Design, Characterization, and Biological Activities of Erythromycin-Loaded Nanodroplets to Counteract Infected Chronic Wounds Due to Streptococcus pyogenes
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Design, Preparation and Characterization of Nanodroplet Formulations
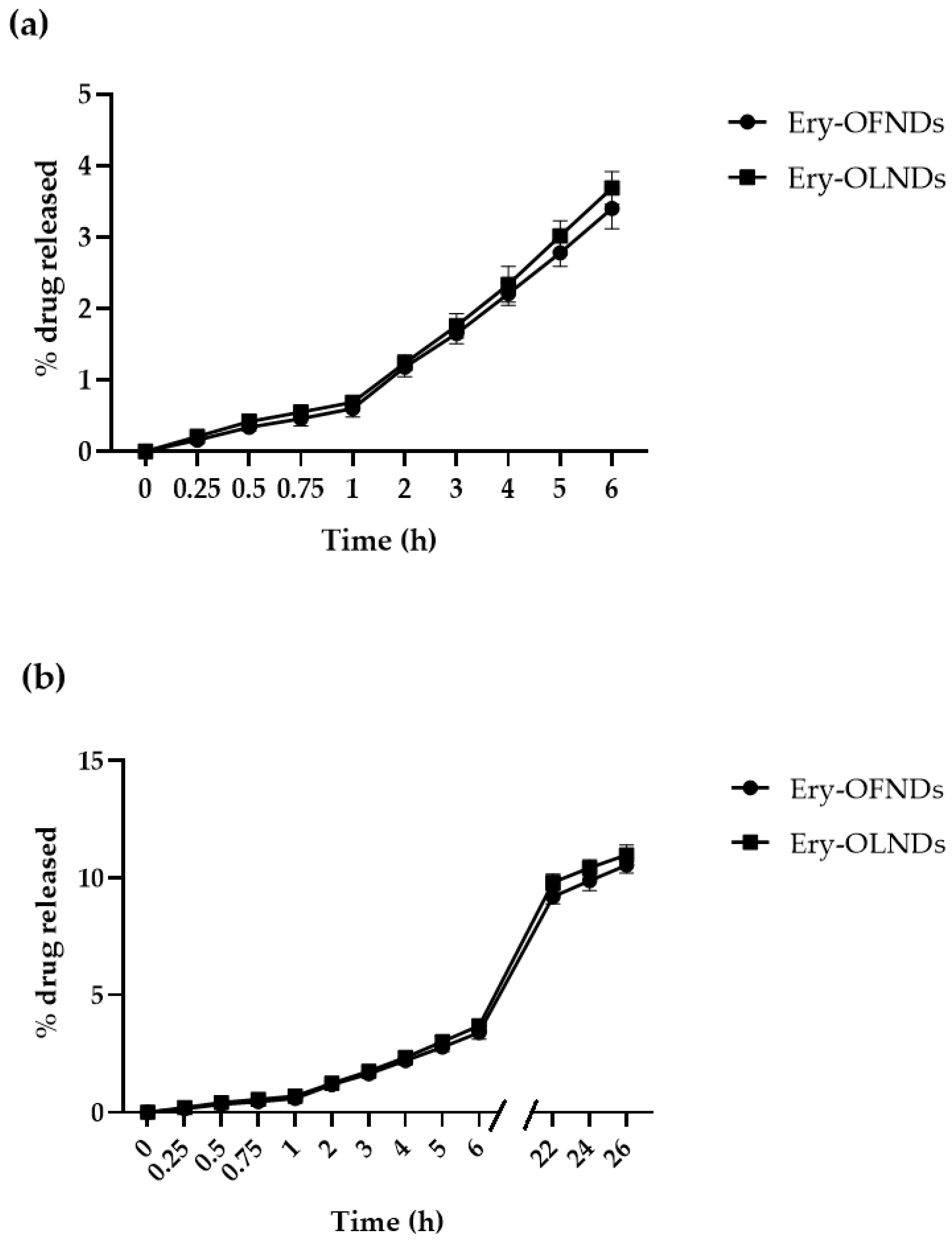
4.2. Evaluation of the In Vitro Release Kinetics of Erythromycin Estolate from Erythromycin-Loaded OFNDs and OLNDs
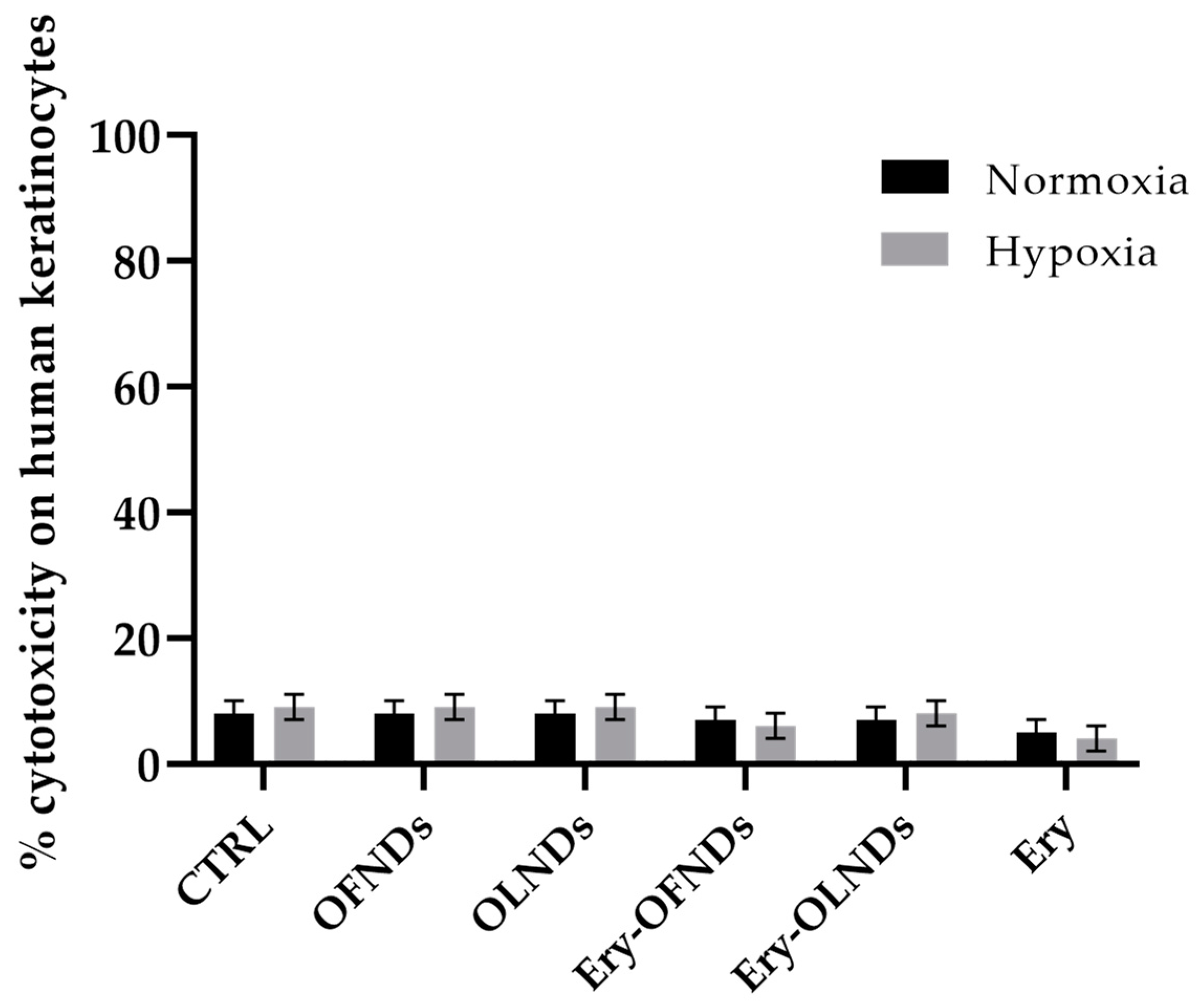
4.3. Assessment of the Potential Cytotoxic Activity of Erythromycin Loaded Nanodroplets on Human Cells by Lactate Dehydrogenase Assay
4.4. Microbiological Assays
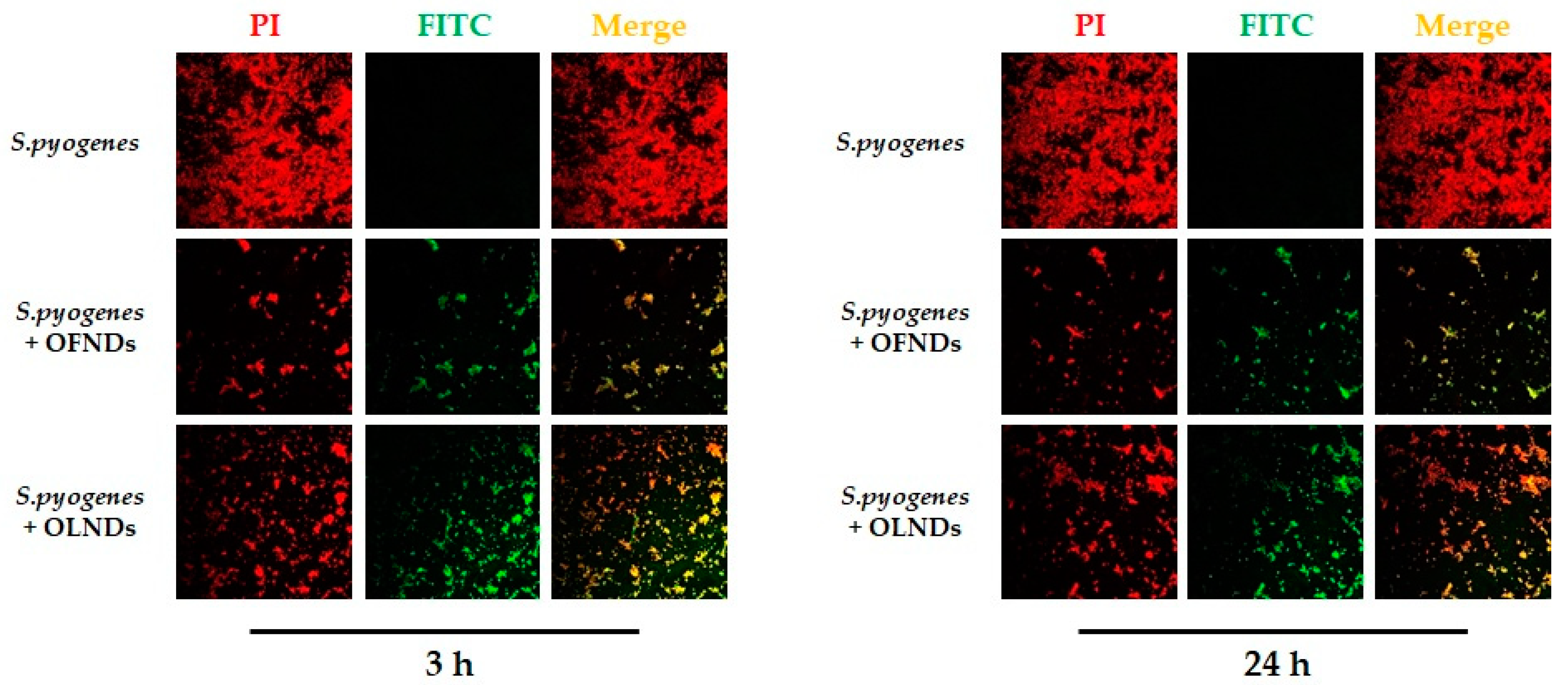
4.4.1. Determination of the Uptake of Nanodroplets by S. pyogenes by Means of Confocal Laser Microscopy Images
4.4.2. Evaluation of The In Vitro Anti-S. pyogenes Activity of Erythromycin Loaded Nanodroplets
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Efiedler, T.; Köller, T.; Ekreikemeyer, B. Streptococcus pyogenes Biofilms-Formation, Biology, and Clinical Relevance. Front. Cell. Infect. Microbiol. 2015, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Rupasinghe, H.V. Application of Medicinal Plants as a Source for Therapeutic Agents Against Streptococcus pyogenes Infections. Curr. Drug Metab. 2018, 19, 695–703. [Google Scholar] [CrossRef]
- Šmitran, A.; Vuković, D.; Opavski, N.; Gajić, I.; Marinković, J.; Božić, L.; Živanović, I.; Kekić, D.; Popović, S.; Ranin, L. Influence of subinhibitory antibiotic concentration on Streptococcus pyogenes adherence and biofilm production. Acta Microbiol. Immunol. Hung. 2018, 65, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims Sanyahumbi, A.; Colquhoun, S.; Wyber, R.; Carapetis, J.R. Global Disease Burden of Group A Streptococcus. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Efstratiou, A.; Lamagni, T. Epidemiology of Streptococcus pyogenes. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Tan, L.K.; Eccersley, L.R.; Sriskandan, S. Current views of haemolytic streptococcal pathogenesis. Curr. Opin. Infect. Dis. 2014, 27, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Ralph, A.P.; Carapetis, J.R. Group A Streptococcal Diseases and Their Global Burden. Host-Pathog. Interact. Streptococcal Dis. 2012, 368, 1–27. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Manigandan, V.; Jeyapragash, D.; Bharathidasan, V.; Anandharaj, B.; Sathya, M. Eucalyptol inhibits biofilm formation of Streptococcus pyogenes and its mediated virulence factors. J. Med. Microbiol. 2020, 69, 1308–1318. [Google Scholar] [CrossRef]
- Roberts, A.L.; Connolly, K.L.; Kirse, D.J.; Evans, A.K.; Poehling, K.A.; Peters, T.R.; Reid, S.D. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, H.; Ohori, J.; Kyutoku, T.; Ito, K.; Kawabata, M. Inhibitory effects of 2-methacryloyloxyethyl phosphorylcholine polymer on the adherence of bacteria causing upper respiratory tract infection. J. Oral Microbiol. 2020, 12, 1808425. [Google Scholar] [CrossRef]
- Iuchi, H.; Ohori, J.; Matsuzaki, H.; Tokushige, T.; Toge, S.; Yamashita, M. Impact of Phosphorylcholine Expression on the Adherence and Invasion of Streptococcus pyogenes to Epithelial Cells. Microorganisms 2022, 10, 527. [Google Scholar] [CrossRef]
- Cole, J.N.; Barnett, T.C.; Nizet, V.; Walker, M.J. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Genet. 2011, 9, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Kozińska, A.; Sitkiewicz, I. Detection of Streptococcus pyogenes Virulence Factors. Methods Mol. Biol. 2020, 2136, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Yates-Alston, S.; Sarkar, S.; Cochran, M.; Kuthirummal, N.; Levi, N. Hybrid donor-acceptor polymer nanoparticles and combination antibiotic for mitigation of pathogenic bacteria and biofilms. J. Microbiol. Methods 2021, 190, 106328. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.; Sickler, J.; Vahidnia, F.; Lee, Y.-C.; Frogner, B.; Thompson, M. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011–2015. BMC Infect. Dis. 2019, 19, 193. [Google Scholar] [CrossRef]
