Studies on the Oxidation of Aromatic Amines Catalyzed by Trametes versicolor Laccase
Abstract
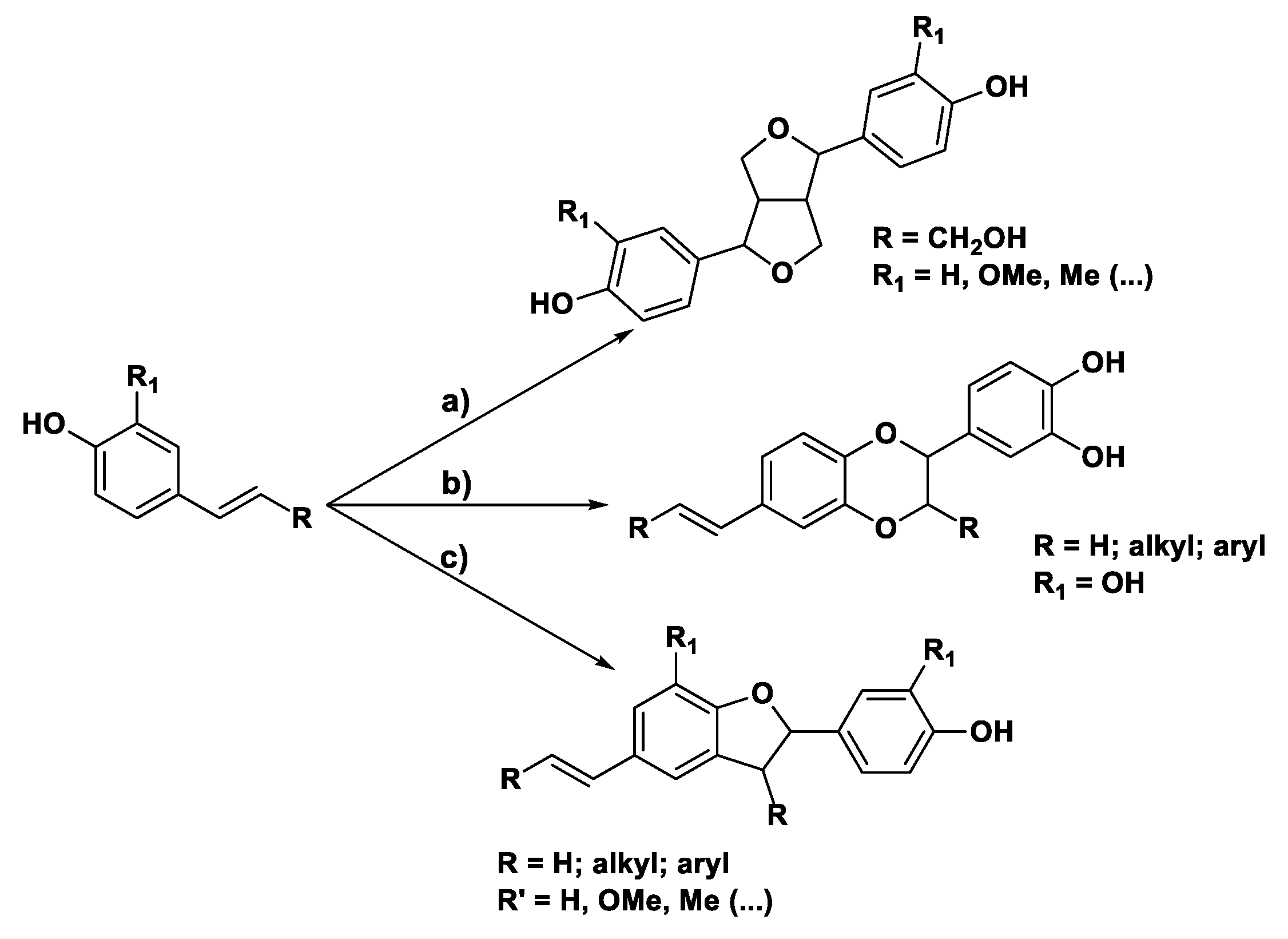
:1. Introduction
2. Results
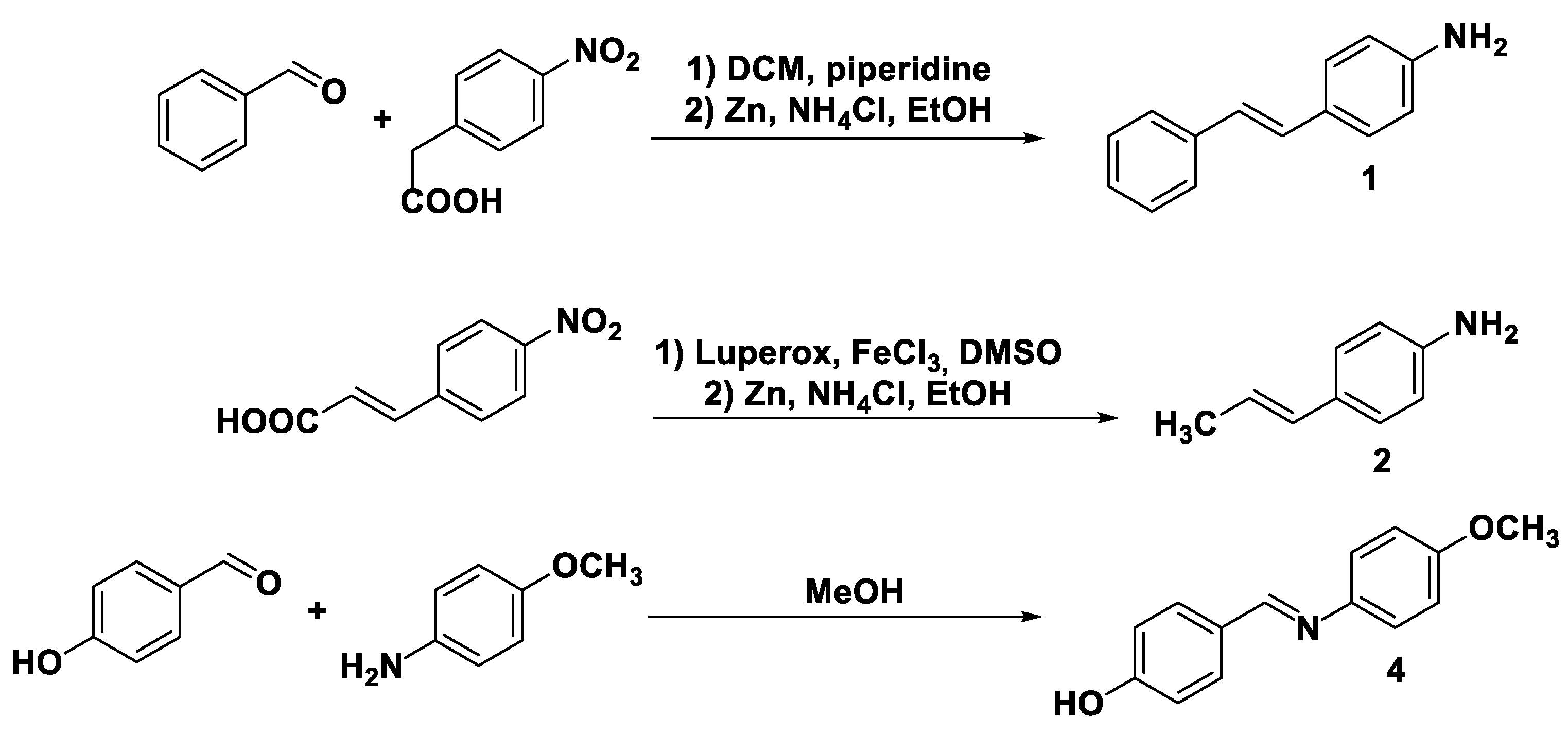
2.1. Synthesis of Substrates
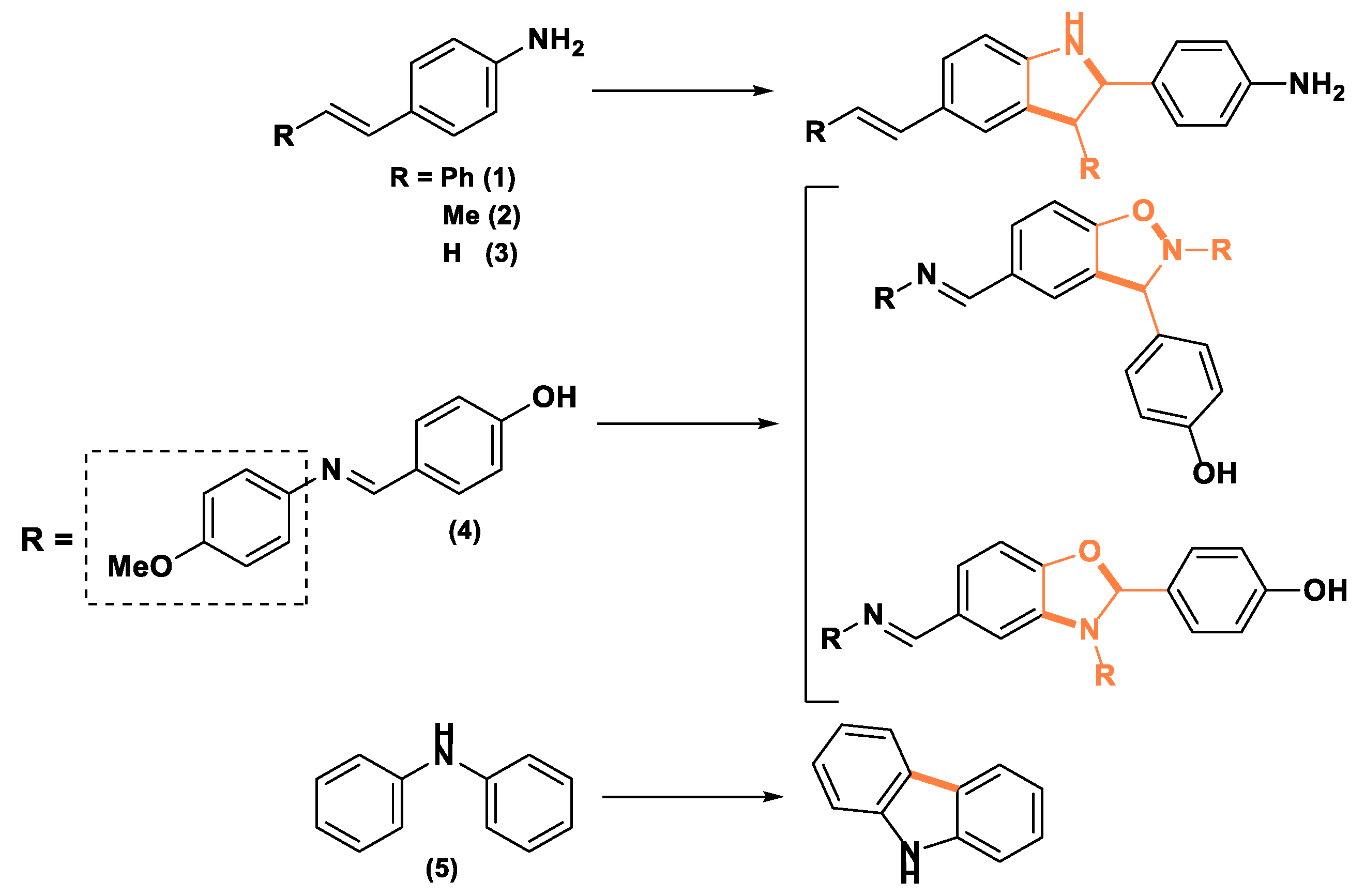
2.2. Laccases-Mediated Biooxidations
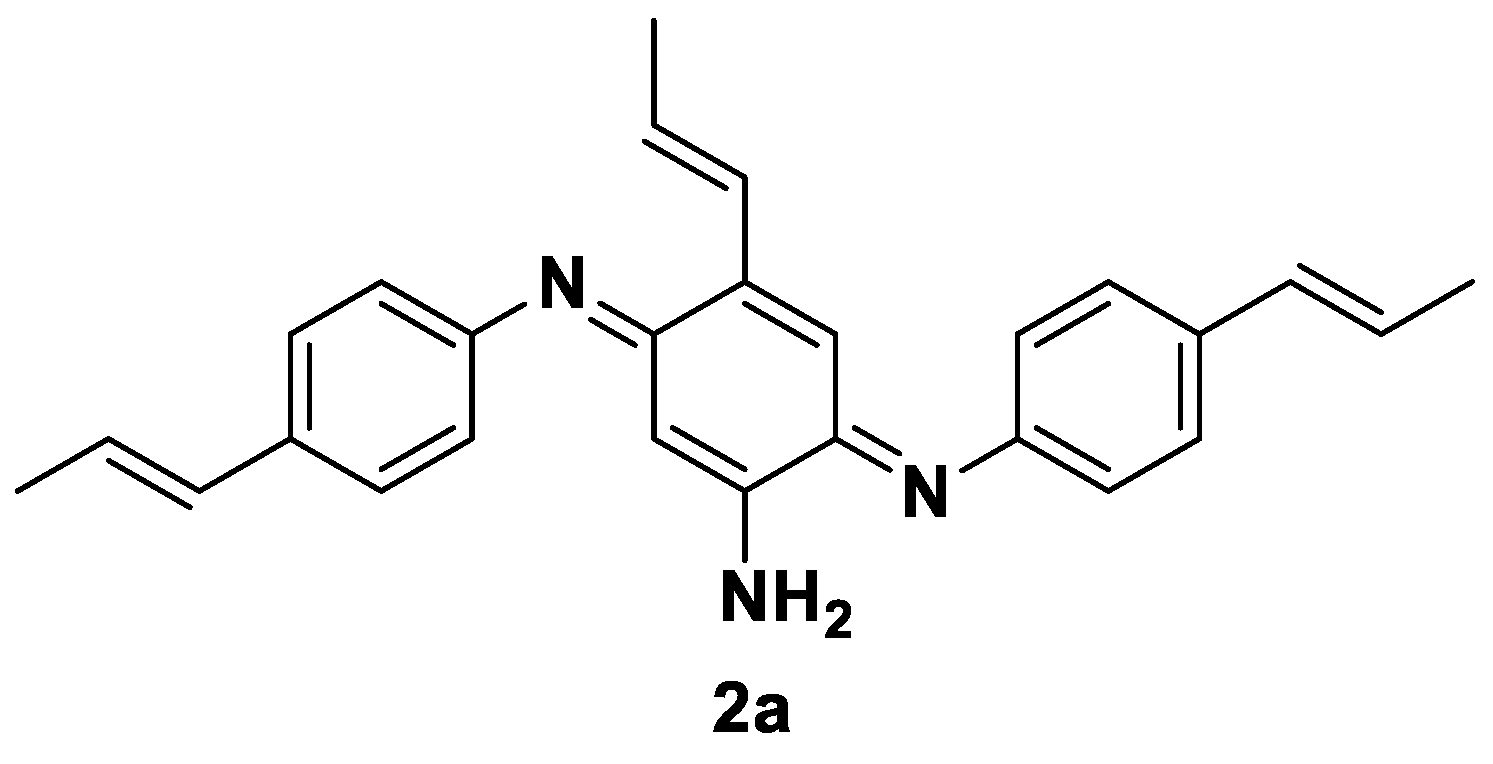
2.2.1. Substrate 1, 2, and 4
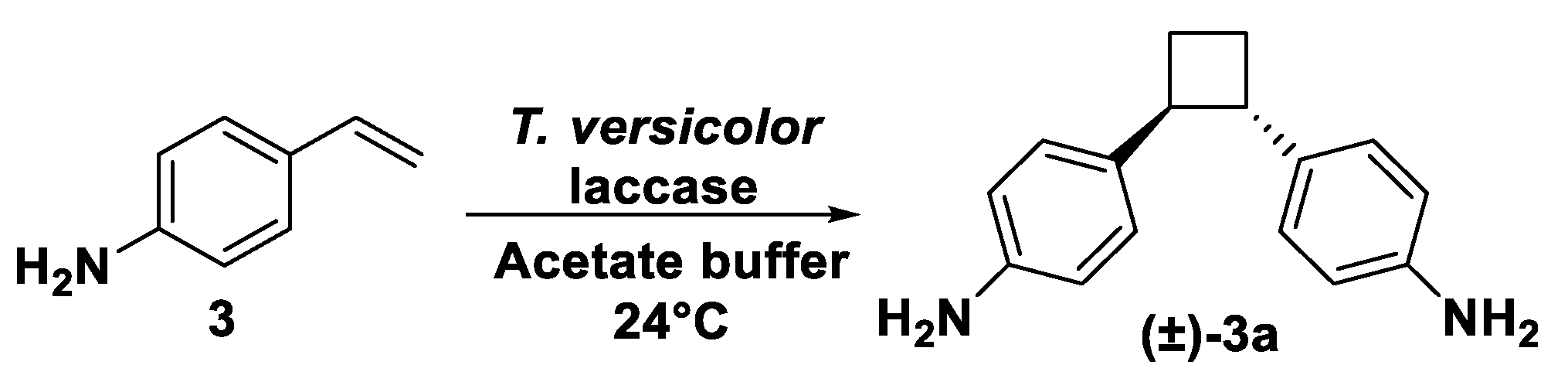
2.2.2. Substrate 3
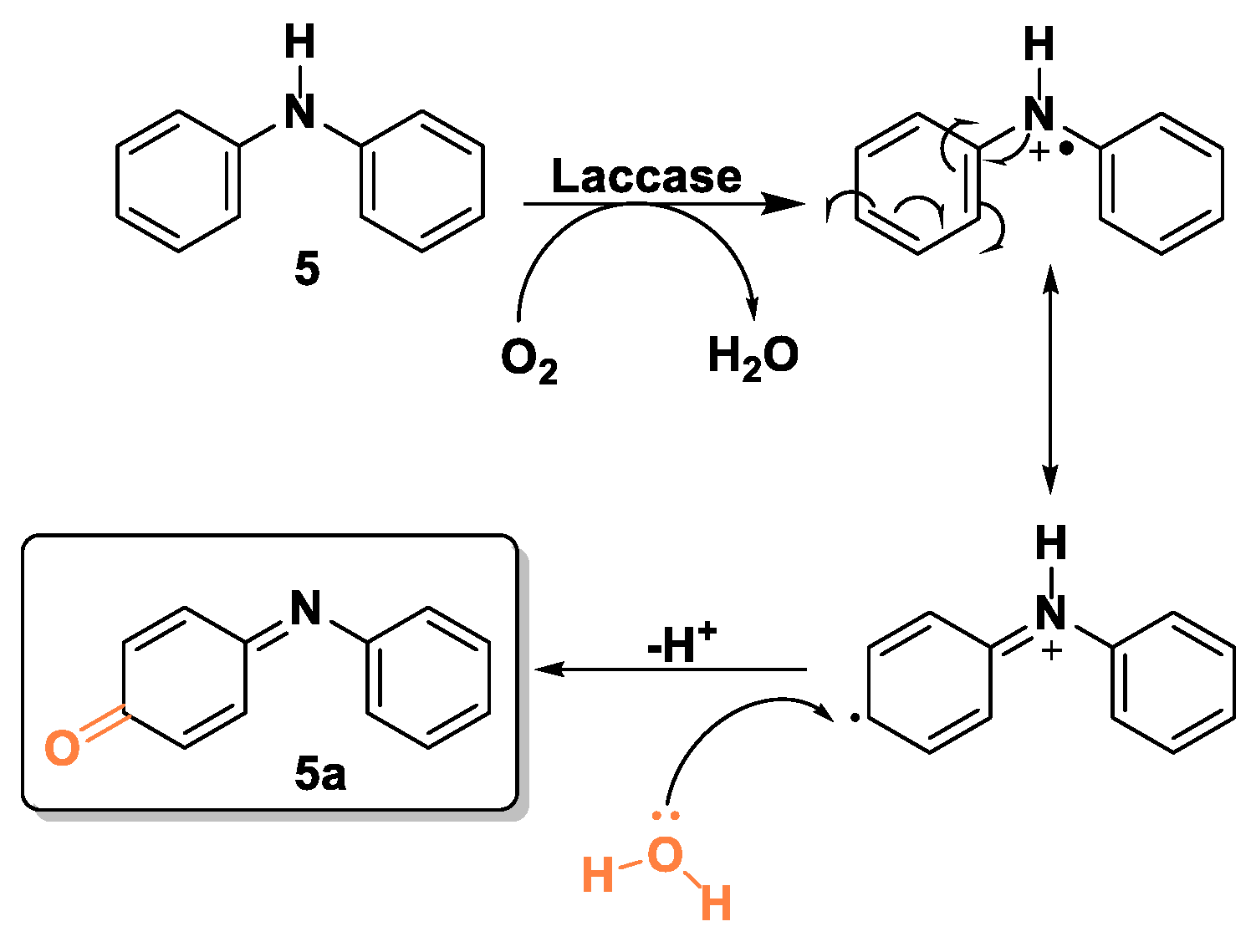
2.2.3. Substrate 5
3. Discussion
4. Materials and Methods
4.1. Synthesis of Dubstrates
4.1.1. (E)-1-nitro-4-styrylbenzene
4.1.2. (E)-4-styrylaniline (Substrate 1)
4.1.3. (E)-1-nitro-4-(prop-1-en-1-yl)benzene
4.1.4. (E)-4-(prop-1-en-1-yl)aniline (Substrate 2)
4.1.5. (E)-4-(((4-methoxyphenyl)imino)methyl)phenol (Substrate 4)
4.2. Biooxidations with T. versicolor Laccase
4.2.1. Oxidation of Vinyl Aniline (Substate 3)
4.2.2. Oxidation of Diphenyl Amine (Substate 5)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Riva, S. Laccases: Blue Enzymes for Green Chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mogharabi, M.; Faramarzi, M.A. Laccase and Laccase-Mediated Systems in the Synthesis of Organic Compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to Enjoy Laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.R.; Herrera, J.L.T. Industrial and Biotechnological Applications of Laccases: A Review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and Their Applications: A Patent Review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef]
