Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells
Abstract
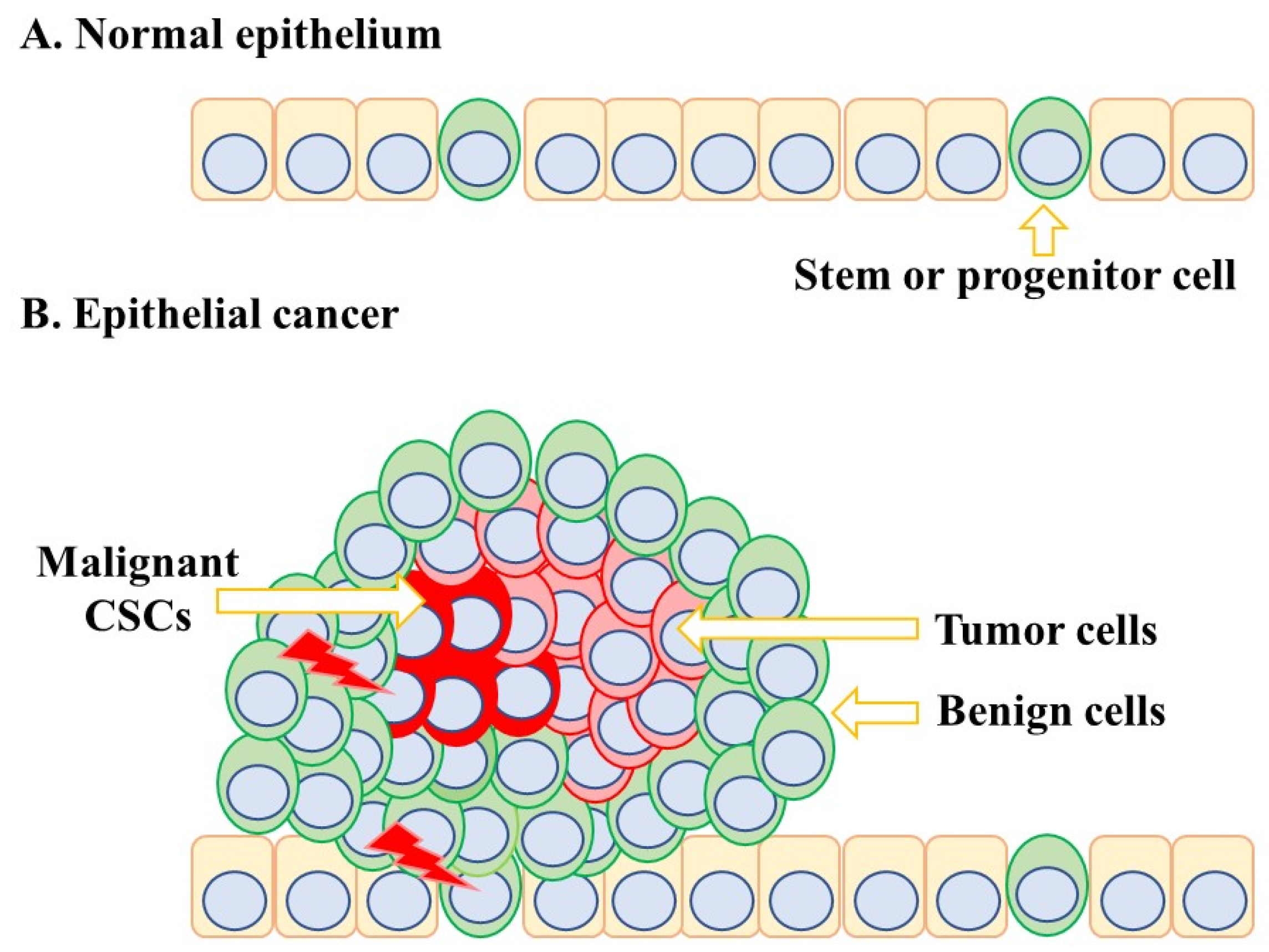
:1. Introduction
2. The Metabolism of Dietary Retinol to ATRA
3. The Biological Actions of ATRA within Normal Cells
4. Defective Retinoid Metabolism within Cancer Cells
5. Some Cancer Cells Reside in a Low ATRA Environment
6. RARγ Is an Oncogene for Various Cancers
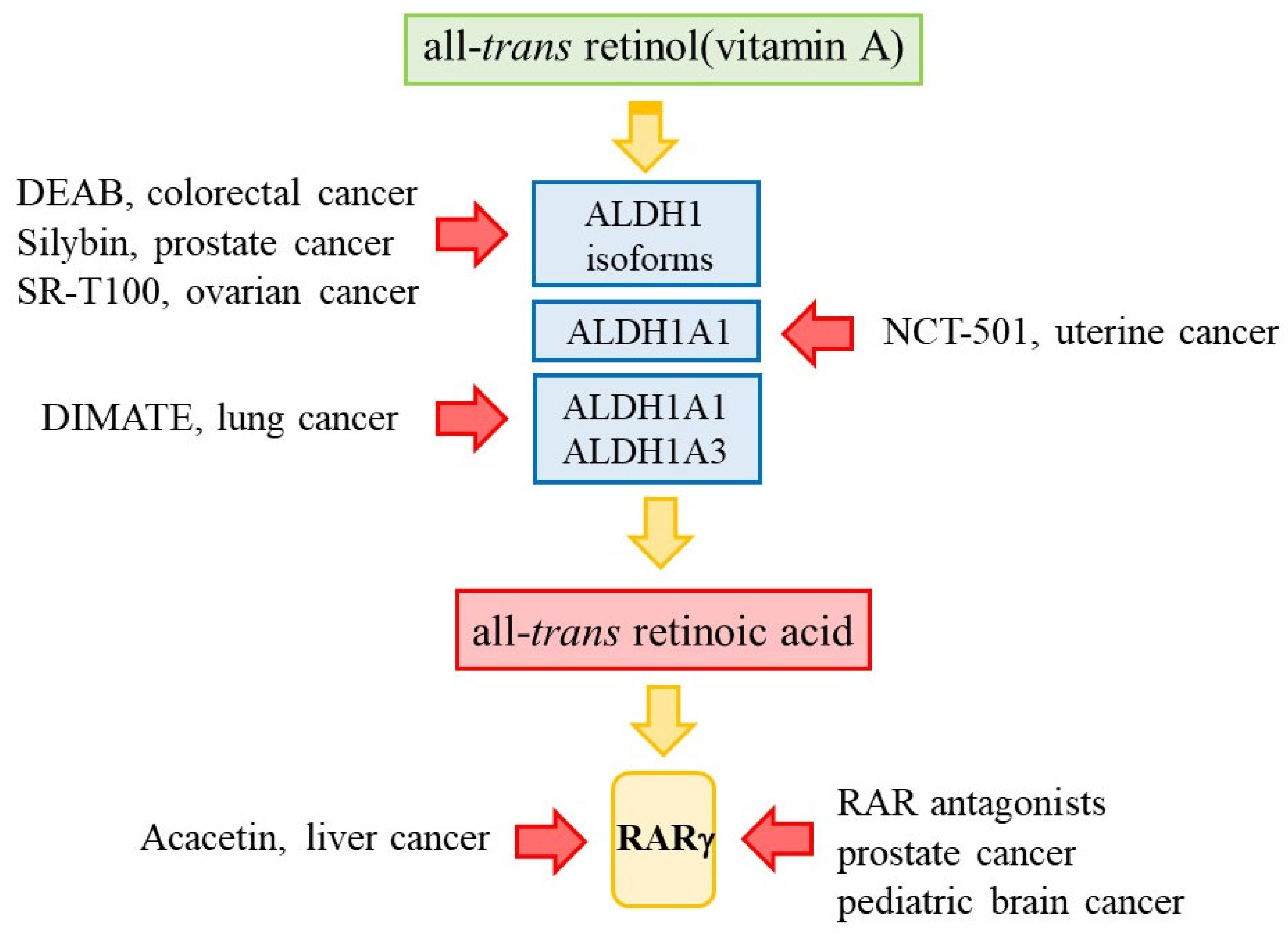
7. Inhibiting the Activity of ALDHs
8. Targeting the Action RARγ
9. Are the Agents That Target the Retinoic Acid Pathway Selective for CSCs?
10. The Prospect of Anti-CSC Agents
11. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [Green Version]
- Means, A.L.; Gudas, L.J. The roles of retinoids in vertebrate development. Annu. Rev. Biochem. 1995, 64, 201–233. [Google Scholar] [CrossRef]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Bellacosa, A. Developmental disease and cancer: Biological and clinical overlaps. Am. J. Mol. Genet. A 2013, 161, 2788–2796. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Tallman, M.S. Acute promyelocytic leukemia (APL): Remaining challenges towards a cure for all. Leuk. Lymphoma 2019, 60, 3107–3115. [Google Scholar] [CrossRef]
- Xu, X.-C. Tumor-suppressive activity of retinoic acid receptor-β in cancer. Cancer Lett. 2007, 253, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbin, A.S.; Agarwal, A.; Loriaux, M.; Cortes, J.; Deininger, M.W.; Druker, B.J. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Investig. 2011, 121, 396–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. The cancer stem cell: Premises, promises, and challenges. Nat. Med. 2011, 17, 313–317. [Google Scholar] [CrossRef]
- Napoli, J.L. Physiological Insights into all-trans retinoic acid biosynthesis. Biochem. Biophys. Acta 2012, 1821, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedishvili, N.J. Retinoic acid synthesis and degradation. Subcell Biochem. 2016, 81, 127–161. [Google Scholar]
- Kawaguchi, R.; Yu, T.; Honda, J.; Whitelegge, J.; Ping, P.; Witta, P.; Bol, D.; Sun, H. A membrane recptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Liden, M.; Erikson, U. Understanding retinol metabolism: Structure and functions of retinol dehydrogenases. JBC 2006, 281, 13001–13004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, J.L. Functions of the intracellular retinoid binding-protein. Subcell Biochem. 2016, 81, 21–76. [Google Scholar]
- Stevison, F.; Hogath, C.; Tripathy, S.; Kent, T.; Isoherranen, N. Inhibition of all-trans retinoic acid (atRA) hydrogenases CYP26A1 and CYPB1 results in dynamic, tissue-specific changes in endogenous atRA signaling. Drug. Metab. Dispos. 2017, 45, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Ortiz, D.; Nath, A.; Isoherran, N. CYP26C1 is a hydroxylase of multiple active retinoids and interacts with cellular retinoic acid binding proteins. Mol. Pharmacol. 2018, 93, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Samarut, E.; Rochette-Egly, C. Nuclear retinoic acid receptors: Conductors of the retinoic acid symphony during development. Mol. Cell. Endocrinol. 2012, 348, 348–360. [Google Scholar] [CrossRef]
