Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Immunofluorescence
4.2. Laser confocal immunofluorescence
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wikondi, J.; Tonfack, D.J.; Meutchieye, F.; Tomedi, T.M. Aquaculture of Heterotis niloticus in Sub-Saharan Africa: Potentials and Perspectives. Genet. Biodivers. J. 2022, 6, 37–44. [Google Scholar] [CrossRef]
- Agbugui, M.O.; Egbo, H.O.; Abhulimen, F.E. The Biology of the African Bonytongue Heterotis niloticus (Cuvier, 1829) from the Lower Niger River at Agenebode in Edo State, Nigeria. Int. J. Zool. 2021, 2021, 1748736. [Google Scholar] [CrossRef]
- Obermiller, L.E.; Pfeiler, E. Phylogenetic relationships of elopomorph fishes inferred from mitochondrial ribosomal DNA sequences. Mol. Phylogenetics Evol. 2003, 26, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, M.C.; Aragona, M.; Briglia, M.; Porcino, C.; Mhalhel, K.; Cometa, M.; Abbate, F.; Montalbano, G.; Laurà, R.; Levanti, M. The Alimentary Tract of African Bony-Tongue, Heterotis niloticus (Cuvier, 1829): Morphology Study. Animals 2022, 12, 1565. [Google Scholar] [CrossRef]
- Wilson, J.; Castro, L. Morphological diversity of the gastrointestinal tract in fishes. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 30, pp. 1–55. [Google Scholar]
- Adite, A.; ImorouToko, I.; Gbankoto, A. Fish assemblages in the degraded mangrove ecosystems of the coastal zone, Benin, West Africa: Implications for ecosystem restoration and resources conservation. J. Environ. Prot. 2013, 4, 1461–1475. [Google Scholar] [CrossRef] [Green Version]
- Silva-Sanchez, A.; Randall, T.D. Chapter 2—Anatomical Uniqueness of the Mucosal Immune System (GALT, NALT, iBALT) for the Induction and Regulation of Mucosal Immunity and Tolerance. In Mucosal Vaccines, 2nd ed.; Kiyono, H., Pascual, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–54. [Google Scholar]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.D.; Criscitiello, M.F. Comparative study of cartilaginous fish divulges insights into the early evolution of primary, secondary and mucosal lymphoid tissue architecture. Fish Shellfish. Immunol. 2020, 107, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Köllner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef]
- Sepahi, A.; Salinas, I. The evolution of nasal immune systems in vertebrates. Mol. Immunol. 2016, 69, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-Y.; Kong, W.-G.; Xu, H.-Y.; Huang, Z.-Y.; Zhang, X.-T.; Ding, L.-G.; Dong, S.; Yin, G.-M.; Dong, F.; Yu, W.; et al. Convergent Evolution of Mucosal Immune Responses at the Buccal Cavity of Teleost Fish. iScience 2019, 19, 821–835. [Google Scholar] [CrossRef]