- Zerva, A.; Simić, S.; Topakas, E.; Nikodinovic-Runic, J. Applications of Microbial Laccases: Patent Review of the Past Decade (2009–2019). Catalysts 2019, 9, 1023. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- Hahn, V.; Davids, T.; Lalk, M.; Schauer, F.; Mikolasch, A. Enzymatic Cyclizations Using Laccases: Multiple Bond Formation between Dihydroxybenzoic Acid Derivatives and Aromatic Amines. Green Chem. 2010, 12, 879. [Google Scholar] [CrossRef]
- Sousa, A.C.; Oliveira, M.C.; Martins, L.O.; Robalo, M.P. Towards the Rational Biosynthesis of Substituted Phenazines and Phenoxazinones by Laccases. Green Chem. 2014, 16, 4127. [Google Scholar] [CrossRef]
- Sousa, A.C.; Piedade, M.F.M.M.; Martins, L.O.; Robalo, M.P. An Enzymatic Route to a Benzocarbazole Framework Using Bacterial CotA Laccase. Green Chem. 2015, 17, 1429–1433. [Google Scholar] [CrossRef]
- Sousa, A.C.; Oliveira, M.C.; Martins, L.O.; Robalo, M.P. A Sustainable Synthesis of Asymmetric Phenazines and Phenoxazinones Mediated by CotA-Laccase. Adv. Synth. Catal. 2018, 360, 575–583. [Google Scholar] [CrossRef]
- Bassanini, I.; Parapini, S.; Basilico, N.; Sparatore, A. Novel Hydrophilic Riminophenazines as Potent Antiprotozoal Agents. ChemMedChem 2019, 14, 1940–1949. [Google Scholar] [CrossRef]
