A Simplified and Efficient Method for Production of Manganese Ferrite Magnetic Nanoparticles and Their Application in DNA Isolation
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Characterization
2.2. Transmission Electron Microscopy (TEM) Results
2.3. FTIR Measurements
2.4. Electrokinetic Potential and Surface Area Measurements
2.5. Thermogravimetric Analysis of the Ferrite Samples
2.6. Magnetization Measurements
2.7. DNA Binding Experiments
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Manganese Ferrite Magnetic Nanoparticles
4.3. Physico-Chemical Characterisation of the Nanoparticles
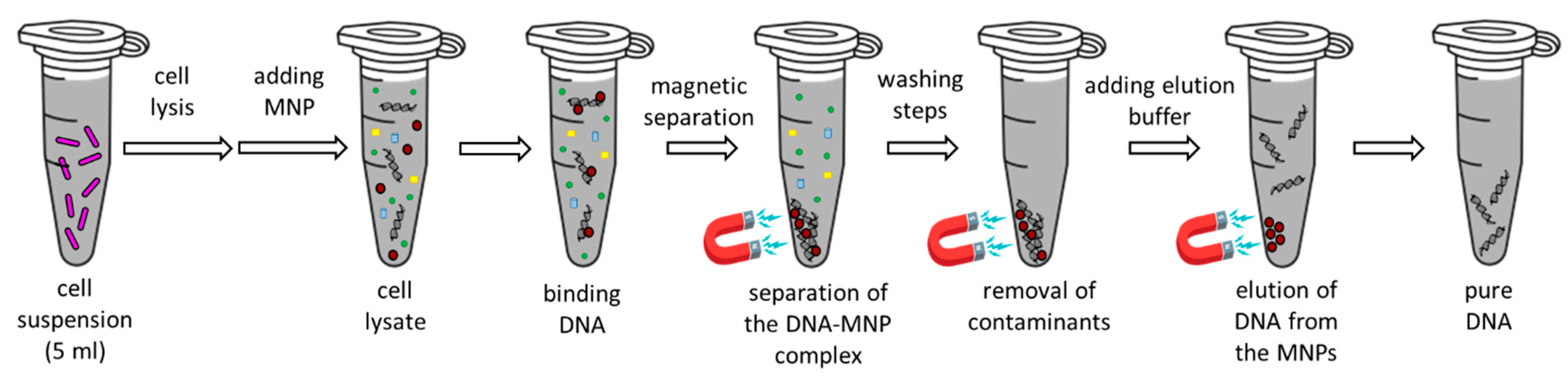
4.4. Biological Characterization of Nanoparticles
4.5. Growth of Escherichia coli
4.6. pDNA Isolation with MnFe2O4 Magnetic Nanoparticles
4.7. Gel Electrophoresis
4.8. Determination of pDNA Concentration
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondal, D.K.; Borgohain, C.; Paul, N.; Borah, J.P. Tuning hyperthermia efficiency of MnFe2O4/ZnS nanocomposites by controlled ZnS concentration. J. Mater. Res. Technol. 2019, 8, 5659–5670. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—A review. Appl. Surf. Sci. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese ferrite (MnFe2O4) Nanoparticles: From synthesis to application—A review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Das, D. Preparation and characterization of mesoporous magnetic MnFe2O4@mSiO2 nanocomposite for drug delivery application. Mater. Today Proc. 2020, 26, 87–93. [Google Scholar] [CrossRef]
- Baig, M.M.; Yousuf, M.A.; Agboola, P.O.; Khan, M.A.; Shakir, I.; Warsi, M.F. Optimization of different wet chemical routes and phase evolution studies of MnFe2O4 nanoparticles. Ceram. Int. 2019, 45, 12682–12690. [Google Scholar] [CrossRef]
- Kalaiselvan, C.R.; Thorat, N.D.; Sahu, N.K. Carboxylated PEG-Functionalized MnFe2O4 Nanocubes Synthesized in a Mixed Solvent: Morphology, Magnetic Properties, and Biomedical Applications. ACS Omega 2021, 6, 5266–5275. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Patade, S.R.; Andhare, D.D.; Somvanshi, S.B.; Jadhav, S.A.; Khedkar, M.V.; Jadhav, K.M. Self-heating evaluation of superparamagnetic MnFe2O4 nanoparticles for magnetic fluid hyperthermia application towards cancer treatment. Ceram. Int. 2020, 46, 25576–25583. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Hou, Y.-L.; Gao, S. Nanomagnetism: Principles, nanostructures, and biomedical applications. Chin. Phys. B 2014, 23, 057505. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, D.S.; Juang, R.-S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gao, K.; Han, G.; Wang, R.; Li, H.; Zhao, X.S.; Guo, P. Solvothermal synthesis of MnFe2O4 colloidal nanocrystal assemblies and their magnetic and electrocatalytic properties. New J. Chem. 2015, 39, 361–368. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, H.C.; Yang, S.Y.; Horng, H.E.; Hung, J.C.; Chen, Y.C.; Hong, C.-Y. Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 2004, 283, 210–214. [Google Scholar] [CrossRef]
- Chen, D.; Xu, R. Hydrothermal synthesis and characterization of nanocrystalline Fe3O4 powders. Mater. Res. Bull. 1998, 33, 1015–1021. [Google Scholar] [CrossRef]