- Purton, L.E.; Dworkin, S.; Olsen, G.H.; Walkley, C.R.; Fabb, S.A.; Collins, S.J.; Chambon, P. RARγ is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006, 203, 1283–1293. [Google Scholar] [CrossRef]
- Hale, L.A.; Tallafuss, A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of the retinoic acid receptor genes raraa, rarab and rarg during zebrafish development. Gene Expr. Patterns 2006, 6, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Samarut, E.; Gandin, C.; Hughes, S.; Gillet, B.; de Bernard, S.; Jouve, P.-E.; Buffat, L.; Allot, A.; Lecompte, O.; Berekelya, L.; et al. Retinoic acid receptor subtype-specific transcriptomes in the early zebrafish embryo. Mol. Endocrinol. 2014, 28, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Gratas, C.; Menot, M.L.; Chomienne, C. Retinoic acid supports granulocyte but not erythroid differentiation of myeloid progenitors in normal bone marrow cells. Leukemia 1993, 7, 1156–1162. [Google Scholar]
- Breitman, T.; Selonick, S.; Collins, S. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. USA 1980, 77, 2936–2940. [Google Scholar] [CrossRef] [Green Version]
- Kastner, P.; Chan, S. Function of RARa during the maturation of neutrophils. Oncogene 2001, 20, 7178–7185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.J. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia 2002, 16, 1896–1905. [Google Scholar] [CrossRef] [Green Version]
- Wai, H.A.; Kawakami, K.; Wada, H.; Muller, F.; Vernalis, A.B.; Brown, G.; Johnson, W.E.B. The development and growth of tissues derived from cranial neural crest and primitive mesoderm is dependent on the ligation status of retinoic acid receptor γ: Evidence that retinoic acid receptor g functions to maintain stem/progenitor cells in the absence of retinoic acid. Stem Cells Dev. 2015, 24, 507–519. [Google Scholar]
- Kashyap, V.; Laursen, K.B.; Brenet, F.; Viale, A.J.; Scandura, J.M.; Gudas, L.J. RARγ is essential for retinoic acid induced chromatin remodelling and transcriptional activation in embryonic stem cells. J. Cell Sci. 2012, 126, 999–1008. [Google Scholar] [PubMed] [Green Version]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors and cancer. Ann. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Berry, D.C.; Levi, L.; Noy, N. Holo-retinol-binding protein and its receptor STRA6 drive oncogenic transformation. Cancer Res. 2014, 74, 6341–6351. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Karunanithi, S.; Levi, L.; DeVecchio, J.; Karagkounis, G.; Reizes, O.; Lathia, J.D.; Kalady, M.E.; Noy, N. RBP4-STRA6 pathway drives cancer stem cell maintenance and mediates high-fat-diet induced colon carcinogenesis. Stem Cell Rep. 2017, 9, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Gudas, L.J. Retinoic acid receptors and GATA transcription factors activate the transcription of the human lecithin:retinol acyltransferase gene. Int. J. Biochem. Cell Biol. 2009, 41, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Mongan, N.P.; Gudas, L.J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation 2007, 75, 853–870. [Google Scholar] [CrossRef]