- Lee, P.T.; Yamamoto, F.Y.; Low, C.F.; Loh, J.Y.; Chong, C.M. Gut Immune System and the Implications of Oral-Administered Immunoprophylaxis in Finfish Aquaculture. Front. Immunol. 2021, 12, 773193. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish. Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Tacchi, L.; Musharrafieh, R.; Larragoite, E.T.; Crossey, K.; Erhardt, E.B.; Martin, S.A.M.; LaPatra, S.E.; Salinas, I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014, 5, 5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, D.; Korytář, T.; Takizawa, F.; Sunyer, J.O. B cells and their role in the teleost gut. Dev. Comp. Immunol. 2016, 64, 150–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, I.; Fernández-Montero, Á.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- German, D.P.; Horn, M.H. Gut length and mass in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects. Mar. Biol. 2006, 148, 1123–1134. [Google Scholar] [CrossRef]
- Sunyer, J.O. Fishing for mammalian paradigms in the teleost immune system. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Picchietti, S.; Buonocore, F.; Guerra, L.; Belardinelli, M.C.; De Wolf, T.; Couto, A.; Fausto, A.M.; Saraceni, P.R.; Miccoli, A.; Scapigliati, G. Molecular and cellular characterization of European sea bass CD3ε+ T lymphocytes and their modulation by microalgal feed supplementation. Cell Tissue Res. 2021, 384, 149–165. [Google Scholar] [CrossRef]
- Graves, C.L.; Chen, A.; Kwon, V.; Shiau, C.E. Zebrafish harbor diverse intestinal macrophage populations including a subset intimately associated with enteric neural processes. iScience 2021, 24, 102496. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Alesci, A.; Mokhtar, D.M.; Aragona, M.; Guerrera, M.C.; Capillo, G.; Albano, M.; de Oliveira Fernandes, J.; Kiron, V.; Sayed, R.K.A.; et al. Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses. Fishes 2023, 8, 43. [Google Scholar] [CrossRef]
- Moradkhani, A.; Abdi, R.; Abadi, M.S.-A.; Nabavi, S.M.; Basir, Z. Quantification and description of gut-associated lymphoid tissue in, shabbout, Arabibarbus grypus (actinopterygii: Cypriniformes: Cyprinidae), in warm and cold season. Acta Ichthyol. Et Piscat. 2020, 50, 423–432. [Google Scholar] [CrossRef]
- Dai, X.; Shu, M.; Fang, W. Histological and ultrastructural study of the digestive tract of rice field eel, Monopterus albus. J. Appl. Ichthyol. 2007, 23, 177–183. [Google Scholar] [CrossRef]