- da Costa, G.M.; De Grave, E.; de Bakker, P.M.A.; Vandenberghe, R.E. Synthesis and Characterization of Some Iron Oxides by Sol-Gel Method. J. Solid State Chem. 1994, 113, 405–412. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, L.; Yang, W.; Fu, S.; Elaıssari, A. Preparation of magnetic polymeric particles via inverse microemulsion polymerization process. J. Magn. Magn. Mater. 2003, 257, 69–78. [Google Scholar] [CrossRef]
- Abu Mukh-Qasem, R.; Gedanken, A. Sonochemical synthesis of stable hydrosol of Fe3O4 nanoparticles. J. Colloid Interface Sci. 2005, 284, 489–494. [Google Scholar] [CrossRef]
- Basak, S.; Chen, D.-R.; Biswas, P. Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: Modified scaling law. Chem. Eng. Sci. 2007, 62, 1263–1268. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.; Morales, M.P.; Serna, C.J. Continuous production of γ-Fe2O3 ultrafine powders by laser pyrolysis. Mater. Lett. 1998, 35, 227–231. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, T.J.; Shin, S.Y.; Bae, N.H.; Lee, S.J.; Park, Y.M. Development of a bacterial DNA extraction modular chip using a magnetic particle and portable vibration motor. Anal. Methods 2020, 12, 1197–1202. [Google Scholar] [CrossRef]
- Kim, S.-E.; Tieu, M.V.; Hwang, S.Y.; Lee, M.-H. Magnetic Particles: Their Applications from Sample Preparations to Biosensing Platforms. Micromachines 2020, 11, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.J.; Pedro, G.C.; Gorza, F.D.; Maciel, B.G.; Ratkovski, G.P.; Mojica-Sánchez, L.C.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E.; de Melo, C.P. DNA purification using a novel γ-Fe2O3/PEDOT hybrid nanocomposite. Anal. Chim. Acta 2021, 1178, 338762. [Google Scholar] [CrossRef]
- da Silva, R.J.; Maciel, B.G.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E.; Alcaraz-Espinoza, J.J.; de Melo, C.P. Extraction of plasmid DNA by use of a magnetic maghemite-polyaniline nanocomposite. Anal. Biochem. 2019, 575, 27–35. [Google Scholar] [CrossRef]
- Sosa-Acosta, J.R.; Silva, J.A.; Fernández-Izquierdo, L.; Díaz-Castañón, S.; Ortiz, M.; Zuaznabar-Gardona, J.C.; Díaz-García, A.M. Iron Oxide Nanoparticles (IONPs) with potential applications in plasmid DNA isolation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 167–178. [Google Scholar] [CrossRef]
- Tan, S.C.; Yiap, B.C. DNA, RNA, and protein extraction: The past and the present. J. Biomed. Biotechnol. 2009, 2009, 574398. [Google Scholar] [CrossRef] [Green Version]
- Berensmeier, S. Magnetic particles for the separation and purification of nucleic acids. Appl. Microbiol. Biotechnol. 2006, 73, 495–504. [Google Scholar] [CrossRef]
- Corchero, J.L.; Villaverde, A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009, 27, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Jamatia, T.; Machovsky, M.; Skoda, D.; Urbánek, P.; Masař, M.; Urbánek, M.; Kalina, L. Impact of sonochemical synthesis condition on the structural and physical properties of MnFe2O4 spinel ferrite nanoparticles. Ultrason. Sonochem. 2020, 61, 104839. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, D.; Lu, W.; Zhang, K. One-pot sonochemical synthesis of magnetite@ reduced graphene oxide nanocomposite for high performance Li ion storage. Ultrason. Sonochem. 