- Jerónimo, C.; Oliveira, J.; Lobo, F.; Pais, I.; Teixeira, M.R.; Lopes, C. Aberrant cellular retinol binding protein 1 (CRBP1) gene expression and promoter methylation in prostate cancer. J. Clin. Pathol. 2004, 57, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Ryzlak, M.T.; Ambroziak, W.; Schaffner, C.P. Human prostatic aldehyde dehydrogenase of healthy controls and diseased prostates. Biochim. Biophys. Acta 1992, 1139, 287–294. [Google Scholar] [CrossRef]
- Trasino, S.E.; Harrison, E.H.; Wang, T.T. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp. Biol. Med. 2007, 232, 762–771. [Google Scholar]
- Kim, H.; Lapointe, J.; Kaygususz, G.; Ong, D.E.; Li, C.; van de Rijn, M.; Brooks, J.D.; Pollack, J.R. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005, 65, 8118–8124. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-A.; Kwen, H.; Cho, H.; Chung, J.-Y.; Hewitt, S.M.; Kim, J.-H. ALDH1A2 is a candidate tumor suppressor gene in ovarian cancer. Cancers 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidensaal, K.; Nollart, A.; Feige, A.H.; Muller, M.; Fleming, T.; Gunkel, N.; Zaoui, K.; Grabe, N.; Weichert, W.; Weber, K.-J.; et al. Impaired aldehyde dehydrogenase 1 subfamily member 2A-dependent retinoic acid signalling is related with a mensenchymal-like phenotype and an unfavourable prognosis of head and neck squamous cell carcinoma. Mol. Cancer 2015, 14, 204. [Google Scholar] [CrossRef] [Green Version]
- Gan, C.; Pierscianek, D.; El Hindy, N.; Ahmadipour, Y.; Keyvani, K.; Sure, U.; Zhu, Y. The predominant expression of cancer stem cell marker ALD1A3 in tumor infiltrative area is associated with shorter overall survival of human glioblastoma. BMC Cancer 2020, 20, 672. [Google Scholar] [CrossRef]
- Opdenaker, L.M.; Arnold, K.M.; Pohlig, R.T.; Padmanabhan, J.S.; Flynn, D.C.; Sims-Mourtada, J. Immunohistochemical analysis of aldehyde dehydrogenase isoforms and their association with estrogen-receptor status and disease progression. Breast Cancer Targets Ther. 2014, 6, 205–209. [Google Scholar]
- Feng, X.; Zhang, M.; Wang, B.; Zhou, C.; Mu, Y.; Liu, X.; Wang, Y.; Song, Z.; Liu, P. CRABP2 regulates invasion and metastasis of breast cancer through hippo pathway dependent on ER status. J. Exp. Clin. Cancer Res. 2019, 38, 361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tan, L.; Jin, Z.; Liu, Y.; Zhang, Z. Downregulation of CRABP2 inhibit the tumorigenesis of hepatocellular carcinoma in vivo and in vitro. BioMed Res. Int. 2020, 2020, 3098327. [Google Scholar] [CrossRef]
- Wu, J.-I.; Lin, Y.-P.; Tseng, L.-W.; Chen, H.-J.; Wang, L.-H. Crabp2 promotes metastasis of lung cancer cells via HUR and integrinb/FAK/ERK signalling. Sci. Rep. 2019, 9, 45. [Google Scholar]
- Bhat, P.V.; Marcinkiewicz, M.; Yuan, L.; Mader, S. Changing patterns of retinal dehydrogenase expression parallel nephron development in the rat. J. Histochem. Cytochem. 1998, 46, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Paquali, D.; Thaller, C.; Eichele, G. Abnormal level of retinoic acid in prostate cancer. J. Clin. Endocrinol. Metab. 1996, 81, 2186–2191. [Google Scholar]
- Boyd, D.L.; Chisholm, G.D.; Habib, F.K. Nuclear retinoic acid binding protein in the human prostate. J. Endocrinol. 1985, 105, 157–162. [Google Scholar] [CrossRef]