- Hilițanu, L.N.; Mititelu-Tarțău, L.; Popa, G.E.; Buca, B.R.; Pavel, L.L.; Pelin, A.-M.; Meca, A.-D.; Bogdan, M.; Pricop, D.A. The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice. Antibiotics 2021, 10, 1471. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Hsieh, K.; Wang, T.-H. Combinatorial nanodroplet platform for screening antibiotic combinations. Lab A Chip 2022, 22, 621–631. [Google Scholar] [CrossRef]
- Djekic, L.; Martinović, M.; Ćirić, A.; Fraj, J. Composite chitosan hydrogels as advanced wound dressings with sustained ibuprofen release and suitable application characteristics. Pharm. Dev. Technol. 2019, 25, 332–339. [Google Scholar] [CrossRef]
- AlGahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.; Nemeş, N.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Mandras, N.; Argenziano, M.; Prato, M.; Roana, J.; Luganini, A.; Allizond, V.; Tullio, V.; Finesso, N.; Comini, S.; Bressan, B.E.; et al. Antibacterial and Antifungal Efficacy of Medium and Low Weight Chitosan-Shelled Nanodroplets for the Treatment of Infected Chronic Wounds. Int. J. Nanomed. 2022, 17, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Bressan, B.; Luganini, A.; Finesso, N.; Genova, T.; Troia, A.; Giribaldi, G.; Banche, G.; Mandras, N.; Cuffini, A.; et al. Comparative Evaluation of Different Chitosan Species and Derivatives as Candidate Biomaterials for Oxygen-Loaded Nanodroplet Formulations to Treat Chronic Wounds. Mar. Drugs 2021, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, C.; Wang, D.-Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.-R. Light-Activated Heterostructured Nanomaterials for Antibacterial Applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.;; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Mandras, N.; Finesso, N.; Luganini, A.; Genova, T.; Argenziano, M.; Magnetto, C.; Gulino, G.R.; Roana, J.; et al. Antimicrobial oxygen-loaded nanobubbles as promising tools to promote wound healing in hypoxic human keratinocytes. Toxicol. Rep. 2022, 9, 154–162. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2020, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaccaro, D.; Ticozzi, R.; D’Alessandro, S.; Delbue, S.; Nano, G.; Costa, E.; Argenziano, M.; Cavalli, R.; Prato, M.; Basilico, N. Effect of antibiotic-loaded chitosan nanodroplets on Enterococci isolated from chronic ulcers of the lower limbs. Futur. Microbiol. 2020, 15, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Prato, M.; Magnetto, C.; Allizond, V.; Giribaldi, G.; Argenziano, M.; Khadjavi, A.; Gulino, G.R.; Finesso, N.; Mandras, N.; et al. Antimicrobial chitosan nanodroplets: New insights for ultrasound-mediated adjuvant treatment of skin infection. Futur. Microbiol. 2015, 10, 929–939. [Google Scholar] [CrossRef]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G.; et al. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.H.; Mohammed, A.M. Biosynthesis and characterization of platinum nanoparticles using Iraqi Zahidi dates and evaluation of their biological applications. Biotechnol. Rep. 2021, 30, e00635. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A.; Moni, S.S.; Sultan, M.H.; Bukhary, H.A.; Ghazwani, M.; Alhakamy, N.A.; Meraya, A.M.; Alshahrani, S.; Alqahtani, S.S.; Bakkari, M.A.; et al. Formulation and evaluation of injectable dextran sulfate sodium nanoparticles as a potent antibacterial agent. Sci. Rep. 2021, 11, 9914. [Google Scholar] [CrossRef]
- Gao, J.; Yu, B.; Li, C.; Xu, M.; Cao, Z.; Xie, X.; Wang, W.; Liu, J. Ultrasound triggered phase-change nanodroplets for doxorubicin prodrug delivery and ultrasound diagnosis: An in vitro study. Colloids Surf. B Biointerfaces 2018, 174, 416–425. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; Du, Q.; Li, P.; Wang, Z.; Wang, A. Stimulated phase-shift acoustic nanodroplets enhance vancomycin efficacy against methicillin-resistant Staphylococcus aureus biofilms. Int. J. Nanomed. 2017, 12, 4679–4690. [Google Scholar] [CrossRef] [PubMed]