2018, 45, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Kubendhiran, S.; Chen, S.-M. Facile one-pot sonochemical synthesis of Ni doped bismuth sulphide for the electrochemical determination of promethazine hydrochloride. Ultrason. Sonochem. 2019, 54, 68–78. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, R. Mini submersible pump assisted sonochemical reactors: Large-scale synthesis of zinc oxide nanoparticles and nanoleaves for antibacterial and anti-counterfeiting applications. Ultrason. Sonochem. 2019, 52, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Martinez–Vargas, S.; Martínez, A.I.; Hernández–Beteta, E.E.; Mijangos–Ricardez, O.F.; Vázquez–Hipólito, V.; Patiño-Carachure, C.; López–Luna, J. As(III) and As(V) adsorption on manganese ferrite nanoparticles. J. Mol. Struct. 2018, 1154, 524–534. [Google Scholar] [CrossRef]
- Elahi, I.; Zahira, R.; Mehmood, K.; Jamil, A.; Amin, N. Co-precipitation synthesis, physical and magnetic properties of manganese ferrite powder. Afr. J. Pure Appl. Chem. 2012, 6, 1–5. [Google Scholar]
- Makridis, A.; Topouridou, K.; Tziomaki, M.; Sakellari, D.; Simeonidis, K.; Angelakeris, M.; Yavropoulou, M.P.; Yovos, J.G.; Kalogirou, O. In vitro application of Mn-ferrite nanoparticles as novel magnetic hyperthermia agents. J. Mater. Chem. B 2014, 2, 8390–8398. [Google Scholar] [CrossRef]
- Szczygieł, I.; Winiarska, K. Synthesis and characterization of manganese–zinc ferrite obtained by thermal decomposition from organic precursors. J. Therm. Anal. Calorim. 2014, 115, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Roos, W.D. Enhanced adsorption of As(V) and Mn(VII) from industrial wastewater using multi-walled carbon nanotubes and carboxylated multi-walled carbon nanotubes. Chemosphere 2020, 254, 126780. [Google Scholar] [CrossRef]
- Bandekar, A.S.; Gaikar, P.S.; Angre, A.P.; Chaughule, A.M.; Pradhan, N.S. Effect of Annealing on Microstructure and Magnetic Properties of Mn Ferrite Powder. J. Biol. Chem. Chron. 2019, 5, 74–78. [Google Scholar]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Hashim, M.; Shaari, A.H. Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2011, 323, 1745–1749. [Google Scholar] [CrossRef]
- Dolores, R.; Raquel, S.; Adianez, G.-L. Sonochemical synthesis of iron oxide nanoparticles loaded with folate and cisplatin: Effect of ultrasonic frequency. Ultrason. Sonochem. 2015, 23, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Zong, Y.; Wei, Y.; Liu, X.; Li, X.; Peng, Y.; Zheng, X. Size-effect induced cation redistribution on the magnetic properties of well-dispersed CoFe2O4 nanocrystals. J. Alloys Compd. 2020, 841, 155710. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Kadau, D.; Herrmann, H.J.; Pratsinis, S.E. Aggregate morphology evolution by sintering: Number and diameter of primary particles. J. Aerosol Sci. 2012, 46, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggersdorfer, M.L.; Pratsinis, S.E. Agglomerates and aggregates of nanoparticles made in the gas phase. Adv. Powder Technol. 2014, 25, 71–90. [Google Scholar] [CrossRef]
