Age-Related Alterations in the Level and Metabolism of Serotonin in the Brain of Males and Females of Annual Turquoise Killifish (Nothobranchius furzeri)
Abstract
:1. Introduction
2. Results
2.1. Body Mass in 2-, 4- and 7-Month-Old Males and Females of N. furzeri
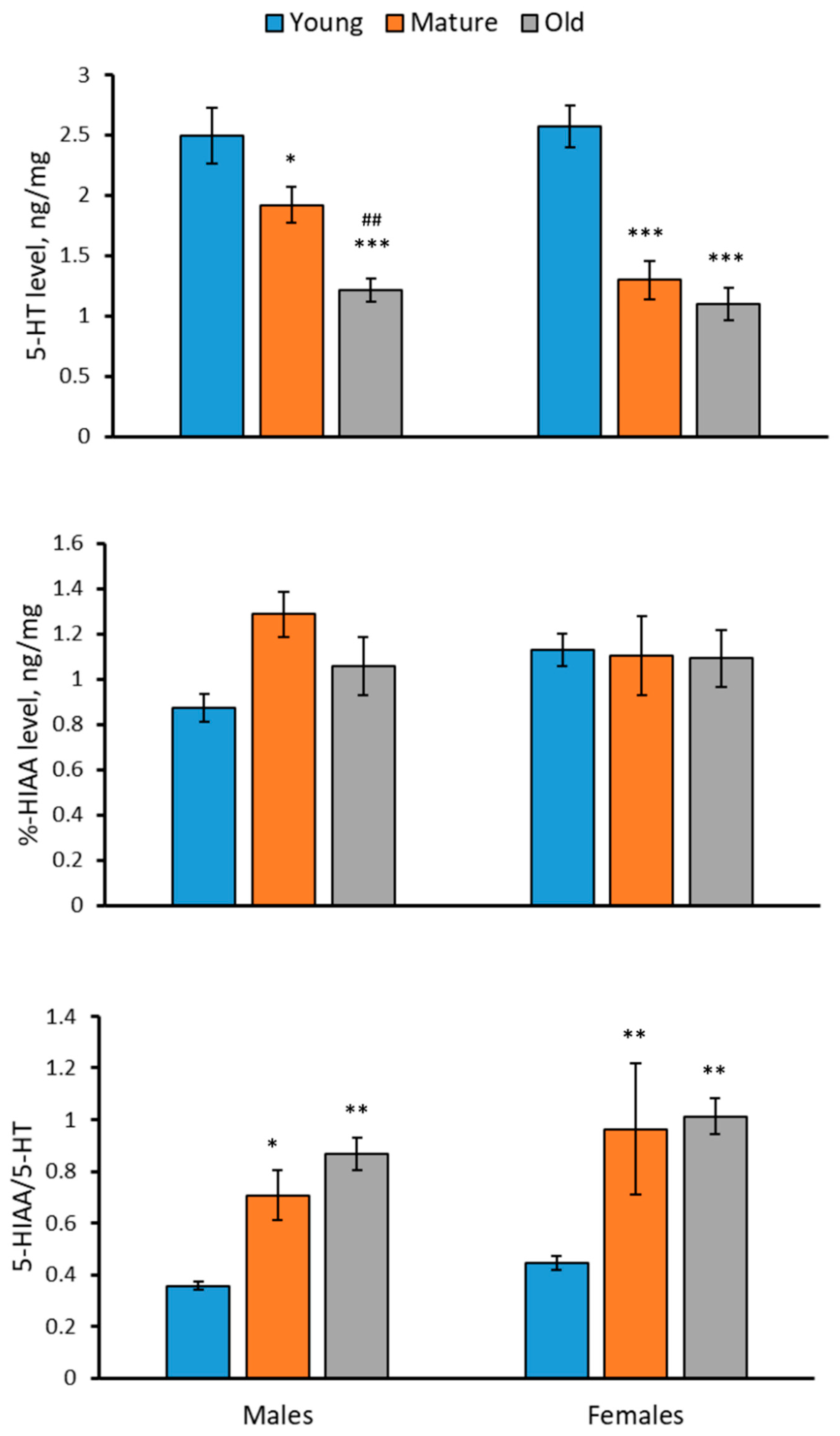
