Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla
Abstract
1. Introduction
2. Results
2.1. Adrenal Medulla Histology
2.2. Proliferation of Chromaffin Cells
2.3. Tyrosine Hydroxylase Content in Chromaffin Cells
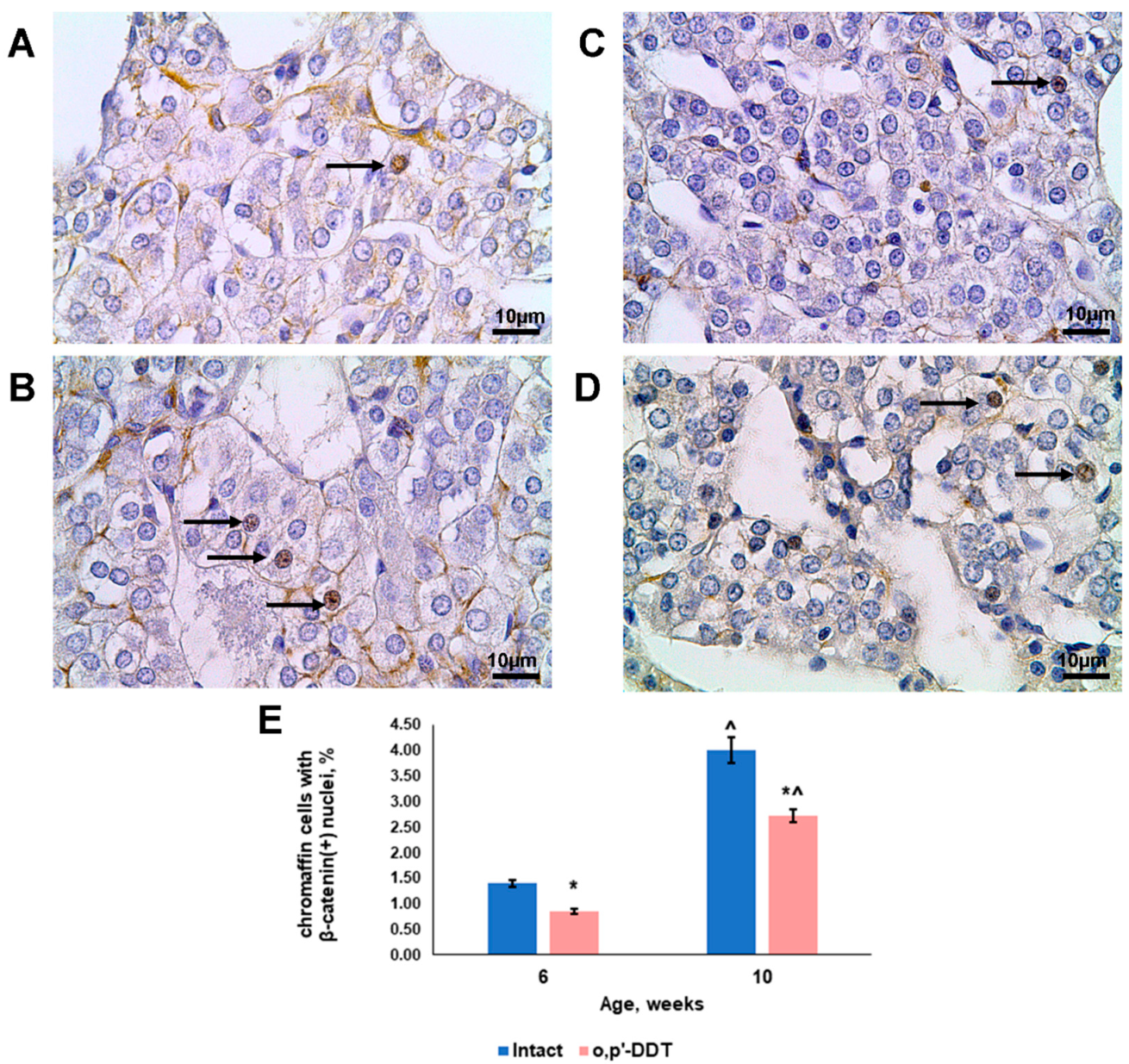
2.4. Activation of Canonical Wnt Signaling in Adrenal Chromaffin Cells
2.5. Sonic Hedgehog (Shh) Expression by Adrenal Chromaffin Cells
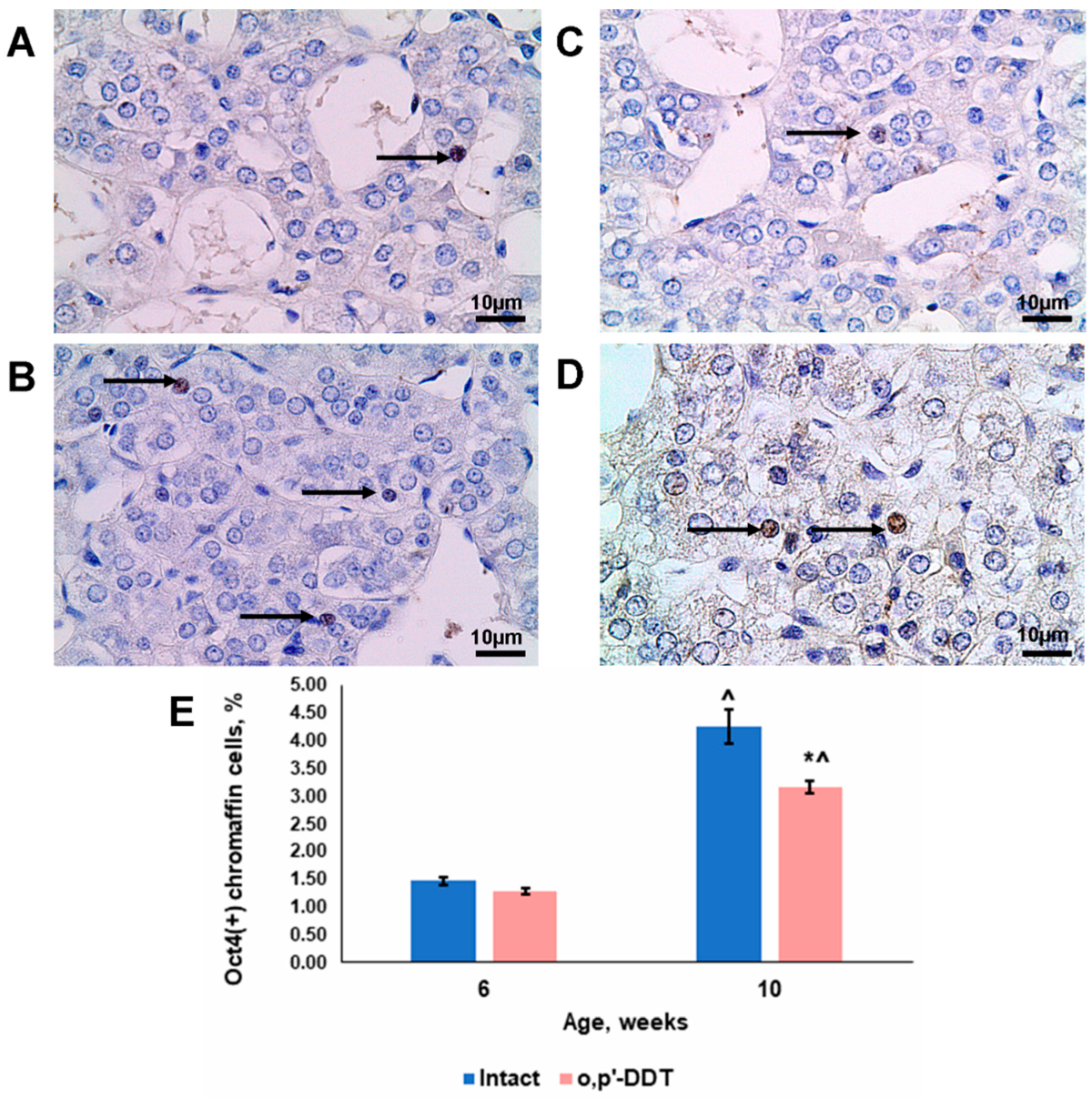
2.6. Oct4 Expression by Adrenal Chromaffin Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Adrenal Histology
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guarnotta, V.; Amodei, R.; Frasca, F.; Aversa, A.; Giordano, C. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 18, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, E.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, D.A.; Timokhina, E.P.; Chereshneva, E.V.; Ivanova, M.Y.; Payushina, O.V. Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus. Int. J. Mol. Sci. 2022, 23, 6678. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Timokhina, E.P.; Yaglov, V.V. Effects of low-dose dichlorodiphenyltrichloroethane on the morphology and function of rat thymus. Bull. Exp. Biol. Med. 2013, 155, 701–704. [Google Scholar] [CrossRef]
- Bansal, R.; Zoeller, R.T. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology 2008, 149, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, C.D.; Bansal, R.; Dunphy, K.A.; Jerry, D.J.; Vandenberg, L.N. Oxybenzone alters mammary gland morphology in mice exposed during pregnancy and lactation. J. Endocr. Soc. 2018, 2, 903–921. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Mita, L.; Cobellis, L.; Merafina, V.; Specchio, R.; Rossi, S.; Mita, D.G.; Mosca, L.; Castaldi, M.A.; De Falco, M.; et al. Triclosan and bisphenol A affect decidualization of human endometrial stromal cells. Mol. Cell. Endocrinol. 2016, 422, 74–83. [Google Scholar] [CrossRef]
- Dickerson, S.M.; Cunningham, S.L.; Patisaul, H.B.; Woller, M.J.; Gore, A.C. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology 2011, 152, 581–594. [Google Scholar] [CrossRef]
- Wenzel, A.G.; Bloom, M.S.; Butts, C.D.; Wineland, R.J.; Brock, J.W.; Cruze, L.; Unal, E.R.; Kucklick, J.R.; Somerville, S.E.; Newman, R.B. Influence of race on prenatal phthalate exposure and anogenital measurements among boys and girls. Environ. Int. 2018, 110, 61–70. [Google Scholar] [CrossRef]
