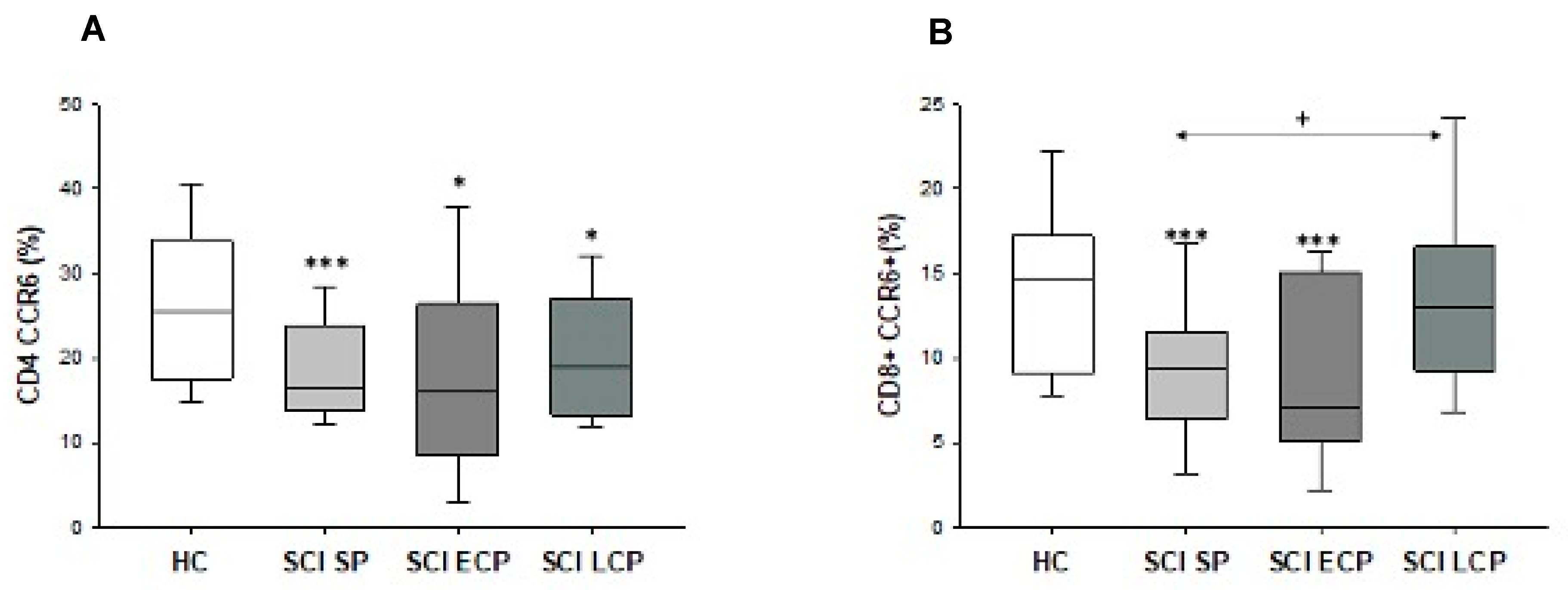
2.5. Patients with Spinal Cord Injury Have a Decreased Percentage and Number of Chemokine Receptor CCR6+ CD8+ T Lymphocytes and CD4+ T Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Isolation of Peripheral Blood Mononuclear Cells
4.3. Immunophenotype Studies
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.S.; Whitmore, R.G. Spinal Cord Injuries. In Surgical Intensive Care Medicine, 3rd ed.; Springer: Cham, Switzerland, 2021; pp. 181–193. [Google Scholar] [CrossRef]
- Kang, Y.; Ding, H.; Zhou, H.; Wei, Z.; Liu, L.; Pan, D.; Feng, S. Epidemiology of Worldwide Spinal Cord Injury: A Literature Review. J. Neurorestoratology 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Cho, N.; Hachem, L.D.; Fehlings, M.G. Spinal Cord Edema After Spinal Cord Injury: From Pathogenesis to Management. In Brain Edema: From Molecular Mechanisms to Clinical Practice; Academic Press: Cambridge, MA, USA, 2017; pp. 261–275. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Haro, S.; Álvarez-Mon, M.Á.; De Leon-Oliva, D.; Gomez-Lahoz, A.M.; Monserrat, J.; Atienza-Pérez, M.; Díaz, D.; et al. A Comprehensive Look at the Psychoneuroimmunoendocrinology of Spinal Cord Injury and Its Progression: Mechanisms and Clinical Opportunities. Mil. Med. Res. 2023, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Sweis, R.; Biller, J. Systemic Complications of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Osinski, T.; Acapo, S.; Bensmail, D.; Bouhassira, D.; Martinez, V. Central Nervous System Reorganization and Pain After Spinal Cord Injury: Possible Targets for Physical Therapy-A Systematic Review of Neuroimaging Studies. Phys. Ther. 2020, 100, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative Analysis of Cellular Inflammation after Traumatic Spinal Cord Injury: Evidence for a Multiphasic Inflammatory Response in the Acute to Chronic Environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Herman, P.; Stein, A.; Gibbs, K.; Korsunsky, I.; Gregersen, P.; Bloom, O. Persons with Chronic Spinal Cord Injury Have Decreased Natural Killer Cell and Increased Toll-Like Receptor/Inflammatory Gene Expression. J. Neurotrauma 2018, 35, 1819. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.S.; Mure, A.; Gomez, C.S. UTIs in Patients with Neurogenic Bladder. Curr. Urol. Rep. 2014, 15, 433. [Google Scholar] [CrossRef]
- Jeffries, M.A.; Tom, V.J. Peripheral Immune Dysfunction: A Problem of Central Importance after Spinal Cord Injury. Biology 2021, 10, 928. [Google Scholar] [CrossRef]
- Diaz, D.; Lopez-Dolado, E.; Haro, S.; Monserrat, J.; Martinez-Alonso, C.; Balomeros, D.; Albillos, A.; Alvarez-Mon, M. Systemic Inflammation and the Breakdown of Intestinal Homeostasis Are Key Events in Chronic Spinal Cord Injury Patients. Int. J. Mol. Sci. 2021, 22, 744. [Google Scholar] [CrossRef]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The Paradox of Chronic Neuroinflammation, Systemic Immune Suppression and Autoimmunity after Traumatic Chronic Spinal Cord Injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef]
- Gao, T.Y.; Huang, F.F.; Xie, Y.Y.; Wang, W.Q.; Di Wang, L.; Mu, D.; Cui, Y.; Wang, B. Dynamic Changes in the Systemic Immune Responses of Spinal Cord Injury Model Mice. Neural Regen. Res. 2021, 16, 382. [Google Scholar] [CrossRef] [PubMed]