2.2. Levels of 5-HT, 5-HIAA and 5-HIAA/5-HT Rate in the Brain of 2-, 4- and 7-Month-Old Males and Females of N. furzeri
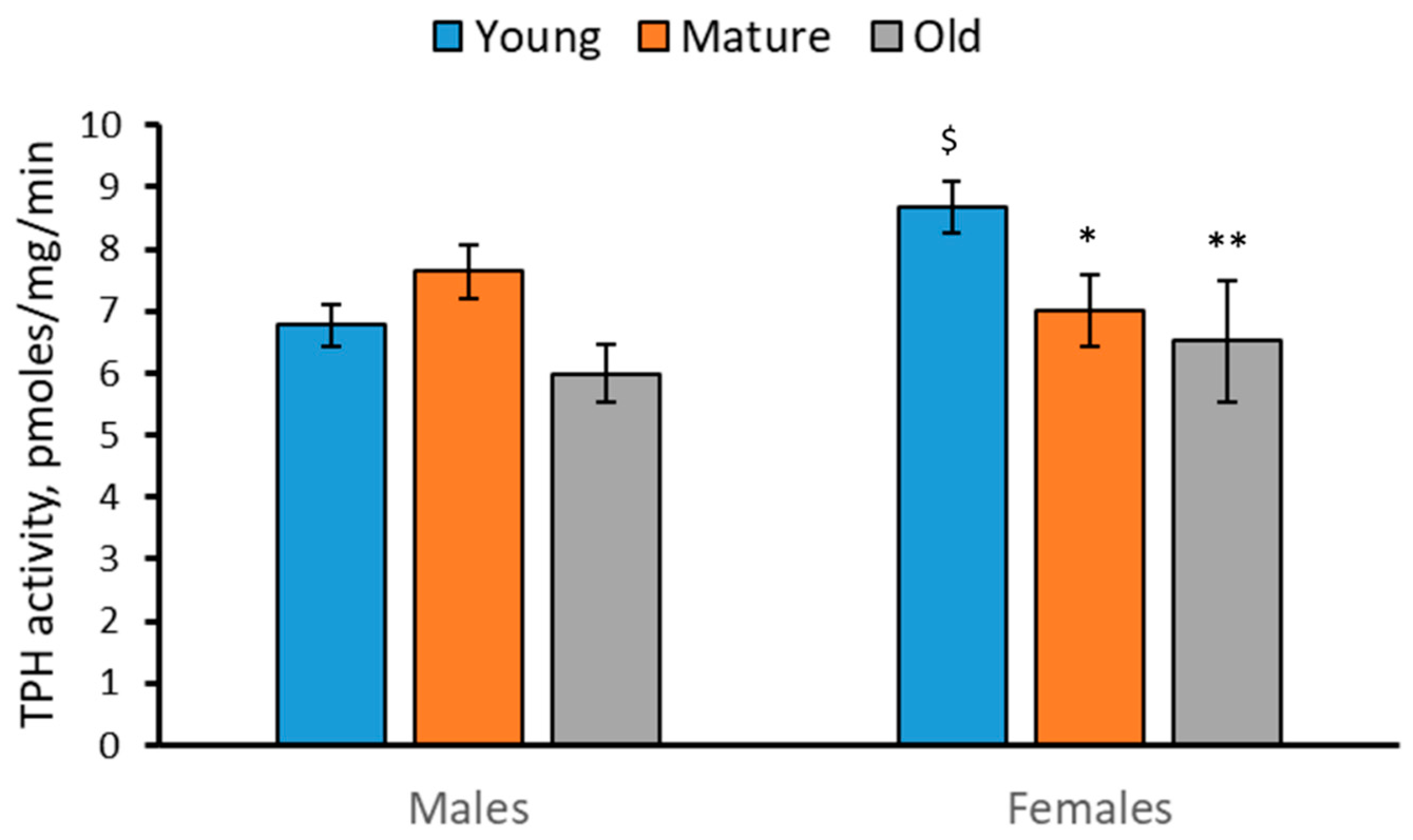
2.3. Activity of TPH in the Brain of 2-, 4- and 7-Month-Old Males and Females of N. furzeri
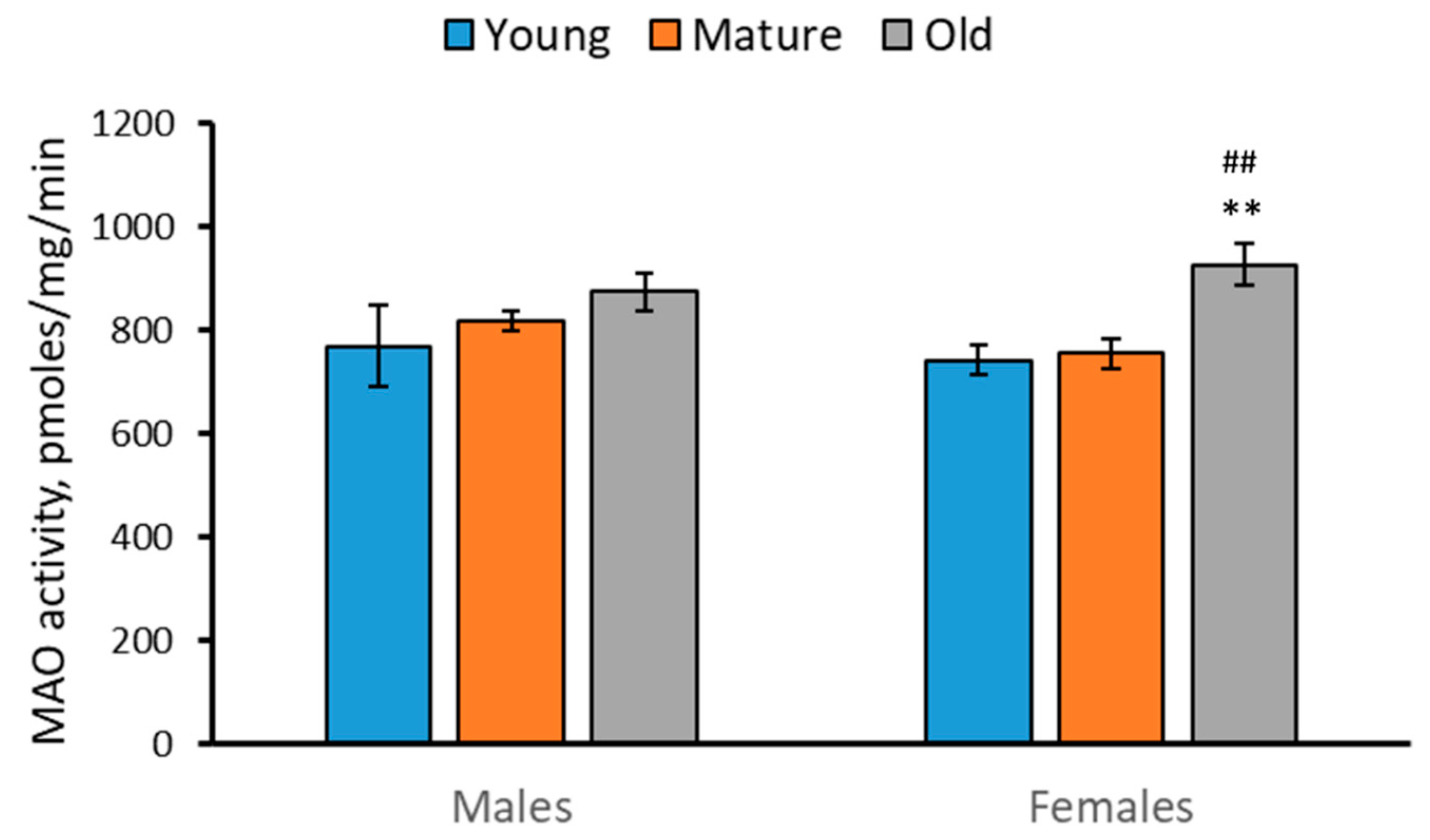