- Jensen, T.K.; Frederiksen, H.; Kyhl, H.B.; Lassen, T.H.; Swan, S.H.; Bornehag, C.G.; Skakkebaek, N.E.; Main, K.M.; Lind, D.V.; Husby, S.; et al. Prenatal Exposure to Phthalates and Anogenital Distance in Male Infants from a Low-Exposed Danish Cohort (2010-2012). Environ. Health Perspect. 2016, 124, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Chakraborty, P.; Ray, S. Human exposure to organochlorine, pyrethroid and neonicotinoid pesticides: Comparison between urban and semi-urban regions of India. Environ. Pollut. 2021, 270, 116156. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Cregut, M.; Abbes, C.; Durand, M.-J.; Landoulsi, A.; Thouand, G. The environmental issues of DDT pollution and bioremediation: A multidisciplinary review. Appl. Biochem. Biotechnol. 2017, 181, 309–339. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pesticide Residues in Food–2018: Toxicological Evaluations; World Health Organization and Food and Agriculture Organization of the United Nations, WHO: Geneva, Switzerland, 2019; p. 780. [Google Scholar]
- Lymperi, S.; Giwercman, A. Endocrine disruptors and testicular function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cohn, B.A.; Cirillo, P.M.; Terry, M.B. DDT and breast cancer: Prospective study of induction time and susceptibility windows. J. Natl. Cancer Inst. 2019, 111, 803–810. [Google Scholar] [CrossRef]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: Highlights from a national Italian meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Tsomartova, D.A.; Yaglov, V.V. Effect of prenatal and postnatal exposure to low doses of DDT on catecholamine secretion in rats in different period of ontogeny. Bull. Exp. Biol. Med. 2017, 163, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Tsomartova, D.A.; Yaglov, V.V. Differences in production of adrenal steroid hormones in pubertal rats exposed to low doses of the endocrine disrupter DDT during prenatal and postnatal development. Biochem. Suppl. Ser. B Biomed. Chem. 2018, 12, 80–86. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2, The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Wolf, C., Jr.; Lambright, C.; Mann, P.; Price, M.; Cooper, R.L.; Ostby, J.; Gray, L.E., Jr. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol. Ind. Health 1999, 15, 94–118. [Google Scholar] [CrossRef]