- Jutley, J.K.; Kelleher, J.; Whelan, P.; Mikel, J. Cytosolic retinoic acid-binding protein in human prostatic dysplasia and neoplasm. Prostate 1987, 11, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Boylan, J.F.; Gudas, L.J. The level of CRABP-1 expression influences the amounts and types of all-trans retinoic acid metabolites in F9 teratocarcinoma stem cells. J. Biol. Chem. 1992, 267, 486–491. [Google Scholar] [CrossRef]
- Brown, G.; Marchwicka, A.; Cunningham, A.; Toellner, K.-M.; Marcinkowksa, E. Antagonising retinoic acid receptors increases myeloid cell production by cultured hematopoietic stem cells. Arch. Immunol. Ther. Exp. 2017, 65, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.C.; Robinson, G.S.; Perruzzi, C.A.; Calvo, A.; Desai, K.; Green, J.E.; Ali, I.U.; Smith, L.E.; Senger, D.R. Molecular profiling of angiogenesis markers. Am. J. Pathol. 2002, 161, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Ghiaur, G.; Yegnasubramanian, S.; Perkins, B.; Gucwa, J.L.; Gerber, J.M.; Jones, R.J. Regulation of human hematopoietic stem cell self-renewal by the microenvironment’s control of retinoic acid signaling. Proc. Nat. Acad. Sci. USA 2013, 110, 16121–16126. [Google Scholar] [CrossRef] [Green Version]
- Su, M.; Alonso, S.; Jones, J.W.; Yu, J.W.; Yu, J.; Kane, M.A.; Jones, R.J.; Ghiaur, G. All-trans retinoic acid activity in acute myeloid leukemia: Role of cytochrome P450 enzyme expression by the microenvironment. PLoS ONE 2015, 10, e0127790. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.B.; Wang, Q.; Khillan, J.S. Amplification of tumor inducing putative cancer stem cells by vitamin A/retinol from mammary tumors. Biochem. Biophys. Res. Comm. 2013, 436, 625–631. [Google Scholar] [CrossRef]
- Williams, S.J.; Cvetkovic, D.; Hamilton, T.C. Vitamin A metabolism is impaired in ovarian cancer. Gynecol. Oncol. 2009, 112, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.J.; Yachide, S.; Mudie, L.J.; Stephens, P.J.; Pleasance, E.D.; Stebbings, L.A.; Morsberger, L.A.; Latimer, C.; McLaren, S.; Lin, M.-L.; et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010, 467, 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Quintan, E.; Shackleton, M.; Schel, M.S.; Fullen, D.R.; Johnson, T.; Morrison, S.J. Efficient tumor formation by single human melanoma cells. Nature 2008, 456, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.L.; Campbell, C.J.V.; Hopkins, C.J.; Fiebig-Comyn, A.; Russel, J.; Ulemek, T.; Foley, R.; Leber, B.; Xenocostas, A.; Collins, T.J.; et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J. Exp. Med. 2014, 211, 1925–1935. [Google Scholar] [CrossRef] [Green Version]
- Lohnes, D.; Kastner, P.; Dierich, A.; Mark, M.; LeMeur, M.; Chambon, P. Function of retinoic acid receptor γ in the mouse. Cell 1993, 73, 643–658. [Google Scholar] [CrossRef]
- Howell, J.M.; Thompson, J.N.; Pitt, G.A. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. The male rat. J. Reprod. Fertil. 1963, 5, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Petrie, K.; Urban-Wojciuk, Z.; Sbirkov, Y.; Graham, A.; Hamann, A.; Brown, G. Retinoic acid receptor γ is a therapeutically targetable driver of growth and survival in prostate cancer. Cancer Rep. 2020, 3, e1284. [Google Scholar] [CrossRef] [PubMed]