2.4. Activity of MAO in the Brain of 2-, 4- and 7-Month-Old Males and Females of N. furzeri
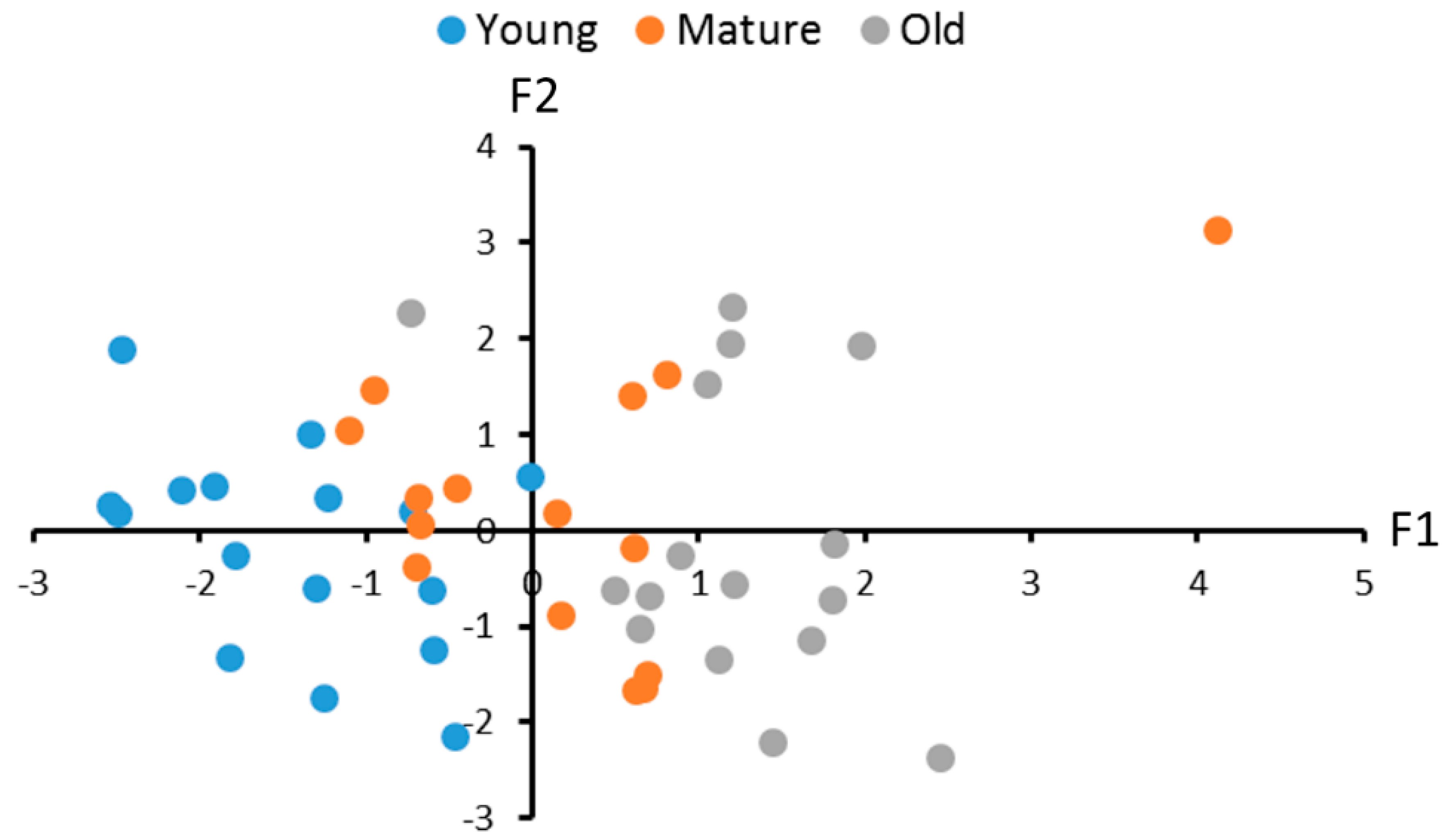
2.5. Correlations between the Levels of the 5-HT, 5-HIAA and 5-HIAA/5-HT Turnover Rate and TPHs and MAO Activities in the Brain of Male and Female N. furzeri
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Assay of 5-HT and 5-HIAA Concentrations
4.3. Assay of TPH Activity
4.4. Assay of MAO Activity
4.5. Statistics
5. Conclusions
- No effect of sex on the 5-HT and 5-HIAA levels or the TPH and MAO activities in the brain was shown.