- Gray, L.E.; Ostby, J.; Furr, J.; Wolf, C.J.; Lambright, C.; Parks, L.; Veeramachaneni, D.N.; Wilson, V.; Price, M.; Hotchkiss, A.; et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 2001, 7, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Klebanoff, M.A.; Brock, J.W.; Zhou, H.; Gray, K.A.; Needham, L.L.; Wilcox, A.J. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am. J. Epidemiol. 2002, 155, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Sampaio, D.R.; Paris, F.; Audran, F.; Orsini, M.; Neto, J.B.; Sultan, C. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int. J. Androl. 2012, 35, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Lubick, N. Examining DDT’s urogenital effects. Environ. Health Perspect. 2010, 118, A18. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Yaglov, V.V. Developmental exposure to endocrine disrupter dichlorodiphenyltrichloroethane alters transcriptional regulation of postnatal morphogenesis of adrenal zona fasciculata. Saudi J. Biol. Sci. 2020, 12, 3655–3659. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Timokhina, E.P.; Tsomartova, D.A. Low-Dose Exposure to Endocrine Disruptor Dichlorodiphenyltrichloroethane (DDT) Affects Transcriptional Regulation of Adrenal Zona Reticularis in Male Rats. Bull. Exp. Biol. Med. 2021, 170, 682–685. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Nazimova, S.V.; Yaglov, V.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Impaired Morphogenesis and Function of Rat Adrenal Zona Glomerulosa by Developmental Low-Dose Exposure to DDT Is Associated with Altered Oct4 Expression. Int. J. Mol. Sci. 2021, 22, 6324. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, E.S.; Timokhina, E.P.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Impact of Prenatal and Postnatal Exposure to Endocrine Disrupter DDT on Adrenal Medulla Function. Int. J. Mol. Sci. 2022, 23, 4912. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R.; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Fuerer, C.; Ching, W.; Harnish, K.; Logan, C.; Zeng, A.; ten Berge, D.; Kalani, Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 59–66. [Google Scholar] [CrossRef]
- Becker, J.; Wilting, Y. WNT signaling, the development of the sympathoadrenal-paraganglionic system and neuroblastoma. Cell. Mol. Life Sci. 2018, 75, 1057–1070. [Google Scholar] [PubMed]
- Brault, V.; Moore, R.; Kutsch, S.; Ishibashi, M.; Rowitch, D.H.; McMahon, A.P.; Sommer, L.; Boussadia, O.; Kemler, R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 2001, 128, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Hari, L.; Brault, V.; Kléber, M.; Lee, H.Y.; Ille, F.; Leimeroth, R.; Paratore, C.; Suter, U.; Kemler, R.; Sommer, L. Lineage-specific requirements of beta-catenin in neural crest development. J. Cell. Biol. 2002, 159, 867–880. [Google Scholar] [CrossRef]