- Meyskens Jr, F.L.; Jacobsen, J.; Nguyen, B.; Weiss, G.R.; Gandara, D.R.; MacDonald, J.S. Phase II trial of oral β-all trans-retinoic acid in hepatocellular carcinoma. Investig. New Drugs 1998, 16, 171–173. [Google Scholar] [CrossRef]
- Yan, T.-D.; Wu, H.; Zhang, H.-P.; Lu, N.; Ye, P.; Yu, F.-H.; Zhou, H.; Li, W.-G.; Cao, X.; Lin, Y.-Y.; et al. Oncogenic potential of retinoic acid receptor-gamma in hepatocellular carcinoma. Cancer Res. 2010, 70, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.M.; Perez, A.; Pereira, L.; Fan, Y.-S.; Brown, G.; Vego, F.; Petrie, K.; Swords, R.T.; Zelent, A. A case of AML characterised by a novel t(4;15)(q31;q22) that confers a growth-stimulatory response to retinoid-based therapy. Int. J. Mol. Sci. 2017, 18, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conserva, M.R.; Redavid, I.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. RARG gene dysregulation in acute myeloid leukemia. Front. Mol. Biosci. 2019, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.L.; Luo, Q.; Rui, G.; Zhang, W.; Zhang, Q.Y.; Chen, Q.X.; Shen, D.Y. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol. Cell. Biol. 2013, 33, 3416–3425. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, A.V.; Nyushko, K.M.; Zaretsky, A.R.; Shagin, D.A.; Kaprin, A.D.; Alekseev, B.Y.; Snezhkina, A.V. Upregulation of Rarb, Rarg, and Rorc Genes in Clear Cell Renal Cell Carcinoma. Biomed. Pharmacol. J. 2016, 9, 967–975. [Google Scholar] [CrossRef]
- Huang, G.L.; Song, W.; Zhou, P.; Fu, Q.R.; Lin, C.L.; Chen, Q.X.; Shen, D.Y. Oncogenic retinoic acid receptor gamma knockdown reverses multi-drug resistance of human colorectal cancer via Wnt/beta-catenin pathway. Cell Cycle 2017, 16, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakowa, K.; Koyangi-Aoi, M.; Machinaga, A.; Kakiuchi, N.; Hirano, T.; Kodama, Y.; Aoi, T. Blockage of retinoic acid signalling via RARg suppressed the proliferation of pancreatic cancer cells by arresting the cell cycle progression in G1-S phase. BMC Cancer Cell Int. 2022. [Google Scholar] [CrossRef]
- Xiu, L.; Zhao, Y.; Li, N.; Zeng, J.; Liu, J.; Fu, Y.; Gao, Q.; Wu, L. High expression of RARG accelerates ovarian cancer progression by regulating cell proliferation. Front. Oncol. 2022, 12, 1063031. [Google Scholar] [CrossRef] [PubMed]
- Van der Waals, L.M.; Borel Rinkes, I.H.M.; Kranenburg, O. ALDH1A1 expression is associated with poor differentiation, ’right-sidedness’ and poor survival in human colorectal cancer. PLoS ONE 2018, 13, e0205536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhe, H.; Gao, P.; Zhang, N.; Li, G.; Qin, J. Cancer stem cell marker ALDH1 expression is associated with lymph node metastasis and poor survival in esophageal squamous cell carcinoma: A study from high incidence area of northern China. Dis. Esophagus 2012, 25, 560–565. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Zhang, R.; Peng, J.; Guo, D.; Zhang, J.; Xiang, B.; Li, L. ALDH1A1 maintains the cancer stem-like cells properties of esophageal squamous cell carcinoma by activating the AKT signal pathway and interacting with β-catenin. Biomed. Pharmacother. 2020, 125, 109940. [Google Scholar] [CrossRef]