- Fraussen, J.; Beckers, L.; van Laake-Geelen, C.C.M.; Depreitere, B.; Deckers, J.; Cornips, E.M.J.; Peuskens, D.; Somers, V. Altered Circulating Immune Cell Distribution in Traumatic Spinal Cord Injury Patients in Relation to Clinical Parameters. Front. Immunol. 2022, 13, 3296. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Ditor, D.S. Immune Dysfunction and Chronic Inflammation Following Spinal Cord Injury. Spinal Cord 2015, 53, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Sauls, R.S.; McCausland, C.; Taylor, B.N. Histology, T-Cell Lymphocyte; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Álvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.A.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Berg, G.; Jenmalm, M.C.; Ernerudh, J. FOXP3+ Regulatory T Cells and T Helper 1, T Helper 2, and T Helper 17 Cells in Human Early Pregnancy Decidua. Biol. Reprod. 2010, 82, 698–705. [Google Scholar] [CrossRef]
- Girón, S.H.; Gómez-Lahoz, A.M.; Sanz, J.M.; Fraile-Martínez, O.; Jiménez, D.J.; Garcia-Montero, C.; de Leon-Oliva, D.; Ortega, M.A.; Atienza-Perez, M.; Diaz, D.; et al. Patients with Chronic Spinal Cord Injury and a Long Period of Evolution Exhibit an Altered Cytokine Production by CD4 and CD8 T Cell Populations. Int. J. Mol. Sci. 2023, 24, 7048. [Google Scholar] [CrossRef]
- Gómez-Lahoz, A.M.; Haro Girón, S.; Monserrat Sanz, J.; Fraile-Martínez, O.; Garcia-Montero, C.; Jiménez, D.J.; De Leon-Oliva, D.; Ortega, M.A.; Atienza-Perez, M.; Diaz, D.; et al. Abnormal Characterization and Distribution of Circulating Regulatory T Cells in Patients with Chronic Spinal Cord Injury According to the Period of Evolution. Biology 2023, 12, 617. [Google Scholar] [CrossRef]
- Monahan, R.; Stein, A.; Gibbs, K.; Bank, M.; Bloom, O. Circulating T Cell Subsets Are Altered in Individuals with Chronic Spinal Cord Injury. Immunol. Res. 2015, 63, 3–10. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Chen, H.; Peng, H.; Wang, P.C.; Zou, T.; Feng, X.M.; Wan, B.W. wen Role of Regulatory T Cells in Spinal Cord Injury. Eur. J. Med. Res. 2023, 28, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Xia, H.; Wang, B.; Zhang, R.; Zeng, Q.; Guo, L.; Shen, K.; Wang, B.T.; Zhong, Y.; et al. CD8 T Cell-Derived Perforin Aggravates Secondary Spinal Cord Injury through Destroying the Blood-Spinal Cord Barrier. Biochem. Biophys. Res. Commun. 2019, 512, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Q.; Huang, T.; Zhang, H.; Chen, X.; Ling, J.; Yang, Y. Regenerative Role of T Cells in Nerve Repair and Functional Recovery. Front. Immunol. 2022, 13, 3389. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.-M.; Chen, S.-Y.; Fu, S.-P.; Zhou, H.; Zhang, Q.; Ao, J.; Luo, X.-P.; Zhang, T. Regulatory Role of Mesenchymal Stem Cells on Secondary Inflammation in Spinal Cord Injury. J. Inflamm. Res. 2022, 15, 573. [Google Scholar] [CrossRef] [PubMed]
- Zha, J. Effect of Chronic Thoracic Spinal Cord Injury (SCI) on the Peripheral Immune System in Mice 2014. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, 2014. [Google Scholar]