- Koval, A.; Bassanini, I.; Xu, J.; Tonelli, M.; Boido, V.; Sparatore, F.; Amant, F.; Annibali, D.; Leucci, E.; Sparatore, A.; et al. Optimization of the Clofazimine Structure Leads to a Highly Water-Soluble C3-Aminopyridinyl Riminophenazine Endowed with Improved Anti-Wnt and Anti-Cancer Activity in Vitro and in Vivo. Eur. J. Med. Chem. 2021, 222, 113562. [Google Scholar] [CrossRef]
- Barteselli, A.; Casagrande, M.; Basilico, N.; Parapini, S.; Rusconi, C.M.; Tonelli, M.; Boido, V.; Taramelli, D.; Sparatore, F.; Sparatore, A. Clofazimine Analogs with Antileishmanial and Antiplasmodial Activity. Bioorg. Med. Chem. 2015, 23, 55–65. [Google Scholar] [CrossRef]
- Bassanini, I.; Kapešová, J.; Petrásková, L.; Pelantová, H.; Markošová, K.; Rebroš, M.; Valentová, K.; Kotik, M.; Káňová, K.; Bojarová, P.; et al. Glycosidase-Catalyzed Synthesis of Glycosyl Esters and Phenolic Glycosides of Aromatic Acids. Adv. Synth. Catal. 2019, 361, adsc.201900259. [Google Scholar] [CrossRef]
- Bassanini, I.; Krejzova, J.; Panzeri, W.; Monti, D.; Kren, V.; Riva, S. A Sustainable One-Pot Two-Enzymes Synthesis of Naturally Occurring Arylalkyl Glucosides. ChemSusChem 2017, 10, 2040–2045. [Google Scholar] [CrossRef]
- Quintanar, L.; Stoj, C.; Taylor, A.B.; Hart, P.J.; Kosman, D.J.; Solomon, E.I. Shall We Dance? How a Multicopper Oxidase Chooses Its Electron Transfer Partner. Acc. Chem. Res. 2007, 40, 445–452. [Google Scholar] [CrossRef]