- Zipare, K.; Dhumal, J.; Bandgar, S.; Mathe, V.; Shahane, G. Superparamagnetic manganese ferrite nanoparticles: Synthesis and magnetic properties. J. Nanosci. 2015, 1, 178–182. [Google Scholar]
- Mukhopadhyay, A.; Joshi, N.; Chattopadhyay, K.; De, G. A Facile Synthesis of PEG-Coated Magnetite (Fe3O4) Nanoparticles and Their Prevention of the Reduction of Cytochrome C. ACS Appl. Mater. Interfaces 2012, 4, 142–149. [Google Scholar] [CrossRef]
- Zakiyah, L.B.; Saion, E.; Al-Hada, N.M.; Gharibshahi, E.; Salem, A.; Soltani, N.; Gene, S. Up-scalable synthesis of size-controlled copper ferrite nanocrystals by thermal treatment method. Mater. Sci. Semicond. 2015, 40, 564–569. [Google Scholar] [CrossRef]
- Venkatesha, P.S.; Srinivasanb, R.; Chandraboseb, A.; Alagara, M. Structural and Morphological Studies of Samarium Doped Yttrium Oxide Nanomaterials Synthesized by Chemical Co-precipitation Method. ANJAC J. Sci. 2013, 12, 9–17. [Google Scholar]
- Jacob, B.P.; Kumar, A.; Pant, R.; Singh, S.; Mohammed, E. Influence of preparation method on structural and magnetic properties of nickel ferrite nanoparticles. Bull. Mater. Sci. 2011, 34, 1345–1350. [Google Scholar] [CrossRef]
- Park, J.Y.; Daksha, P.; Lee, G.H.; Woo, S.; Chang, Y. Highly water-dispersible PEG surface modified ultra small superparamagnetic iron oxide nanoparticles useful for target-specific biomedical applications. Nanotechnology 2008, 19, 365603. [Google Scholar] [CrossRef] [PubMed]
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N.; Du, H.; Niu, L. Synthesis of spinel ferrite MFe2O4 (M=Co, Cu, Mn, and Zn) for persulfate activation to remove aqueous organics: Effects of m-site metal and synthetic method. Front. Chem. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Pershina, A.G.; Sazonov, A.E.; Filimonov, V.D. Magnetic nanoparticles–DNA interactions: Design and applications of nanobiohybrid systems. Russ. Chem. Rev. 2014, 83, 299–322. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Vanyorek, L.; Ilosvai, Á.M.; Szőri-Dorogházi, E.; Váradi, C.; Kristály, F.; Prekob, Á.; Fiser, B.; Varga, T.; Kónya, Z.; Viskolcz, B. Synthesis of iron oxide nanoparticles for DNA purification. J. Dispers. Sci. Technol. 2021, 42, 693–700. [Google Scholar] [CrossRef]
- Min, J.H.; Woo, M.-K.; Yoon, H.Y.; Jang, J.W.; Wu, J.H.; Lim, C.-S.; Kim, Y.K. Isolation of DNA using magnetic nanoparticles coated with dimercaptosuccinic acid. Anal. Biochem. 2014, 447, 114–118. [Google Scholar] [CrossRef]
- Sharma, J.N.; Pattadar, D.K.; Mainali, B.P.; Zamborini, F.P. Size Determination of Metal Nanoparticles Based on Electrochemically Measured Surface-Area-to-Volume Ratios. Anal. Chem. 2018, 90, 9308–9314. [Google Scholar] [CrossRef]
- Neelamegan, H.; Yang, D.-K.; Lee, G.-J.; Anandan, S.; Sorrentino, A.; Wu, J.J. Synthesis of Magnetite-Based Polymers as Mercury and Anion Sensors Using Single Electron Transfer-Living Radical Polymerization. ACS Omega 2020, 5, 7201–7210. [Google Scholar] [CrossRef] [Green Version]
- Maity, D.; Chandrasekharan, P.; Feng, S.-S.; Jun, D. Synthesis and studies of APTES functionalized magnetite nanoparticles. In Proceedings of the 2010 International Conference on Nanoscience and Nanotechnology, Sydney, Australia, 22–26 February 2010; pp. 94–97. [Google Scholar]