- The 5-HT level was decreased, while 5-HIAA/5-HT was increased in the brain of males and females during aging.
- No age-related dynamic in the 5-HIAA level in the brain of males and females was observed.
- The TPH activity was decreased, while the MAO activity was increased in the brain of females during aging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesch, K.P.; Waider, J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelop-mental disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef]
- Lucki, I. The spectrum of behavior influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Meltzer, H.Y. The serotonin hypothesis of major depression. In Psychopharmacology; The fourth generation of progress; Bloom, E.E., Kupfer, N.N., Eds.; Raven Press: New York, NY, USA, 1995; pp. 933–944. [Google Scholar]
- Van Praag, H.M. Can stress cause depression? Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 891–907. [Google Scholar] [CrossRef]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37 Pt 1, 2331–2371. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsycho-Pharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; Hen, R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015, 30, 51–58. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Waider, J.; Kraft., S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural. Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef]
- Savelieva, K.V.; Zhao, S.; Pogorelov, V.M.; Rajan, I.; Yang, Q.; Cullinan, E.; Lanthorn, T.H. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 2008, 3, e3301. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef]
- Mosienko, V.; Beis, D.; Pasqualetti, M.; Waider, J.; Matthes, S.; Qadri, F.; Bader, M.; Alenina, N. Life without brain serotonin: Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav. Brain. Res. 2015, 277, 78–88. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Kulikov, A.V. Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: Focus on animal models. Expert Opin. Ther. Targets 2019, 23, 655–667. [Google Scholar] [CrossRef]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Osipova, D.V.; Naumenko, V.S.; Terenina, E.; Mormède, P.; Popova, N.K. A pharmacological evidence of positive association between mouse intermale aggression and brain serotonin metabolism. Behav. Brain Res. 2012, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hagsäter, S.M.; Pettersson, R.; Holmäng, A.; Eriksson, E. Serotonin depletion reduces both acquisition and expression of context-conditioned fear. Acta Neuropsychiatr. 2021, 33, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Grouleff, J.; Ladefoged, L.K.; Koldsø, H.; Schiøtt, B. Monoamine transporters: Insights from molecular dynamics simulations. Front. Pharmacol. 2015, 6, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, J.C.; Thompson, R.F. Monoamine oxidase in neuropsychiatry and behavior. Am. J. Hum. Genet. 1999, 65, 593–598. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Wu, J.B.; Chen, K. Transcriptional regulation and multiple functions of MAO genes. J. Neural. Transm. 2011, 118, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Felice, D.; O’Leary, O.F.; Cryan, J.F.; Dinan, T.G.; Gardier, A.M.; Sánchez, C.; David, D.J. When ageing meets the blues: Are current antidepressants effective in depressed aged patients? Neurosci. Biobehav. Rev. 2015, 55, 478–497. [Google Scholar] [CrossRef]
- Buhot, M.C.; Martin, S.; Segu, L. Role of serotonin in memory impairment. Ann. Med. 2000, 32, 210–221. [Google Scholar] [CrossRef]
- Fidalgo, S.; Ivanov, D.K.; Wood, S.H. Serotonin: From top to bottom. Biogerontology 2013, 14, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Lin, C.H.; Lane, H.Y. Molecular Basis of Late-Life Depression. Int. J. Mol. Sci. 2021, 22, 7421. [Google Scholar] [CrossRef]