- Constantin, M.-A.; Conrad, J.; Beifuss, U. Laccase-Catalyzed Oxidative Phenolic Coupling of Vanillidene Derivatives. Green Chem. 2012, 14, 2375–2379. [Google Scholar] [CrossRef]
- Ricklefs, E.; Girhard, M.; Urlacher, V.B. Three-Steps in One-Pot: Whole-Cell Biocatalytic Synthesis of Enantiopure (+)- and (−)-Pinoresinol via Kinetic Resolution. Microb. Cell Factories 2016, 15, 78. [Google Scholar] [CrossRef]
- Navarra, C.; Goodwin, C.; Burton, S.; Danieli, B.; Riva, S. Laccase-Mediated Oxidation of Phenolic Derivatives. J. Mol. Catal. B Enzym. 2010, 65, 52–57. [Google Scholar] [CrossRef]
- Wan, Y.; Lu, R.; Akiyama, K.; Miyakoshi, T.; Du, Y. Enzymatic Synthesis of Bioactive Compounds by Rhus Laccase from Chinese Rhus Vernicifera. Sci. China Ser. B Chem. 2007, 50, 179–182. [Google Scholar] [CrossRef]
- Ricklefs, E.; Girhard, M.; Koschorreck, K.; Smit, M.S.; Urlacher, V.B. Two-Step One-Pot Synthesis of Pinoresinol from Eugenol in an Enzymatic Cascade. ChemCatChem 2015, 7, 1857–1864. [Google Scholar] [CrossRef]
- Pickel, B.; Constantin, M.A.; Pfannstiel, J.; Conrad, J.; Beifuss, U.; Schaller, A. An Enantiocomplementary Dirigent Protein for the Enantioselective Lacease-Catalyzed Oxidative Coupling of Phenols. Angew Chem. Int. Ed. 2010, 49, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Davin, L.B. Stereoselective Bimolecular Phenoxy Radical Coupling by an Auxiliary (Dirigent) Protein Without an Active Center. Science 1997, 275, 362–367. [Google Scholar] [CrossRef]