- Van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzman-Ramirez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010, 70, 5163–5173. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.P.; Spinola, M.; Dodge, M.; Raso, M.G.; Behrens, C.; Gao, B.; Schuster, K.; Shao, C.; Larsen, J.E.; Sullivan, L.A.; et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on Notch signalling. Cancer Res. 2010, 70, 9937–9948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flahaut, M.; Jauquier, N.; Chevalier, N.; Nardou, K.; Balmas Bourloud, K.; Joseph, J.M.; Barras, D.; Widmann, C.; Gross, N.; Renella, R.; et al. Aldehyde dehydrogenase activity plays a key role in the aggressive phenotype of neuroblastoma. BMC Cancer 2016, 16, 781. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Song, H.; Jiang, L.; Qiao, Y.; Yang, D.; Wang, D.; Li, J. Silybin prevents prostate cancer by inhibited the ALDH1A1 expression in the retinol metabolism pathway. Front. Cell Dev. Biol. 2020, 8, 574394. [Google Scholar] [CrossRef]
- Rebollido-Rios, R.; Venton, G.; Sancchez-Redondo, S.; Felip, C.I.; Fournet, G.; Gonzalez, E.; Romero-Fernandez, W.; Esuaela, D.O.B.; Di Stefano, B.; Penarroche-Diaz, R.; et al. Dual disruption of aldehyde dehydrogenases 1 and 3 promotes functional changes in the glutathione redox system and enhances chemosensitivity in nonsmall cell lung cancer. Oncogene 2020, 39, 2756–2771. [Google Scholar] [CrossRef] [Green Version]
- Kosovska, Z.; Patsalis, A.; Bajzik, V.; Durininkova, E.; Demkova, L.; Jargasova, S.; Smalkova, B.; Plava, J.; Kucerova, L.; Matushova, M. ALDH1A inhibition sensitizes colon cancer cells to chemotherapy. BMC Cancer 2018, 18, 656. [Google Scholar] [CrossRef]
- Zong, X.; Nephew, K.P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting. Cancers 2019, 11, 934. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-H.; Chiu, W.-T.; Young, M.-Y.; Change, T.-H.; Huang, Y.-F.; Chou, C.-Y. Solanum incanum extract downregulates aldehyde dehydrogenase 1-mediated stemness and inhibits tumor formation in ovarian cancer cells. J. Cancer 2015, 6, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Muralikrishman, V.; Hurley, T.D.; Nephew, K.P. Targeting aldehyde dehydrogenases to eliminate cancer stem in gynecologic malignancies. Cancers 2020, 12, 961. [Google Scholar] [CrossRef] [Green Version]
- Shiu, L.Y.; Chang, L.C.; Liang, C.H.; Huang, Y.S.; Sheu, H.M.; Kuo, K.W. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol 2007, 45, 2155–2164. [Google Scholar] [CrossRef]
- Wu, C.H.; Liang, C.H.; Shiu, L.Y.; Change, L.-C.; Lin, T.-S.; Lan, C.-C.E.; Tsai, J.-C.; Wong, T.-W.; Wei, K.-J.; Lin, T.-K.; et al. Solanum incanum extract (SR-T100) induces human cutaneous squamous cell carcinoma apoptosis through modulating tumor necrosis factor receptor signaling pathway. J. Dermatol. Sci. 2011, 63, 83–92. [Google Scholar] [CrossRef]
- Yasgar, A.; Titus, S.A.; Wang, Y.; Danchik, C.; Yang, S.M.; Vasiliou, V.; Jadhav, A.; Maloney, D.J.; Simeonov, A.; Martinez, N.J. A high-content assay enables the automated screening and identification of small molecules with specific ALDH1A1-inhibitory activity. PLoS ONE 2017, 12, e0170937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Yamawaki, K.; Ishiguro, T.; Yoshihara, K.; Ueda, H.; Sato, A.; Ohata, H.; Yoshida, Y.; Minamino, T.; Okamoto, K.; et al. ALDH-dependent glycolytic activation