- Aghaallaei, N.; Agarwal, R.; Benjaminsen, J.; Lust, K.; Bajoghli, B.; Wittbrodt, J.; Feijoo, C.G. Antigen-Presenting Cells and T Cells Interact in a Specific Area of the Intestinal Mucosa Defined by the Ccl25-Ccr9 Axis in Medaka. Front. Immunol. 2022, 13, 812899. [Google Scholar] [CrossRef]
- Tacchi, L.; Larragoite, E.T.; Muñoz, P.; Amemiya, C.T.; Salinas, I. African Lungfish Reveal the Evolutionary Origins of Organized Mucosal Lymphoid Tissue in Vertebrates. Curr. Biol. 2015, 25, 2417–2424. [Google Scholar] [CrossRef] [Green Version]
- Lauriano, E.; Faggio, C.; Capillo, G.; Spanò, N.; Kuciel, M.; Aragona, M.; Pergolizzi, S. Immunohistochemical characterization of epidermal dendritic-like cells in giant mudskipper, Periophthalmodon schlosseri. Fish Shellfish. Immunol. 2018, 74, 380–385. [Google Scholar] [CrossRef]
- Lauriano, E.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: A histochemical, immunohistochemical and confocal study. Fish Shellfish. Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef]
- Lauriano, E.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish. Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef]
- Lauriano, E.; Pergolizzi, S.; Cascio, P.L.; Kuciel, M.; Zizzo, N.; Guerrera, M.; Aragona, M.; Capillo, G. Expression of Langerin/CD207 in airways, lung and associated lymph nodes of a stranded striped dolphin (Stenella coeruleoalba). Acta Histochem. 2020, 122, 151471. [Google Scholar] [CrossRef]
- Lauriano, E.; Silvestri, G.; Kuciel, M.; Żuwała, K.; Zaccone, D.; Palombieri, D.; Alesci, A.; Pergolizzi, S. Immunohistochemical localization of Toll-like receptor 2 in skin Langerhans’ cells of striped dolphin (Stenella coeruleoalba). Tissue Cell 2014, 46, 113–121. [Google Scholar] [CrossRef]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S.; et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Capillo, G.; Mokhtar, D.M.; Fumia, A.; D’Angelo, R.; Lo Cascio, P.; Albano, M.; Guerrera, M.C.; Sayed, R.K.A.; Spanò, N.; et al. Expression of Antimicrobic Peptide Piscidin1 in Gills Mast Cells of Giant Mudskipper Periophthalmodon schlosseri (Pallas, 1770). Int. J. Mol. Sci. 2022, 23, 13707. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Capillo, G.; Lo Cascio, P.; Lauriano, E.R. Rodlet cells in kidney of goldfish (Carassius auratus, Linnaeus 1758): A light and confocal microscopy study. Acta Histochem. 2022, 124, 151876. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal regeneration: Vertebrates comparative overview and new perspectives for neurodegenerative diseases. Acta Zool. 2022, 103, 129–140. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef]