- Massoumi, B.; Ramezani, M.; Jaymand, M.; Ahmadinejad, M. Multi-walled carbon nanotubes-g-[poly (ethylene glycol)-b-poly (ε-caprolactone)]: Synthesis, characterization, and properties. J. Polym. Res. 2015, 22, 214. [Google Scholar] [CrossRef]
- Mazo-Zuluaga, J.; Barrero, C.A.; Díaz-Terán, J.; Jerez, A. Thermally Induced Magnetite–Haematite Transformation. Hyperfine Interact. 2003, 148, 153–161. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinuhaji, P.; Simbolon, T.R.; Hamid, M.; Hutajulu, D.A.; Sembiring, T.; Rianna, M.; Ginting, M. Influences of Co compositions in CoFe2O4 on microstructures, thermal, and magnetic properties. Case Stud. Therm. Eng. 2021, 26, 101040. [Google Scholar] [CrossRef]
- Nikolic, A.; Boskovic, M.; Spasojevic, V.; Jancar, B.; Antic, B. Magnetite/Mn-ferrite nanocomposite with improved magnetic properties. Mater. Lett. 2014, 120, 86–89. [Google Scholar] [CrossRef]
- Quy, D.V.; Hieu, N.M.; Tra, P.T.; Nam, N.H.; Hai, N.H.; Thai Son, N.; Nghia, P.T.; Anh, N.T.V.; Hong, T.T.; Luong, N.H. Synthesis of Silica-Coated Magnetic Nanoparticles and Application in the Detection of Pathogenic Viruses. J. Nanomater. 2013, 2013, 603940. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Nitika; Rana, A.; Kumar, V. Influence of temperature on structural, magnetic and thermal properties of superparamagnetic MnFe2O4 nanoparticles. Mater. Today Proc. 2021, 45, 4773–4776. [Google Scholar] [CrossRef]
- Ceylan, A.; Ozcan, S. Effects of disordered surface structure on the magnetic properties of nanocrystalline MnFe2O4. Ceram. Int. 2015, 41, 3875–3878. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Saeednia, S.; Mehran, M.; Dafeh, S.R. Modified structural and magnetic properties of nanocrystalline MnFe2O4 by pH in capping agent free co-precipitation method. J. Magn. Magn. Mater. 2017, 425, 31–36. [Google Scholar] [CrossRef]
- Bernaoui, C.R.; Bendraoua, A.; Zaoui, F.; Gallardo, J.J.; Navas, J.; Boudia, R.A.; Djediai, H.; Goual, N.e.H.; Adjdir, M. Synthesis and characterization of NiFe2O4 nanoparticles as reusable magnetic nanocatalyst for organic dyes catalytic reduction: Study of the counter anion effect. Mater. Chem. Phys. 2022, 292, 126793. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sukumar, M.; Dash, C.S.; Sutha, A.; Suresh, S.; Ubaidullah, M.; Al-Enizi, A.M.; Raza, M.K.; Kumar, D. A comparative study on NiFe2O4 and ZnFe2O4 spinel nanoparticles: Structural, surface chemistry, optical, morphology and magnetic studies. Physica B Condens. Matter 2022, 644, 414232. [Google Scholar] [CrossRef]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Zhang, S.; Cao, C.; Su, S.; Jiang, R.; Xia, A.; Zhang, H.; Li, H.; Liu, Z.; Jin, C. Structural and magnetic properties of SrFe12O19/CoFe2O4 composites with exchange coupling interaction. J. Magn. Magn. Mater. 2022, 564, 170073. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Eskandarzadeh, N.; Jalili, H.; Ghotbi Varzaneh, A.; Kameli, P.; Orue, I.; Chernenko, V.; Hajalilou, A.; Ferreira, L.P.; Cruz, M.M. Magnetic hyperthermia properties of CoFe2O4 nanoparticles: Effect of polymer coating and interparticle interactions. Ceram. Int. 2022, 48, 27995–28005. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Varvaro, G.; Peddis, D.; Kameli, P. Particle size, spin wave and surface effects on magnetic properties of MgFe2O4 nanoparticles. J. Magn. Magn. Mater. 2017, 422, 7–12. [Google Scholar] [CrossRef]