mediates stemness and paclitaxel resistance in patient-derived spheroid models of uterine endometrial cancer. Stem Cell Rep. 2019, 13, 730–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chefetz, I.; Grimley, E.; Yang, K.; Hong, L.; Vinogradova, E.V.; Suciu, R.; Kovalenko, I.; Karnak, D.; Morgan, C.A.; Chtcherbinine, M.; et al. A pan-ALDH1A inhibitor induces necroptosis in ovarian cancer stem-like cells. Cell Rep. 2019, 26, 3061–3075. [Google Scholar] [CrossRef] [Green Version]
- Huddle, B.C.; Grimley, E.; Buchman, C.D.; Chtcherbinine, M.; Debnath, B.; Mehta, P.; Yang, K.; Morgan, C.A.; Li, S.; Felton, J.; et al. Structure-based optimization of a novel class of aldehyde dehydrogenase 1A (ALDH1A) subfamily-selective inhibitors as potential adjuncts to ovarian cancer chemotherapy. J. Med. Chem. 2018, 61, 8754–8773. [Google Scholar] [CrossRef]
- Keedwell, R.G.; Zhao, Y.; Hammond, L.A.; Wen, K.; Qin, S.; Atangan, L.I.; Shurland, D.-L.; Wallace, D.M.A.; Bird, R.; Reitmair, A.; et al. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br. J. Cancer 2004, 91, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, L.A.; Van Krinks, C.H.; Durham, J.; Tomkins, E.; Burnett, R.D.; Jones, E.L.; Chandraratna, R.A.S.; Brown, G. Antagonists of retinoic acid receptors (RARs) are potent growth inhibitors of prostate carcinoma cells. Br. J. Cancer 2001, 85, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Brown, G. Antagonizing RARg drives necroptosis of cancer stem cells. Int. J. Mol. Sci. 2022, 23, 4814. [Google Scholar] [CrossRef]
- Chiu, H.J.; Fishman, D.A.; Hammerling, U. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprevation. FASEB J. 2008, 22, 3878–3887. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.; Kraus, W.L. On PAR with PARP: Cellular stress signalling through poly(ADP-ribose) and PARP1. Genes Dev. 2012, 26, 417–432. [Google Scholar]
- Zeng, W.; Zhang, C.; Cheng, H.; Wu, Y.-L.; Liu, J.; Chen, Z.; Huang, J.-G.; Erickson, R.E.; Chen, L.; Zhang, H.; et al. Targeting to the non-genomic action of retinoic acid-recptor-gamma by acacetin in hepatocellular carcinoma. Sci. Rep. 2017, 7, 348. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, R.; Yuasa, T.; Williams, J.A.; Byers, S.W.; Shah, S.; Pacifici, M.; Iwamoto, M.; Enamoto-Iwamoto, M. Wnt/b-catenin and retinoic acid signalin pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 2010, 285, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, N.; De, P.K.; Wang, M.; Zhang, H.; Dobtrota, E.A.; Robertson, K.A.; Durden, D.L. CSK controls retinoic acid receptor (RAR) signaling: A RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol. Cell Biol. 2007, 27, 4179–4197. [Google Scholar] [CrossRef] [Green Version]
- Kadigamuwa, C.; Choksi, S.; Xu, Q.; Cataisson, C.; Greenbaum, S.S.; Yuspa, S.H.; Liu, Z.G. Role of retinoic acid receptor-gamma in DNA damage-induced necroptosis. iScience 2019, 17, 74–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Jitkaew, S.; Choksi, S.; Kadigamuwa, C.; Qu, J.; Choe, M.; Jang, J.; Liu, C.; Liu, Z.G. The cytoplasmic nuclear receptor RARgamma controls RIP1 initiated cell death when cIAP activity is inhibited. Nat. Commun. 