- Beneventi, E.; Conte, S.; Cramarossa, M.R.; Riva, S.; Forti, L. Chemo-Enzymatic Synthesis of New Resveratrol-Related Dimers Containing the Benzo[b]Furan Framework and Evaluation of Their Radical Scavenger Activities. Tetrahedron 2015, 71, 3052–3058. [Google Scholar] [CrossRef]
- Gavezzotti, P.; Bertacchi, F.; Fronza, G.; Křen, V.; Monti, D.; Riva, S. Laccase-Catalyzed Dimerization of Piceid, a Resveratrol Glucoside, and Its Further Enzymatic Elaboration. Adv. Synth. Catal. 2015, 357, 1831–1839. [Google Scholar] [CrossRef]
- Bassanini, I.; Gavezzotti, P.; Monti, D.; Krejzová, J.; Křen, V.; Riva, S. Laccase-Catalyzed Dimerization of Glycosylated Lignols. J. Mol. Catal. B Enzym. 2016, 134, 295–301. [Google Scholar] [CrossRef]
- Ponzoni, C.; Beneventi, E.; Cramarossa, M.R.; Raimondi, S.; Trevisi, G.; Pagnoni, U.M.; Riva, S.; Forti, L. Laccase-Catalyzed Dimerization of Hydroxystilbenes. Adv. Synth. Catal. 2007, 349, 1497–1506. [Google Scholar] [CrossRef]
- Gavezzotti, P.; Navarra, C.; Caufin, S.; Danieli, B.; Magrone, P.; Monti, D.; Riva, S. Synthesis of Enantiomerically Enriched Dimers of Vinylphenols by Tandem Action of Laccases and Lipases. Adv. Synth. Catal. 2011, 353, 2421–2430. [Google Scholar] [CrossRef]