- Berthon, A.; Martinez, J.; Bertherat, P.; Val, P. Wnt/b-catenin signalling in adrenal physiology and tumour development. Mol. Cell. Endocrinol. 2012, 35, 87–95. [Google Scholar] [CrossRef]
- Walczak, E.M.; Kuick, R.; Finco, I.; Bohin, N.; Hrycaj, S.M.; Wellik, D.M.; Hammer, G.D. Wnt-signaling inhibits adrenal steroidogenesis by cell-autonomous and noncell-autonomous mechanisms. Mol. Endocrinol. 2014, 28, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, E.; Leng, S.; Yuchi, Y.; Borges, K.; Guagliardo, N.; Shan, M.; Ruiz-Babot, G.; Katyiawasam, D.; Taketo, M.; Mial, J.; et al. Beta-catenin causes adrenal hyperplasia by blocking zonal transdifferentiation. Cell Rep. 2020, 31, 107524. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Nazimova, S.V. The role of the canonical Wnt-signaling pathway in morphogenesis and regeneration of the adrenal cortex in rats exposed to the endocrine disruptor dichlorodiphenyltrichloroethane during prenatal and postnatal development. Biol. Bull. 2019, 46, 74–81. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Simandi, Z.; Horvath, A.; Wright, L.C.; Cuaranta-Monroy, I.; De Luca, I.; Karolyi, K.; Sauer, S.; Deleuze, J.F.; Gudas, L.J.; Cowley, S.M.; et al. OCT4 Acts as an Integrator of Pluripotency and Signal-Induced Differentiation. Mol. Cell 2016, 63, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, K.; Ruiz-Babot, G.; McGaugh, E.C.; Nicholson, J.G.; Gualtieri, A.; Gaston-Massuet, C.; Nostro, M.C.; Guasti, L. Stem cells, self-renewal, and lineage commitment in the endocrine system. Front. Endocrinol. 2019, 10, 772. [Google Scholar] [CrossRef]
- Alexander, R.; Cheng, L.; Grignon, D.J.; Idrees, M. Cytoplasmic Staining of OCT4 is a highly sensitive marker of adrenal medullary–derived tissue. Am. J. Surg. Pathol. 2013, 37, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.; Cheng, L.; Grignon, D.; Idrees, M. Cytoplasmic OCT4 staining is a sensitive marker of neuroendocrine differentiation. Hum. Pathol. 2014, 45, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Timokhina, E.P.; Yaglov, V.V. Changes in the Expression of Transcription Factor Oct4 during Postnatal Development of Adrenal Medulla. Bull. Exp. Biol. Med. 2022, 173, 783–786. [Google Scholar] [CrossRef]
- Petrova, R.; Joyner, A. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2013, 14, 188–202. [Google Scholar] [CrossRef]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 2013, 12, 426–439, Erratum in Cell Stem Cell 2013, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Traiffort, E.; Charytoniuk, D.; Watroba, L.; Faure, H.; Sales, N.; Ruat, M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur. J. Neurosci. 1999, 11, 3199–3214. [Google Scholar] [CrossRef]