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bank, M.; Bloom, O. Pilot Study: Elevated Circulating Levels of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor in Patients with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Kim, S.W.; Suda, W.; Kawasumi, M.; Onawa, S.; Taguchi-Atarashi, N.; Morita, H.; Taylor, T.D.; Hattori, M.; Ohno, H. Gut Microorganisms Act Together to Exacerbate Inflammation in Spinal Cords. Nature 2020, 585, 102–106. [Google Scholar] [CrossRef]
- Carpenter, R.S.; Marbourg, J.M.; Brennan, F.H.; Mifflin, K.A.; Hall, J.C.E.; Jiang, R.R.; Mo, X.M.; Karunasiri, M.; Burke, M.H.; Dorrance, A.M.; et al. Spinal Cord Injury Causes Chronic Bone Marrow Failure. Nat. Commun. 2020, 11, 3702. [Google Scholar] [CrossRef]
- Gucluler, G.; Adiguzel, E.; Gungor, B.; Kahraman, T.; Gursel, M.; Yilmaz, B.; Gursel, I. Impaired Toll like Receptor-7 and 9 Induced Immune Activation in Chronic Spinal Cord Injured Patients Contributes to Immune Dysfunction. PLoS ONE 2017, 12, e0171003. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Mechanisms Underlying T Cell Ageing. Nat. Rev. Immunol. 2019, 19, 573. [Google Scholar] [CrossRef]
- Monserrat, J.; Bohórquez, C.; Gómez Lahoz, A.M.; Movasat, A.; Pérez, A.; Ruíz, L.; Díaz, D.; Chara, L.; Sánchez, A.I.; Albarrán, F.; et al. The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-Onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells 2019, 8, 871. [Google Scholar] [CrossRef]
- Covre, L.P.; De Maeyer, R.P.H.; Gomes, D.C.O.; Akbar, A.N. The Role of Senescent T Cells in Immunopathology. Aging Cell 2020, 19, e13272. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Angiari, S. The Role of T Cell Senescence in Neurological Diseases and Its Regulation by Cellular Metabolism. Front. Immunol. 2021, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28- and CD8+ CD57+ T Cells and Their Role in Health and Disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Paramos-de-Carvalho, D.; Martins, I.; Cristóvão, A.M.; Dias, A.F.; Neves-Silva, D.; Pereira, T.; Chapela, D.; Farinho, A.; Jacinto, A.; Saúde, L. Targeting Senescent Cells Improves Functional Recovery after Spinal Cord Injury. Cell Rep. 2021, 36, 109334. [Google Scholar] [CrossRef] [PubMed]
- Fenn, A.M.; Hall, J.C.E.; Gensel, J.C.; Popovich, P.G.; Godbout, J.P. IL-4 Signaling Drives a Unique Arginase+/IL-1β+ Microglia Phenotype and Recruits Macrophages to the Inflammatory CNS: Consequences of Age-Related Deficits in IL-4Rα after Traumatic Spinal Cord Injury. J. Neurosci. 2014, 34, 8904. [Google Scholar] [CrossRef] [PubMed]
- Pavlicek, D.; Krebs, J.; Capossela, S.; Bertolo, A.; Engelhardt, B.; Pannek, J.; Stoyanov, J. Immunosenescence in Persons with Spinal Cord Injury in Relation to Urinary Tract Infections -a Cross-Sectional Study. Immun. Ageing 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Knerlich-Lukoschus, F.; Held-Feindt, J. Chemokine-Ligands/Receptors: Multiplayers in Traumatic Spinal Cord Injury. Mediators Inflamm. 2015, 2015, 486758. [Google Scholar] [CrossRef]
- Pelisch, N.; Rosas Almanza, J.; Stehlik, K.E.; Aperi, B.V.; Kroner, A. CCL3 Contributes to Secondary Damage after Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 362. [Google Scholar] [CrossRef]