- Araújo, J.C.R.; Araujo-Barbosa, S.; Souza, A.L.R.; Iglesias, C.A.M.; Xavier, J.; Souza, P.B.; Plá Cid, C.C.; Azevedo, S.; da Silva, R.B.; Correa, M.A.; et al. Tuning structural, magnetic, electrical, and dielectric properties of MgFe2O4 synthesized by sol-gel followed by heat treatment. J. Phys Chem. Solids 2021, 154, 110051. [Google Scholar] [CrossRef]
- Naik, M.Z.; Salker, A.V. Tailoring the super-paramagnetic nature of MgFe2O4 nanoparticles by In3+ incorporation. Mater. Sci. Eng. B 2016, 211, 37–44. [Google Scholar] [CrossRef]
- Ajeesha, T.; A, A.; George, M.; Manikandan, A.; Mary, J.A.; Slimani, Y.; Almessiere, M.A.; Baykal, A. Nickel substituted MgFe2O4 nanoparticles via co-precipitation method for photocatalytic applications. Phys. B Condens. Matter 2021, 606, 412660. [Google Scholar] [CrossRef]
- Sarkar, K.; Mondal, R.; Dey, S.; Kumar, S. Cation vacancy and magnetic properties of ZnFe2O4 microspheres. Phys. B Condens. Matter 2020, 583, 412015. [Google Scholar] [CrossRef]
- Kirtimala Devi, N.; Wareppam, B.; Herojit Singh, L. Effect of sintering temperature on the magnetic properties of ZnFe2O4 composite with cobaltic oxide synthesized by chemical co precipitation method. Mater. Today Proc. 2022, 68, 196–199. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Madej, A.; Szymczak, M.; Ostaszewski, R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials 2020, 13, 5169. [Google Scholar] [CrossRef] [PubMed]
- Thorne, H.V. Electrophoretic characterization and fractionation of polyoma virus DNA. J. Mol. Biol. 1967, 24, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Aguilar, G.; Sánchez-López, A.M.; Barberán-Aceituno, C.; Carrillo-Avila, J.A.; López-Guerrero, J.A.; Aguilar-Quesada, R. DNA source selection for downstream applications based on DNA quality indicators analysis. Biopreserv. Biobank. 2016, 14, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Pervaiz, Z.; Turi, N.; Khaliq, I.; Rabbani, M.; Malik, S. Methodology: A modified method for high-quality DNA extraction for molecular analysis in cereal plants. Genet. Mol. Res. 2011, 10, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, A.; Osman, G. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods 2017, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- GenElute Plasmid Miniprep Kit; Sigma Aldrich: Saint Louis, MO, USA, 2018; (quality release date); pp. 4–5. Available online: https://www.sigmaaldrich.com (accessed on 4 September 2022).
- Omega Bio-tek. Mag Bind Ultra-Pure Plasmid DNA Kit; Omega Bio-tek: Norcross, GA, USA, 2014; pp. 5–7. Available online: https://ca.vwr.com/ (accessed on 4 September 2022).
- Yasui, K. Numerical simulations for sonochemistry. Ultrason. Sonochem. 2021, 78, 105728. [Google Scholar] [CrossRef] [PubMed]
- Treitli, S.C. BOMB Plasmid DNA Extraction Using Sera-Mag Carboxylated Beads, in Protocol 5.3. Doctoral Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2019; pp. 2–5. Available online: https://bomb.bio/protocols/ (accessed on 4 September 2022).