- Alesci, A.; Cicero, N.; Salvo, A.; Palombieri, D.; Zaccone, D.; Dugo, G.; Bruno, M.; Vadalà, R.; Lauriano, E.R.; Pergolizzi, S. Extracts deriving from olive mill waste water and their effects on the liver of the goldfish Carassius auratus fed with hypercholesterolemic diet. Nat. Prod. Res. 2014, 28, 1343–1349. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Aragona, M.; Capillo, G.; Pergolizzi, S. Marking vertebrates langerhans cells, from fish to mammals. Acta Histochem. 2020, 122, 151622. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Marino, A.; Capillo, G.; Aragona, M.; Marconi, P.; Lauriano, E. Expression of Langerin/CD 207 and α-smooth muscle actin in ex vivo rabbit corneal keratitis model. Tissue Cell 2020, 66, 1–6. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Characterization of the fish ovarian stroma during the spawning season: Cytochemical, immunohistochemical and ultrastructural studies. Fish Shellfish. Immunol. 2019, 94, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, W.X.; Cai, H.; Tedla, N.; Armishaw, C.; Di Girolamo, N.; Wang, H.W.; Hampartzoumian, T.; Simpson, J.L.; Gibson, P.G.; et al. S100A12 provokes mast cell activation: A potential amplification pathway in asthma and innate immunity. J. Allergy Clin. Immunol. 2007, 119, 106–114. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauriano, E.R.; Capillo, G.; Kuciel, M. Air-breathing in fish: Air-breathing organs and control of respiration: Nerves and neurotransmitters in the air-breathing organs and the skin. Acta Histochem. 2018, 120, 630–641. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Role of 5-HT7 receptors in the immune system in health and disease. Mol. Med. 2019, 26, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast cells in goldfish (Carassius auratus) gut: Immunohistochemical characterization. Acta Zool. 2022, 103. [Google Scholar] [CrossRef]
- Lovy, J.; Wright, G.M.; Speare, D.J. Comparative cellular morphology suggesting the existence of resident dendritic cells within immune organs of salmonids. Anat. Rec. 2008, 291, 456–462. [Google Scholar] [CrossRef]
- Kordon, A.O.; Scott, M.A.; Ibrahim, I.; Abdelhamed, H.; Ahmed, H.; Baumgartner, W.; Karsi, A.; Pinchuk, L.M. Identification of Langerhans-like cells in the immunocompetent tissues of channel catfish, Ictalurus punctatus. Fish Shellfish. Immunol. 2016, 58, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaghloul, D.M.; Derbalah, A.E.; Rutland, C.S. Unique characterization of Langerhans cells in the spleen of the African catfish (Clarias gariepinus). Matters Sel. 2017, 3, e201703000005. [Google Scholar] [CrossRef] [Green Version]