- Grosso, S.; Radaelli, F.; Fronza, G.; Passarella, D.; Monti, D.; Riva, S. Studies on the Laccase-Catalyzed Oxidation of 4-Hydroxy-Chalcones. Adv. Synth. Catal. 2019, 361, adsc.201900190. [Google Scholar] [CrossRef]
- Bassanini, I.; D’Annessa, I.; Costa, M.; Monti, D.; Colombo, G.; Riva, S. Chemo-Enzymatic Synthesis of (E)-2,3-Diaryl-5-Styryl- Trans -2,3-Dihydrobenzofuran-Based Scaffolds and Their in Vitro and in Silico Evaluation as a Novel Sub-Family of Potential Allosteric Modulators of the 90 KDa Heat Shock Protein (Hsp90). Org. Biomol. Chem. 2018, 16, 3741–3753. [Google Scholar] [CrossRef]
- Ncanana, S.; Baratto, L.; Roncaglia, L.; Riva, S.; Burton, S.G. Laccase-Mediated Oxidation of Totarol. Adv. Synth. Catal. 2007, 349, 1507–1513. [Google Scholar] [CrossRef]
- Navarra, C.; Gavezzotti, P.; Monti, D.; Panzeri, W.; Riva, S. Biocatalyzed Synthesis of Enantiomerically Enriched β-5-like Dimer of 4-Vinylphenol. J. Mol. Catal. B Enzym. 2012, 84, 115–120. [Google Scholar] [CrossRef]
- Ficarra, S.; Tellone, E.; Pirolli, D.; Russo, A.; Barreca, D.; Galtieri, A.; Giardina, B.; Gavezzotti, P.; Riva, S.; De Rosa, M.C. Insights into the Properties of the Two Enantiomers of Trans-δ-Viniferin, a Resveratrol Derivative: Antioxidant Activity, Biochemical and Molecular Modeling Studies of Its Interactions with Hemoglobin. Mol. Biosyst. 2016, 12, 1276–1286. [Google Scholar] [CrossRef]