- Cellerino, A.; Valenzano, D.R.; Reichard, M. From the bush to the bench: The annual Nothobranchius fishes as a new model system in biology. Biol. Rev. Camb. Philos. Soc. 2016, 91, 511–533. [Google Scholar] [CrossRef]
- Terzibasi, E.; Valenzano, D.R.; Cellerino, A. The short-lived fish Nothobranchius furzeri as a new model system for aging studies. Exp. Gerontol. 2007, 42, 81–89. [Google Scholar] [CrossRef]
- Platzer, M.; Englert, C. Nothobranchius furzeri: A model for aging research and more. Trends Genet. 2016, 32, 543–552. [Google Scholar] [CrossRef]
- Genade, T.; Benedetti, M.; Terzibasi, E.; Roncaglia, P.; Valenzano, D.R.; Cattaneo, A.; Cellerino, A. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging. Cell 2005, 4, 223–233. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Terzibasi, E.; Valenzano, D.R.; Benedetti, M.; Roncaglia, P.; Cattaneo, A.; Domenici, L.; Cellerino, A. Large differences in aging phenotype between strains of the shortlived annual fish Nothobranchius furzeri. PLoS ONE 2008, 3, e3866. [Google Scholar] [CrossRef] [PubMed]
- Terzibasi Tozzini, E.; Cellerino, A. Nothobranchius annual killifishes. Evodevo 2020, 11, 25. [Google Scholar] [CrossRef]
- Dohi, E.; Matsui, H. The Utility of Small Fishes for the Genetic Study of Human Age-Related Disorders. Front. Genet. 2022, 13, 928597. [Google Scholar] [CrossRef] [PubMed]
- Kodera, K.; Matsui, H. Zebrafish, Medaka and Turquoise Killifish for Understanding Human Neurodegenerative/Neurodevelopmental Disorders. Int. J. Mol. Sci. 2022, 23, 1399. [Google Scholar] [CrossRef] [PubMed]
- Oginuma, M.; Nishida, M.; Ohmura-Adachi, T.; Abe, K.; Ogamino, S.; Mogi, C.; Matsui, H.; Ishitani, T. Rapid reverse genetics systems for Nothobranchius furzeri, a suitable model organism to study vertebrate aging. Sci. Rep. 2022, 12, 11628. [Google Scholar] [CrossRef] [PubMed]
- Poeschla, M.; Valenzano, D.R. The turquoise killifish: A genetically tractable model for the study of aging. J. Exp. Biol. 2020, 223 (Suppl. 1). [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Philippe, C.; Brendonck, L.; Pinceel, T. Antidepressant exposure reduces body size, increases fecundity and alters social behavior in the short-lived killifish Nothobranchius furzeri. Environ Pollut. 2020, 265 Pt A, 115068. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Steenaerts, L.; Philippe, C.; Grégoir, A.F.; Brendonck, L.; Pinceel, T. Improving the reliability and ecological validity of pharmaceutical risk assessment: Turquoise killifish (Nothobranchius furzeri) as a model in behavioral eco-toxicology. Environ. Toxicol. Chem. 2019, 38, 262–270. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Lillesaar, C. Probing the diversity of serotonin neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Evsiukova, V.S.; Kulikova, E.A.; Kulikov, A.V. Age-Related Alterations in the Behavior and Serotonin-Related Gene mRNA Levels in the Brain of Males and Females of Short-Lived Turquoise Killifish (Nothobranchius furzeri). Biomolecules 2021, 11, 1421. [Google Scholar] [CrossRef]
- Evsiukova, V.; Antonov, E.; Kulikov, A.V. Effects of Sex and Group Size on Behavior and Brain Biogenic Amines in Short-Lived Turquoise Killifish (Nothobranchius furzeri). Zebrafish 2021, 18, 265–273. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Fursenko, D.V.; Bazhenova, E.Y.; Kulikov, A.V. Decrease in the Activity of Striatal-enriched Pro-tein-tyrosine-Phosphatase (STEP) in the Brain of Danio rerio Treated with p-Chlorophenylalanine and Pargyline. Mol. Biol. 2021, 55, 573–578. [Google Scholar] [CrossRef]