- Soleto, I.; Granja, A.G.; Simón, R.; Morel, E.; Díaz-Rosales, P.; Tafalla, C. Identification of CD8α+ dendritic cells in rainbow trout (Oncorhynchus mykiss) intestine. Fish Shellfish. Immunol. 2019, 89, 309–318. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Lo Cascio, P.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef]
- Moore, L.J.; Dijkstra, J.M.; Koppang, E.O.; Hordvik, I. CD4 homologues in Atlantic salmon. Fish Shellfish. Immunol. 2009, 26, 10–18. [Google Scholar] [CrossRef]
- Zaccone, G.; Capillo, G.; Aragona, M.; Alesci, A.; Cupello, C.; Lauriano, E.R.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; Germana, A.; et al. Gill structure and neurochemical markers in the African bonytongue (Heterotis niloticus): A preliminary study. Acta Histochem. 2022, 124, 151954. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ardavin, C.F.; Zapata, A.; Villena, A.; Solas, M.T. Gut-Associated lymphoid tissue (GALT) in the amphibian urodele Pleurodeles waltl. J. Morphol. 1982, 173, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Dixon, K.E. Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis tadpoles. J. Anat. 1978, 126, 133–144. [Google Scholar] [PubMed]
- Befus, A.D.; Johnston, N.; Leslie, G.; Bienenstock, J. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer’s patches. J. Immunol. 1980, 125, 2626–2632. [Google Scholar] [CrossRef]
- Borysenko, M.; Cooper, E.L. Lymphoid tissue in the snapping turtle, Chelydra serpentina. J. Morphol. 1972, 138, 487–497. [Google Scholar] [CrossRef]
- Colombo, B.M.; Scalvenzi, T.; Benlamara, S.; Pollet, N. Microbiota and mucosal immunity in amphibians. Front. Immunol. 2015, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Arroyo Portilla, C.; Tomas, J.; Gorvel, J.-P.; Lelouard, H. From species to regional and local specialization of intestinal macrophages. Front. Cell Dev. Biol. 2021, 8, 624213. [Google Scholar] [CrossRef]
- Aas, I.B.; Austbø, L.; König, M.; Syed, M.; Falk, K.; Hordvik, I.; Koppang, E.O. Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J. Immunol. 2014, 193, 3463–3469. [Google Scholar] [CrossRef] [Green Version]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [Green Version]
- Løken, O.M.; Bjørgen, H.; Hordvik, I.; Koppang, E.O. A teleost structural analogue to the avian bursa of Fabricius. J. Anat. 2020, 236, 798–808. [Google Scholar] [CrossRef]
- Matsunaga, T.; Rahman, A. What brought the adaptive immune system to vertebrates?—The jaw hypothesis and the seahorse. Immunol. Rev. 1998, 166, 177–186. [Google Scholar] [CrossRef]