2017, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Parra, M.A.; Walia, M.; Sankar, M.; Gronemeyer, H. Dissecting the retinoid-induced differentiation of F9 embryonal stem cells by integrative genomics. Mol. Sys. Biol. 2011, 7, 538. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Q.; An, Q.; Zhang, C.; Mohagheghian, E.; Niu, B.; Qi, F.; Wei, F.; Chen, S.; Chen, X.; et al. Synthetic retinoid kills drug-resistant cancer stem cells via inducing RARg translocation-mediated tension reduction and chromatin decondensation. Adv. Sci. 2022, 9, 2203173. [Google Scholar] [CrossRef] [PubMed]
- Chatagnon, A.; Veber, P.; Marin, V.; Bede, J.; Triqueneaux, G.; Semon, M.; Laudet, V.; d’alche-Buc, F.; Benoit, G. RAR/RXR binding dynamics distinguish pluripotency from differentiation associated cis-regulatory elements. Nucleic Acid Res. 2015, 43, 4833–4854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G. Oncogenes and the origins of leukemia. Int. J. Mol. Sci. 2022, 23, 2293. [Google Scholar] [CrossRef] [PubMed]
- Tsou, H.C.; Lee, X.; Si, S.P.; Peacocke, M. Regulation of retinoic acid receptor expression in dermal fibroblasts. Exp. Cell Res. 1994, 211, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, T.; Wang, I.-M.; Tamara, T.; Pannamperuma, R.H.; Levine, R.; Holmes, K.L.; Morse, H.C., III; De Luca, L.H.; Ozato, K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 2000, 95, 3349–3356. [Google Scholar] [CrossRef]
- Gordy, G.; Dzhagalov, I.; He, Y.-W. Regulation of CD8+ T cell functions by RARg. Semin. Immunol. 2009, 21, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.S.W.; Wang, X.; Roberts, S.S.; Griffey, S.M.; Reczek, P.R.; Wolgemuth, D.J. Oral administration of a retinoic receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 2011, 152, 2492–2502. [Google Scholar] [CrossRef] [Green Version]
- Schulze, G.E.; Clay, R.J.; Mezza, L.E.; Bregman, C.L.; Buroker, R.A.; Frantz, J.D. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol. Sci. 2001, 59, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicology of silymarin, the major constituent of thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, J.; Zheng, M.; Shi, L. Inhibitory effects of Actinidia Chinensis planch root extracts (acRoots) on human lung cancer cells through retonic acid receptor beta. Mol. Cell. Ther. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Winkel, M.J.; Chowanski, S.; Slokinska, M. Anticancer activity of glycoalkaloids from Solanum plants: A review. Front. Pharmacol. 2022, 13, 979451. [Google Scholar] [CrossRef] [PubMed]
- Engberg, N.; Kahn, M.; Peterson, D.R.; Hansson, M.; Serup, P. Retinoic acid synthesis promotes development of neural progenitors from mouse embyronic stem cells by suppressing endogenous Wnt-dependent nodal signaling. Stem Cells 2010, 28, 1498–1509. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 2373. https://doi.org/10.3390/ijms24032373
Brown G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. International Journal of Molecular Sciences. 2023; 24(3):2373. https://doi.org/10.3390/ijms24032373
Chicago/Turabian StyleBrown, Geoffrey. 2023. "Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells" International Journal of Molecular Sciences 24, no. 3: 2373. https://doi.org/10.3390/ijms24032373