- Surzycki, S. Agarose Gel Electrophoresis of DNA. In Basic Techniques in Molecular Biology; Surzycki, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 163–191. [Google Scholar]

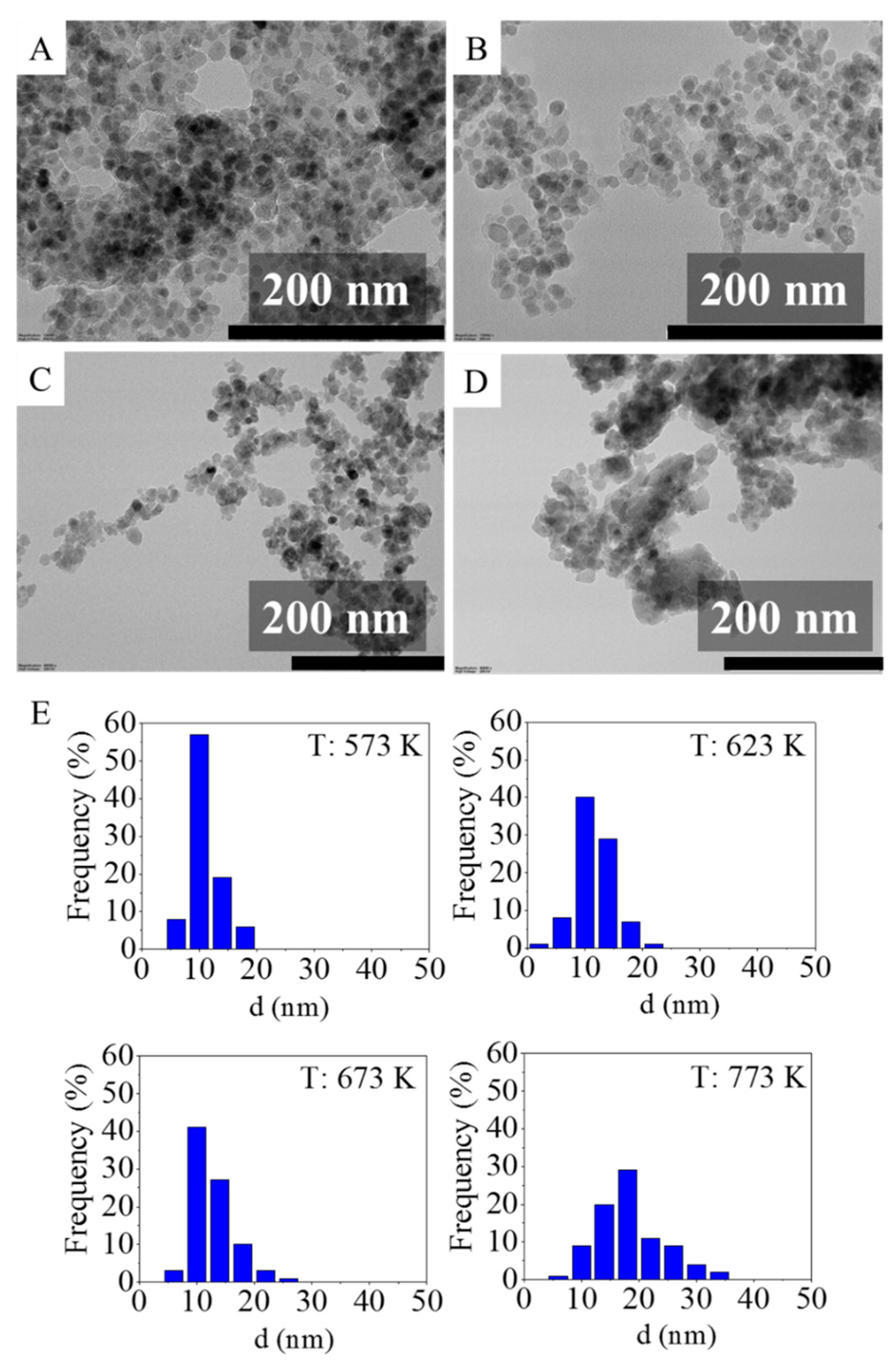





| T (K) | MnFe2O4 (nm) | Fe3O4 (nm) |
|---|---|---|
| 573 | 11 | - |
| 623 | 11 | 14 |
| 673 | 13 | 12 |
| 773 | 14 | 14 |
| T (K) | Mean (nm) | Min. (nm) | Max. (nm) | Median (nm) | P90 (nm) | P95 (nm) | P99 (nm) |
|---|---|---|---|---|---|---|---|
| 573 | 11.2 ± 2.6 | 5.6 | 18.6 | 10.7 | 14.5 | 17.1 | 18.6 |
| 623 | 11.7 ± 3.2 | 3.4 | 20.9 | 11.3 | 15.9 | 16.4 | 20.9 |
| 673 | 12.6 ± 3.8 | 5.9 | 25.3 | 11.9 | 17.8 | 19.2 | 25.3 |
| 773 | 18.4 ± 5.9 | 4.2 | 34.1 | 17.5 | 27.5 | 29.5 | 34.1 |
| Adsorbed Water (wt%) | Physisorbed Organic Compounds (wt%) | Remaining Carbon Forms (wt%) | |
|---|---|---|---|
| MnFe2O4 (573 K) | 2.5 | 6.9 | 19.25 |
| MnFe2O4 (623 K) | 1.9 | 3.3 | 10.9 |
| MnFe2O4 (673 K) | 1.7 | 0.24 | 1.0 |
| MnFe2O4 (773 K) | 0.9 | 0.15 | 0.11 |
| Volume of Initial Cell Suspension (mL) | Concentration of MnFe2O4 MNP (mg/mL) | Concentration of Purified pDNA in First (80 µL) Elution (µg/mL) | A260/280 | Concentration of Purified pDNA in Second (80 µL) Elution (µg/mL) | A260/280 |
|---|---|---|---|---|---|