- Doostan, M.; Maleki, H.; Doostan, M.; Khoshnevisan, K.; Faridi-Majidi, R.; Arkan, E. Effective antibacterial electrospun cellulose acetate nanofibrous patches containing chitosan/erythromycin nanoparticles. Int. J. Biol. Macromol. 2020, 168, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Q.; Cao, J.; Shi, Y.; Wang, J.; Ma, H.; Sun, Y.; Song, Y. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int. J. Biol. Macromol. 2021, 191, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Imam, S.S.; Yasir, M.; Alruwaili, N.K.; Alsaidan, O.A.; Warsi, M.H.; Ullah, S.N.M.N.; Alshehri, S.; Ghoneim, M.M. Preparation of NLCs-Based Topical Erythromycin Gel: In Vitro Characterization and Antibacterial Assessment. Gels 2022, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Gilani, S.J.; Jahangir, M.A.; Zafar, A.; Alshehri, S.; Yasir, M.; Kala, C.; Taleuzzaman, M.; Imam, S.S. Clarithromycin-Loaded Ocular Chitosan Nanoparticle: Formulation, Optimization, Characterization, Ocular Irritation, and Antimicrobial Activity. Int. J. Nanomed. 2020, 15, 7861–7875. [Google Scholar] [CrossRef]
- Hsiao, K.-H.; Huang, C.-M.; Lee, Y.-H. Novel Rifampicin and Indocyanine Green Co-Loaded Perfluorocarbon Nanodroplets Provide Effective In Vivo Photo–Chemo–Probiotic Antimicrobility against Pathogen of Acne Vulgaris Cutibacterium acnes. Nanomaterials 2020, 10, 1095. [Google Scholar] [CrossRef]
- Platon, V.-M.; Dragoi, B.; Marin, L. Erythromycin Formulations—A Journey to Advanced Drug Delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef]
- Scutera, S.; Argenziano, M.; Sparti, R.; Bessone, F.; Bianco, G.; Bastiancich, C.; Castagnoli, C.; Stella, M.; Musso, T.; Cavalli, R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics 2021, 10, 57. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Allizond, V.; Bertea, C.; Novara, C.; Cochis, A.; Geobaldo, F.; Bistolfi, A.; Cuffini, A.; Rimondini, L.; et al. Grafting of the peppermint essential oil to a chemically treated Ti6Al4V alloy to counteract the bacterial adhesion. Surf. Coat. Technol. 2019, 378, 125011. [Google Scholar] [CrossRef]
- Alhodieb, F.S.; Barkat, A.; Barkat, H.A.; Ab Hadi, H.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469. [Google Scholar] [CrossRef]