- Traiffort, E.; Angot, E.; Ruat, M. Sonic Hedgehog signaling in the mammalian brain. J. Neurochem. 2010, 113, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; Lerario, A.; Hammer, G. Sonic Hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 2018, 159, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N.P.; Rosenfeld, P.F. (Eds.) . Handbook of Pollution Prevention and Cleaner Production: Best Practices in the Agrochemical Industry; William Andrew: Amsterdam, The Netherlands, 2010; p. 313. [Google Scholar] [CrossRef]
- Pignatelli, D.; Xiao, F.; Gouvtia, A.; Ferreira, J.; Vinson, G. Adrenarche in the rat. J. Endocrinol. 2006, 191, 301–308. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takano, R.; Shimizu, M.; Murayama, N.; Kitajima, M.; Shono, M. Human blood concentrations of dichlorodiphenyltrichloroethane (DDT) extrapolated from metabolism in rats and humans and physiologically based pharmacokinetic modeling. J. Health Sci. 2010, 56, 566–575. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.; Jho, E.H. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaglova, N.V.; Nazimova, S.V.; Obernikhin, S.S.; Tsomartova, D.A.; Yaglov, V.V.; Timokhina, E.P.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla. Int. J. Mol. Sci. 2023, 24, 2774. https://doi.org/10.3390/ijms24032774
Yaglova NV, Nazimova SV, Obernikhin SS, Tsomartova DA, Yaglov VV, Timokhina EP, Tsomartova ES, Chereshneva EV, Ivanova MY, Lomanovskaya TA. Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla. International Journal of Molecular Sciences. 2023; 24(3):2774. https://doi.org/10.3390/ijms24032774
Chicago/Turabian StyleYaglova, Nataliya V., Svetlana V. Nazimova, Sergey S. Obernikhin, Dibakhan A. Tsomartova, Valentin V. Yaglov, Ekaterina P. Timokhina, Elina S. Tsomartova, Elizaveta V. Chereshneva, Marina Y. Ivanova, and Tatiana A. Lomanovskaya. 2023. "Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla" International Journal of Molecular Sciences 24, no. 3: 2774. https://doi.org/10.3390/ijms24032774
APA StyleYaglova, N. V., Nazimova, S. V., Obernikhin, S. S., Tsomartova, D. A., Yaglov, V. V., Timokhina, E. P., Tsomartova, E. S., Chereshneva, E. V., Ivanova, M. Y., & Lomanovskaya, T. A. (2023). Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla. International Journal of Molecular Sciences, 24(3), 2774. https://doi.org/10.3390/ijms24032774