- Das, M.; Tang, X.; Han, J.Y.; Mayilsamy, K.; Foran, E.; Biswal, M.R.; Tzekov, R.; Mohapatra, S.S.; Mohapatra, S. CCL20-CCR6 Axis Modulated Traumatic Brain Injury-Induced Visual Pathologies. J. Neuroinflamm. 2019, 16, 115. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Z.; Li, X.; Lu, H. C-C Motif Chemokine Ligand 20 Regulates Neuroinflammation Following Spinal Cord Injury via Th17 Cell Recruitment. J. Neuroinflamm. 2016, 13, 162. [Google Scholar] [CrossRef]
- Parsa, N.; Zaheri, P.M.; Hewitt, R.G.; Karimi Akhormeh, A.; Taravatmanesh, S.; Wallin, L. The Rapid CD4 + T-Lymphocyte Decline and Human Immunodeficiency Virus Progression in Females Compared to Males. Sci. Rep. 2020, 10, 16816. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.Q.; Ruan, L.; Zhou, H.R.; Gao, W.L.; Zhang, Q.; Zhang, C.T. Age-Related Changes in Peripheral T-Cell Subpopulations in Elderly Individuals: An Observational Study. Open Life Sci. 2023, 18, 20220557. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, F.; Charlifue, S.; DeVivo, M.; Noonan, V.; Post, M.; Stripling, T.; Wing, P. International Spinal Cord Injury Data Sets. Spinal Cord 2006, 44, 530–534. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International Standards for Neurological Classification of Spinal Cord Injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]

| Variable | HC (n = 38) | SCI (n = 105) | SCI-SP (n = 31) | SCI-ECP (n = 32) | SCI-LCP (n = 42) |
|---|---|---|---|---|---|
| Age (years) | 31.41 ± 7.99 | 36.33 ± 13.24 | 29.24 ± 14.40 | 36.81 ± 12.26 | 40.28 ± 17.65 |
| Sex (men/ women) | 62.10%/ 37.90% | 74.28%/25.72% | 90.32%/9.68% | 77.78%/22.22% | 61.70%/ 38.30% |
| Time of injury(years) | 13.24 ± 9.47 | 2.30 ± 1.54 | 10.11 ± 2.55 | 22.26 ± 5.33 | |
| ASIA | |||||
| A | 46.67% | 41.93% | 55.55% | 44.68% | |
| B | 16.19% | 3.70% | 3.70% | 21.28% | |
| C | 16.19% | 18.51% | 18.52% | 19.15% | |
| D | 20.95% | 22.22% | 22.22% | 14.89% | |
| Injury level | |||||
| C1–C4 | 23.80% | 22.22% | 22.22% | 14.89% | |
| C5–C8 | 20.00% | 18.51% | 18.52% | 25.53% | |
| T1–T6 | 26.27% | 25.92% | 25.93% | 29.79% | |
| T7–T12 | 20.95% | 29.62% | 29.63% | 14.89% | |
| L1–L6 | 8.57% | 3.70% | 3.70% | 14.89% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro, S.; Gomez-Lahoz, A.M.; Monserrat, J.; Atienza-Pérez, M.; Fraile-Martinez, O.; Ortega, M.A.; García-Montero, C.; Díaz, D.; Lopez-Dolado, E.; Álvarez-Mon, M. Patients with Chronic Spinal Cord Injury Display a Progressive Alteration over the Years of the Activation Stages of the T Lymphocyte Compartment. Int. J. Mol. Sci. 2023, 24, 17596. https://doi.org/10.3390/ijms242417596
Haro S, Gomez-Lahoz AM, Monserrat J, Atienza-Pérez M, Fraile-Martinez O, Ortega MA, García-Montero C, Díaz D, Lopez-Dolado E, Álvarez-Mon M. Patients with Chronic Spinal Cord Injury Display a Progressive Alteration over the Years of the Activation Stages of the T Lymphocyte Compartment. International Journal of Molecular Sciences. 2023; 24(24):17596. https://doi.org/10.3390/ijms242417596
Chicago/Turabian StyleHaro, Sergio, Ana M. Gomez-Lahoz, Jorge Monserrat, Mar Atienza-Pérez, Oscar Fraile-Martinez, Miguel A. Ortega, Cielo García-Montero, David Díaz, Elisa Lopez-Dolado, and Melchor Álvarez-Mon. 2023. "Patients with Chronic Spinal Cord Injury Display a Progressive Alteration over the Years of the Activation Stages of the T Lymphocyte Compartment" International Journal of Molecular Sciences 24, no. 24: 17596. https://doi.org/10.3390/ijms242417596