| Nanodroplets | Outer Shell Polysaccharide | Inner Core Fluorocarbon | Fluorocarbon Boiling Point | O2 Content (g/mL ± SD) | Average Diameter (nm ± SD) | Polydispersity Index | Zeta Potential (mV ± SD) | Osmolarity (mOsm ± SD) | Viscosity (cP ± SD) |
|---|---|---|---|---|---|---|---|---|---|
| OFNDs | LW-chitosan | DFP | 51 °C | / | 404.5 ± 22.95 | 0.22 | 31.95 ± 3.44 | 283 ± 0.4 | 1.33 ± 0.01 |
| OLNDs | LW-chitosan | DFP | 51 °C | 0.45 ± 0.01 | 437.3 ± 33.08 | 0.22 | 32.07 ± 2.10 | 282 ± 0.6 | 1.32 ± 0.02 |
| Ery-OFNDs | LW-chitosan | DFP | 51 °C | / | 409.8 ± 13.96 | 0.23 | 30.15 ± 3.97 | 285 ± 0.5 | 1.34 ± 0.01 |
| Ery-OLNDs | LW-chitosan | DFP | 51 °C | 0.46 ± 0.01 | 440.2 ± 30.23 | 0.23 | 30.82 ± 3.57 | 285 ± 0.4 | 1.32 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandras, N.; Luganini, A.; Argenziano, M.; Roana, J.; Giribaldi, G.; Tullio, V.; Cavallo, L.; Prato, M.; Cavalli, R.; Cuffini, A.M.; et al. Design, Characterization, and Biological Activities of Erythromycin-Loaded Nanodroplets to Counteract Infected Chronic Wounds Due to Streptococcus pyogenes. Int. J. Mol. Sci. 2023, 24, 1865. https://doi.org/10.3390/ijms24031865
Mandras N, Luganini A, Argenziano M, Roana J, Giribaldi G, Tullio V, Cavallo L, Prato M, Cavalli R, Cuffini AM, et al. Design, Characterization, and Biological Activities of Erythromycin-Loaded Nanodroplets to Counteract Infected Chronic Wounds Due to Streptococcus pyogenes. International Journal of Molecular Sciences. 2023; 24(3):1865. https://doi.org/10.3390/ijms24031865
Chicago/Turabian StyleMandras, Narcisa, Anna Luganini, Monica Argenziano, Janira Roana, Giuliana Giribaldi, Vivian Tullio, Lorenza Cavallo, Mauro Prato, Roberta Cavalli, Anna Maria Cuffini, and et al. 2023. "Design, Characterization, and Biological Activities of Erythromycin-Loaded Nanodroplets to Counteract Infected Chronic Wounds Due to Streptococcus pyogenes" International Journal of Molecular Sciences 24, no. 3: 1865. https://doi.org/10.3390/ijms24031865