- Evsiukova, V.S.; Bazovkina, D.; Bazhenova, E.; Kulikova, E.A.; Kulikov, A.V. Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT and BDNF-Systems in the Brain of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2021, 22, 12851. [Google Scholar] [CrossRef]
- Banerjee, S.; Poddar, M.K. Carnosine: Effect on aging-induced increase in brain regional monoamine oxidase-A activity. Neurosci. Res. 2015, 92, 62–70. [Google Scholar] [CrossRef]
- Saura, J.; Andrés, N.; Andrade, C.; Ojuel, J.; Eriksson, K.; Mahy, N. Biphasic and region-specific MAO-B response to aging in normal human brain. Neurobiol. Aging. 1997, 18, 497–507. [Google Scholar] [CrossRef]
- Kumar, M.J.; Andersen, J.K. Perspectives on MAO-B in aging and neurological disease: Where do we go from here? Mol. Neurobiol. 2004, 30, 77–89. [Google Scholar] [CrossRef]
- Nicotra, A.; Pierucci, F.; Parvez, H.; Senatori, O. Monoamine oxidase expression during development and aging. Neuro-toxicology 2004, 25, 155–165. [Google Scholar] [CrossRef]
- Mitchell, E.S.; McDevitt, R.A.; Neumaier, J.F. Adaptations in 5-HT receptor expression and function: Implications for treatment of cognitive impairment in aging. J. Neurosci. Res. 2009, 87, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.M.; Mitra, A.K. Effect of aging on tryptophan hydroxylase in rat brain: Implications on serotonin level. Drug. Metab. Dispos. 2000, 28, 1038–1042. [Google Scholar] [PubMed]
- Polačik, M.; Blažek, R.; Reichard, M. Laboratory breeding of the short-lived annual killifish Nothobranchius furzeri. Nat. Protoc. 2016, 11, 1396–1413. [Google Scholar] [CrossRef]
- Freeman, K.B.; Bulawa, M.C.; Zeng, Q.; Blank, C.L. Rapid and simultaneous determination of monoamine oxidase A and monoamine oxidase B activities in mouse brain homogenates by liquid chromatography with electrochemical detection. Anal. Biochem. 1993, 208, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S287–S296. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review). Mol. Med. Rep. 2014, 9, 1533–1541. [Google Scholar] [CrossRef] [Green Version]

| Trait | Age | Sex | Interaction |
|---|---|---|---|
| 5-HT | F(2,41) = 38.23, p < 0.001 | F(1,41) = 2.77, p = 0.1 | F(2,41) = 2.42, p = 0.1 |
| 5-HIAA | F(2,41) = 1.37, p = 0.27 | F(1,41) < 1 | F(2,41) = 1.72, p = 0.19 |
| 5-HIAA/5-HT | F(2,41) = 11.69, p < 0.001 | F(1,41) = 2.84, p = 0.1 | F(2,41) < 1 |
| TPH | MAO | 5-HT | 5-HIAA | |
|---|---|---|---|---|
| TPH | ||||
| MAO | −0.04 | |||
| 5-HT | 0.59 | −0.18 | ||
| 5-HIAA | 0.50 | 0.31 | 0.13 | |
| 5-HIAA/5-HT | −0.22 | 0.24 | −0.65 | 0.55 |
| Factor 1 (41.5%) | Factor 2 (35.2%) | Factor 3 (16.4%) | |
|---|---|---|---|
| TPH | −0.61 | 0.68 | −0.11 |
| MAO | 0.40 | 0.42 | 0.81 |
| 5-HT | −0.90 | 0.26 | 0.10 |
| 5-HIAA | 0.16 | 0.95 | −0.17 |
| 5-HIAA/5-HT | 0.84 | 0.40 | −0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evsiukova, V.S.; Arefieva, A.B.; Sorokin, I.E.; Kulikov, A.V. Age-Related Alterations in the Level and Metabolism of Serotonin in the Brain of Males and Females of Annual Turquoise Killifish (Nothobranchius furzeri). Int. J. Mol. Sci. 2023, 24, 3185. https://doi.org/10.3390/ijms24043185
Evsiukova VS, Arefieva AB, Sorokin IE, Kulikov AV. Age-Related Alterations in the Level and Metabolism of Serotonin in the Brain of Males and Females of Annual Turquoise Killifish (Nothobranchius furzeri). International Journal of Molecular Sciences. 2023; 24(4):3185. https://doi.org/10.3390/ijms24043185
Chicago/Turabian StyleEvsiukova, Valentina S., Alla B. Arefieva, Ivan E. Sorokin, and Alexander V. Kulikov. 2023. "Age-Related Alterations in the Level and Metabolism of Serotonin in the Brain of Males and Females of Annual Turquoise Killifish (Nothobranchius furzeri)" International Journal of Molecular Sciences 24, no. 4: 3185. https://doi.org/10.3390/ijms24043185