- Sousa, A.C.; Martins, L.O.; Robalo, M.P. Laccase-Catalysed Homocoupling of Primary Aromatic Amines towards the Biosynthesis of Dyes. Adv. Synth. Catal. 2013, 355, 2908–2917. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Milojević-Rakić, M.; Janošević-Ležaić, A.; Luginbühl, S.; Walde, P. Enzymatic Oligomerization and Polymerization of Arylamines: State of the Art and Perspectives. Chem. Zvesti 2017, 71, 199–242. [Google Scholar] [CrossRef]
- Raza, G.; Bella, J.; Segre, A.; Ferrando, A.; Goffredi, G. Structures and NMR Parameters of 1,2-Diphenylcyclobutanes. Struct. Chem. 1998, 9, 419–427. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef]
- Crellin, R.A.; Lamber, C. Photochemical 2 + 2 Cycloaddition via a Cation-Radical Chain Reaction. J. Chem. Soc. D Chem. Commun. 1970, 11, 682–683. [Google Scholar] [CrossRef]
- Horibe, T.; Katagiri, K.; Ishihara, K. Radical-Cation-Induced Crossed [2 + 2] Cycloaddition of Electron-Deficient Anetholes Initiated by Iron(III) Salt. Adv. Synth. Catal. 2020, 362, 960–963. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Horiguchi, G.; Kamiya, H.; Okada, Y. Design of a Photocatalytic [2 + 2] Cycloaddition Reaction Using Redox-Tag Strategy. Chem. Eur. J. 2022, 28, e202202018. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.; Reiser, O.; Díaz, D. Effect of Reaction Media on Photosensitized [2 + 2]-Cycloaddition of Cinnamates. ChemistryOpen 2020, 9, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; López-Arteaga, R.; Weiss, E.A. Quantum Dots Photocatalyze Intermolecular [2 + 2] Cycloadditions of Aromatic Alkenes Adsorbed to Their Surfaces via van Der Waals Interactions. J. Am. Chem. Soc. 2022, 144, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, C.; Rogers, C.R.; Kodaimati, M.S.; Weiss, E.A. Regio- and Diastereoselective Intermolecular [2 + 2] Cycloadditions Photocatalysed by Quantum Dots. Nat. Chem. 2019, 11, 1034–1040. [Google Scholar] [CrossRef]


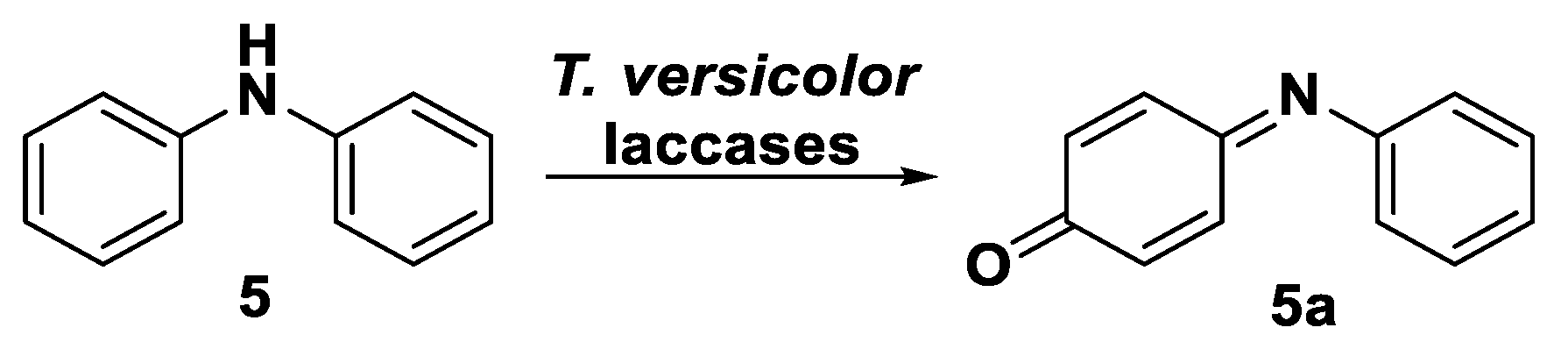
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassanini, I.; Grosso, S.; Tognoli, C.; Fronza, G.; Riva, S. Studies on the Oxidation of Aromatic Amines Catalyzed by Trametes versicolor Laccase. Int. J. Mol. Sci. 2023, 24, 3524. https://doi.org/10.3390/ijms24043524
Bassanini I, Grosso S, Tognoli C, Fronza G, Riva S. Studies on the Oxidation of Aromatic Amines Catalyzed by Trametes versicolor Laccase. International Journal of Molecular Sciences. 2023; 24(4):3524. https://doi.org/10.3390/ijms24043524
Chicago/Turabian StyleBassanini, Ivan, Simone Grosso, Chiara Tognoli, Giovanni Fronza, and Sergio Riva. 2023. "Studies on the Oxidation of Aromatic Amines Catalyzed by Trametes versicolor Laccase" International Journal of Molecular Sciences 24, no. 4: 3524. https://doi.org/10.3390/ijms24043524