- Pellicioli, A.C.A.; Bingle, L.; Farthing, P.; Lopes, M.A.; Martins, M.D.; Vargas, P.A. Immunosurveillance profile of oral squamous cell carcinoma and oral epithelial dysplasia through dendritic and T-cell analysis. J. Oral Pathol. Med. 2017, 46, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish. Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutsch, K.M.; Hsieh, C.-S. T cell tolerance and immunity to commensal bacteria. Curr. Opin. Immunol. 2012, 24, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedini, V.; Scocco, P.; Gargiulo, A.; Ceccarelli, P.; Lorvik, S. Glycoconjugate characterization in the intestine of Umbrina cirrosa by means of lectin histochemistry. J. Fish Biol. 2002, 61, 1363–1372. [Google Scholar] [CrossRef]
- Mabbott, N.; Donaldson, D.; Ohno, H.; Williams, I.; Mahajan, A.M. Cells: Important Immunosurveillance Posts in the Intestinal Epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic bioactive compounds shape fish mucosal immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef]
- Abelli, L.; Picchietti, S.; Romano, N.; Mastrolia, L.; Scapigliati, G. Immunohistochemistry of gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax (L.). Fish Shellfish. Immunol. 1997, 7, 235–245. [Google Scholar] [CrossRef]
- Faraj, B.A.; Olkowski, Z.L.; Jackson, R.T. Expression of a high-affinity serotonin transporter in human lymphocytes. Int. J. Immunopharmacol. 1994, 16, 561–567. [Google Scholar] [CrossRef]
- Gordon, J.; Barnes, N.M. Box 1. Where, when and why might lymphocytes’ see’serotonin and dopamine? Trends Immunol. 2003, 24, 438. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordlind, K.; Sundström, E.; Bondesson, L. Inhibiting Effects of Serotonin Antagonists on the Proliferation of Mercuric Chloride Stimulated Human Peripheral Blood T Lymphocytes. Int. Arch. Allergy Immunol. 1992, 97, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Capillo, G.; Icardo, J.M.; Fernandes, J.M.O.; Kiron, V.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Germana’, A.; et al. Neuroepithelial cells (NECs) and mucous cells express a variety of neurotransmitters and neurotransmitter receptors in the gill and respiratory air-sac of the catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): A possible role in local immune defence. Zoology 2021, 148, 125958. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Zaccone, G.; Cupello, C.; Fernandes, J.M.O.; Viswanath, K.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Icardo, J.M.; et al. Expression of acetylcholine, its contribution to regulation of immune function and O2 sensing and phylogenetic interpretations of the African butterfly fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish. Immunol. 2021, 111, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.; Guerrera, M.; Laurà, R.; Capillo, G.; Pergolizzi, S.; Aragona, M.; Abbate, F.; Germanà, A. Effect of light on the calretinin and calbindin expression in skin club cells of adult zebrafish. Histochem. Cell Biol. 2020, 154, 495–505. [Google Scholar] [CrossRef]