| 1.5 | 6 | 44.95 ± 19.87 | 1.87 ± 0.12 | 16.63 ± 12.76 | 2.04 ± 0.14 |
| 1.5 | 20 | 63.8 ± 13.01 | 1.87 ± 0.07 | 36.93 ± 8.94 | 1.99 ± 0.07 |
| 5 | 20 | 390.60 ± 41.55 | 2.06 ± 0.03 | 178.77 ± 16.06 | 2.09 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerzsenyi, T.B.; Ilosvai, Á.M.; Szilágyi, G.; Szőri, M.; Váradi, C.; Viskolcz, B.; Vanyorek, L.; Szőri-Dorogházi, E. A Simplified and Efficient Method for Production of Manganese Ferrite Magnetic Nanoparticles and Their Application in DNA Isolation. Int. J. Mol. Sci. 2023, 24, 2156. https://doi.org/10.3390/ijms24032156
Gerzsenyi TB, Ilosvai ÁM, Szilágyi G, Szőri M, Váradi C, Viskolcz B, Vanyorek L, Szőri-Dorogházi E. A Simplified and Efficient Method for Production of Manganese Ferrite Magnetic Nanoparticles and Their Application in DNA Isolation. International Journal of Molecular Sciences. 2023; 24(3):2156. https://doi.org/10.3390/ijms24032156
Chicago/Turabian StyleGerzsenyi, Tímea B., Ágnes M. Ilosvai, Gergely Szilágyi, Milán Szőri, Csaba Váradi, Béla Viskolcz, László Vanyorek, and Emma Szőri-Dorogházi. 2023. "A Simplified and Efficient Method for Production of Manganese Ferrite Magnetic Nanoparticles and Their Application in DNA Isolation" International Journal of Molecular Sciences 24, no. 3: 2156. https://doi.org/10.3390/ijms24032156