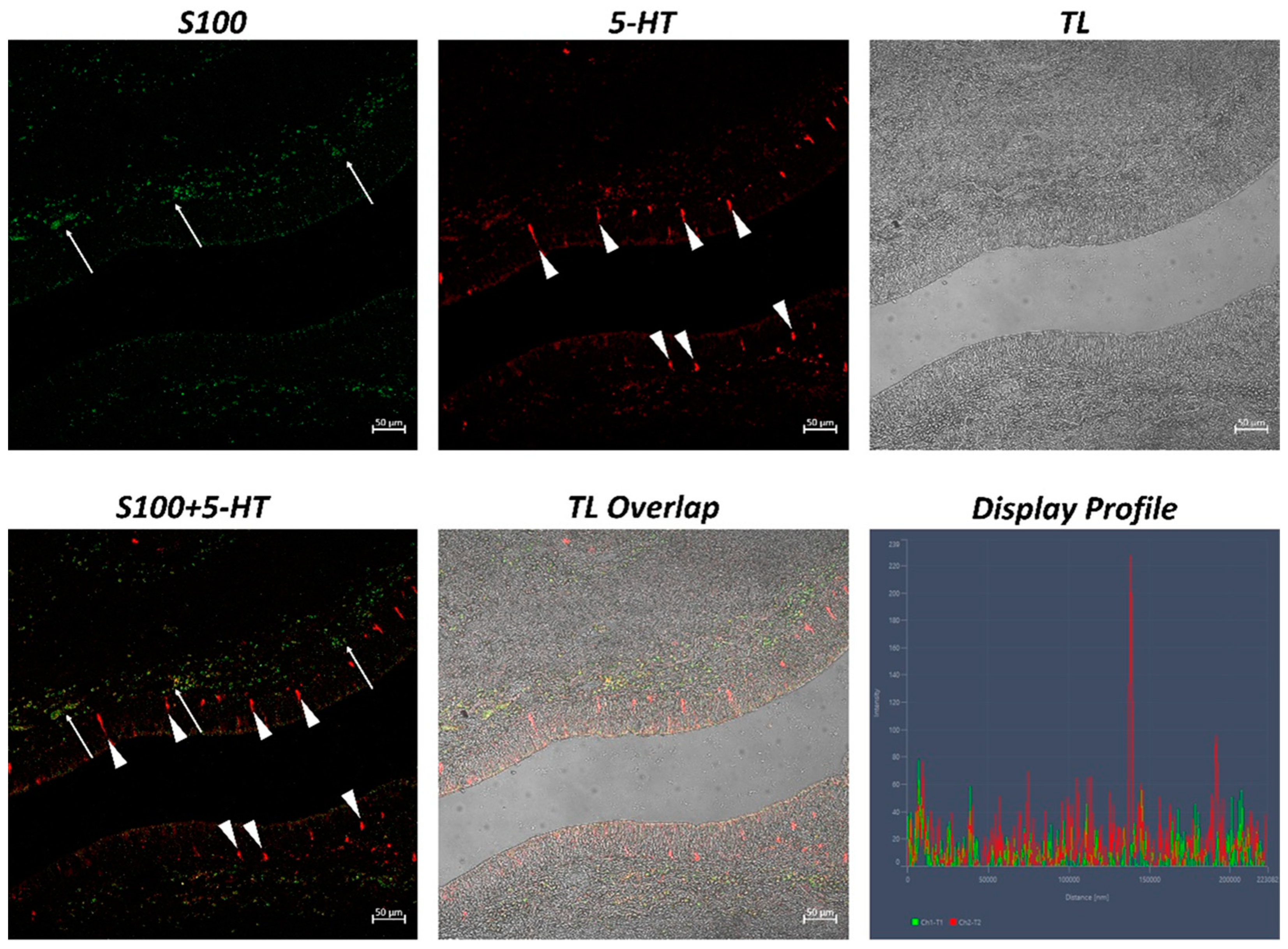
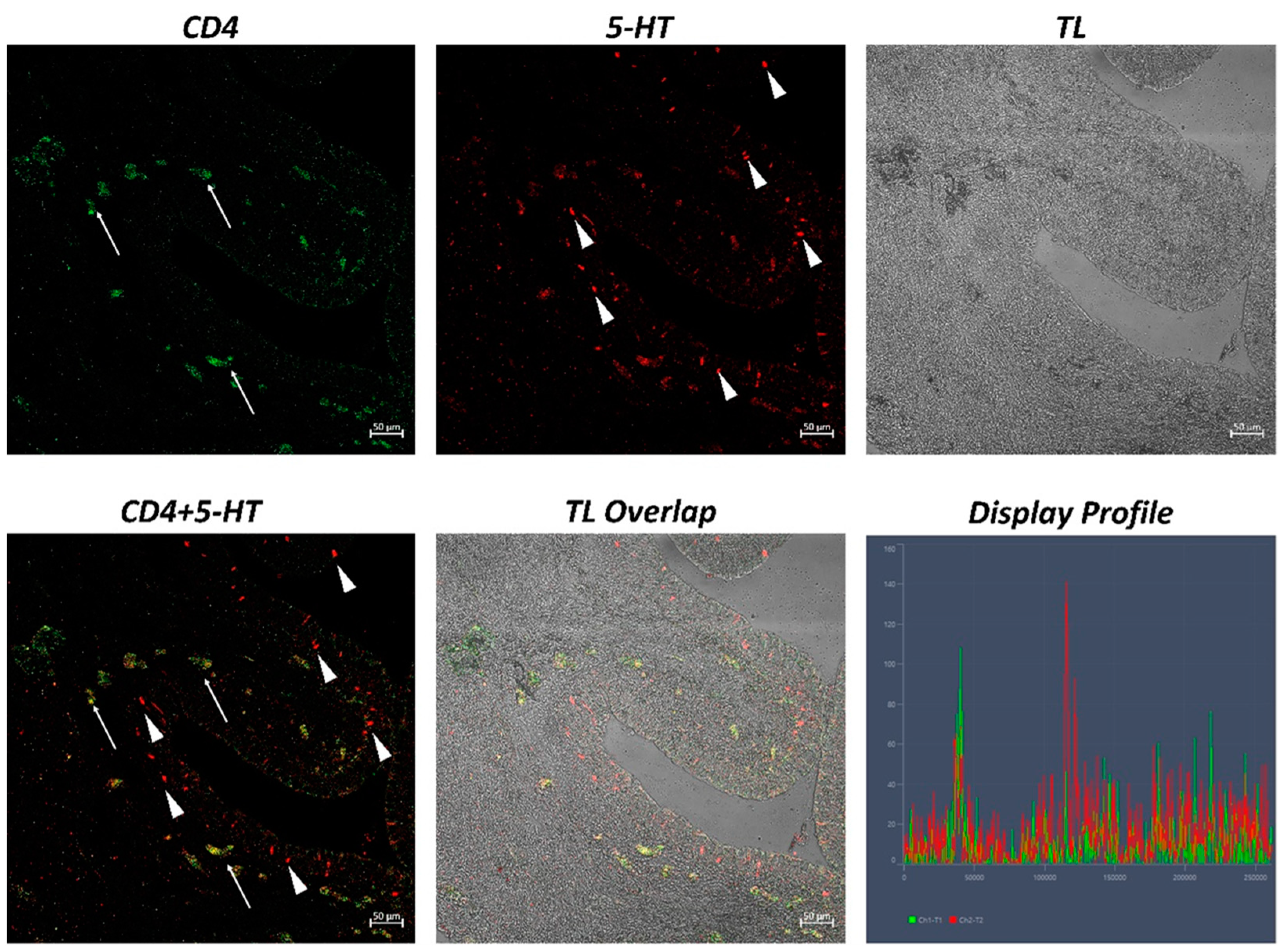

| No. of Positive Immune Cells | |
|---|---|
| TLR-2 | 476.38 ± 45.29 * |
| MHC class II | 447.44 ± 38.20 ** |
| Langerin | 329.46 ± 28.01 * |
| CD4 | 365.25 ± 33.06 * |
| 5-HT | 313.94 ± 29.36 * |
| S100 | 406.36 ± 39.85 * |
| iNOS | 418.42 ± 47.31 ** |
| TLR2 + MHC II | 416.03 ± 37.78 * |
| TLR2 + Langerin | 301.53 ± 30.25 ** |
| CD4 + 5-HT | 307.48 ± 20.34 * |
| TLR2 + iNOS | 403.94 ± 32.75 ** |
| Primary Antibodies | Supplier | Catalog Number | Source | Dilution | Antibody ID |
| TLR-2 (pAb) | Active Motif | 40981 | Rabbit | 1:125 | AB_2750977 |
| Anti-serotonin (5HT) | Sigma-Aldrich | S5545 | Rabbit | 1:300 | AB_477522 |
| Langerin/H-4 | Santa Cruz Biotechnology | sc-271272 | Mouse | 1:250 | AB_10611518 |
| MHC class II (Y-Ae) | Santa Cruz Biotechnology | Sc-32247 | Mouse | 1:250 | AB_627939 |
| S100 (s161) | Santa Cruz Biotechnology | Sc-53438 | Mouse | 1:100 | AB_630214 |
| CD4 MT310 | Santa Cruz Biotechnology | Sc-19641 | Mouse | 1:100 | AB_627055 |
| iNOS | Santa Cruz Biotechnology | Sc7271 | Mouse | 1:200 | |
| Secondary Antibodies | Supplier | Source | Dilution | Antibody ID | |
| Alexa Fluor 488 anti-mouse IgG FITC conjugated | Invitrogen | A-21202 | Donkey | 1:300 | AB_141607 |
| Alexa Fluor 594 anti-rabbit IgG TRITC conjugated | Invitrogen | A32754 | Donkey | 1:300 | AB_2762827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauriano, E.R.; Alesci, A.; Aragona, M.; Pergolizzi, S.; Miller, A.; Zuwala, K.; Kuciel, M.; Zaccone, G.; Germanà, A.; Guerrera, M.C. Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829). Int. J. Mol. Sci. 2023, 24, 2316. https://doi.org/10.3390/ijms24032316
Lauriano ER, Alesci A, Aragona M, Pergolizzi S, Miller A, Zuwala K, Kuciel M, Zaccone G, Germanà A, Guerrera MC. Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829). International Journal of Molecular Sciences. 2023; 24(3):2316. https://doi.org/10.3390/ijms24032316
Chicago/Turabian StyleLauriano, Eugenia Rita, Alessio Alesci, Marialuisa Aragona, Simona Pergolizzi, Anthea Miller, Kristina Zuwala, Michal Kuciel, Giacomo Zaccone, Antonino Germanà, and Maria Cristina Guerrera. 2023. "Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829)" International Journal of Molecular Sciences 24, no. 3: 2316. https://doi.org/10.3390/ijms24032316






