Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil
Abstract
:1. Introduction
2. Results
2.1. General Findings
2.2. Liver Triglyceride (TG) and Phospholipid (PL) Fatty Acids
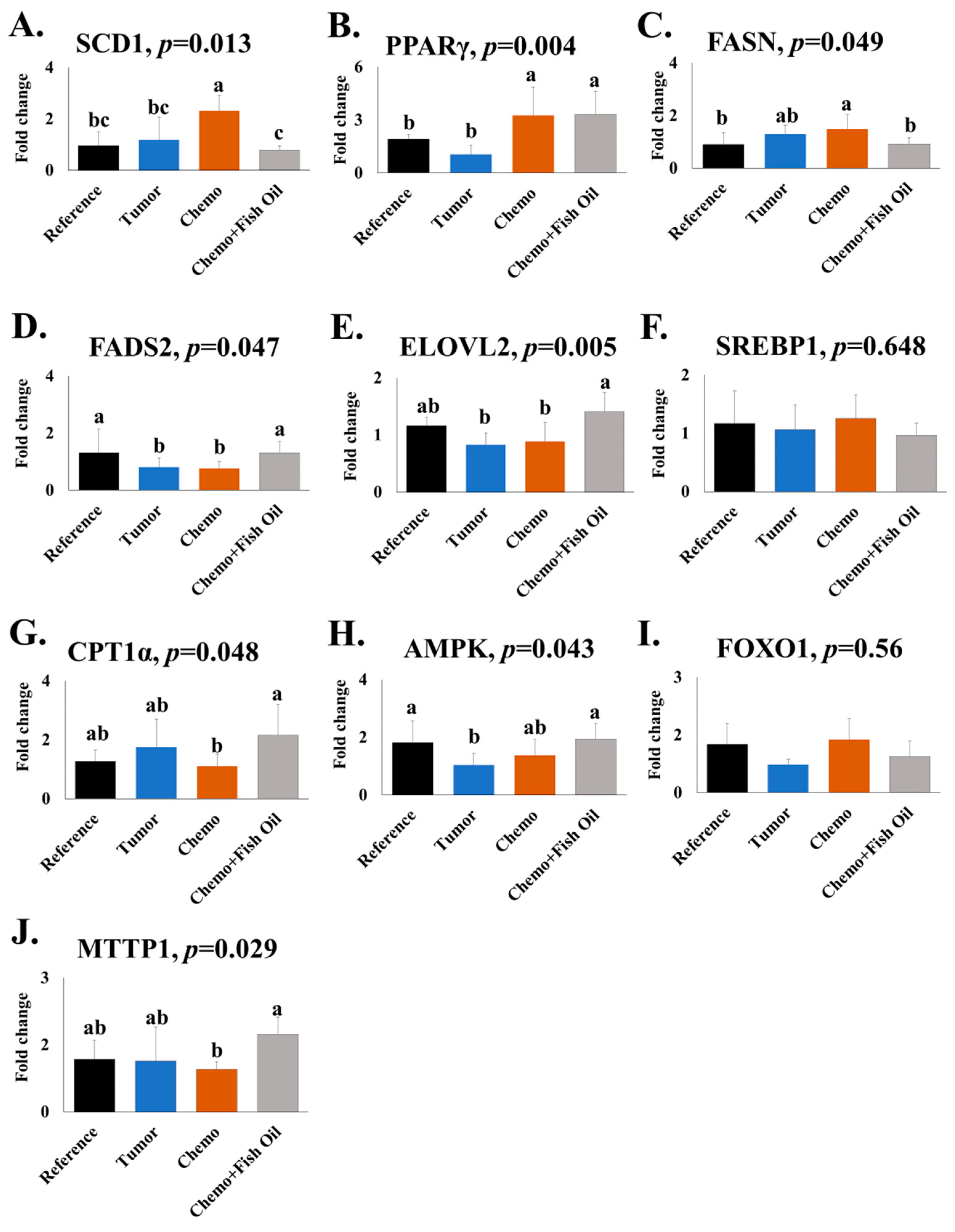
2.3. Gene Expression
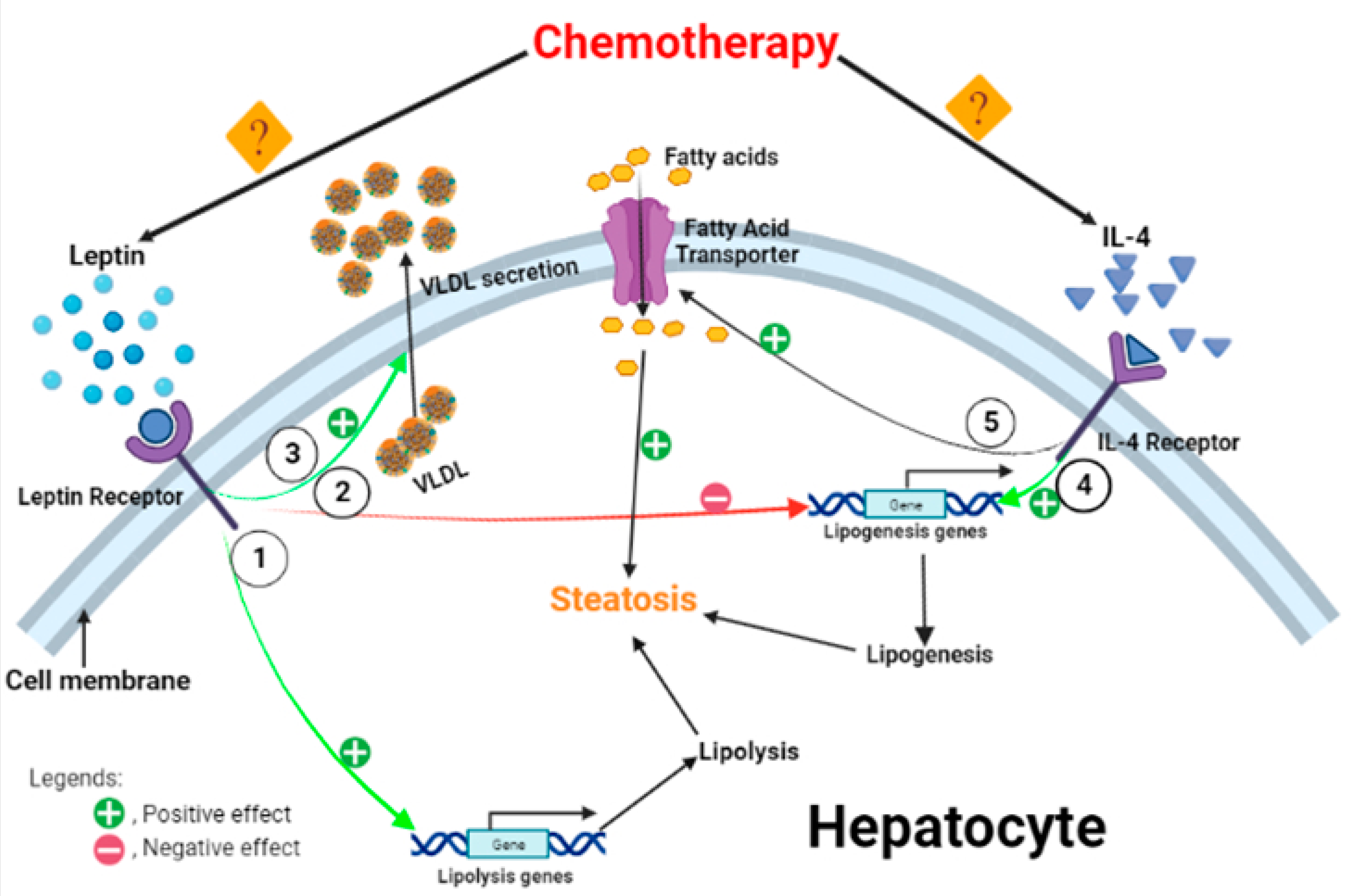
2.4. Hepatic Leptin and Interleukin (IL)-4
3. Discussion
4. Materials and Methods
4.1. Animal Handling, Diet, and Experimental Design
4.2. Fatty Acid Analysis
4.3. RNA Preparation and Gene Expression
4.4. Determination of Leptin and IL-4
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.; Bryan, S.; De, P. Canadian Cancer Statistics Advisory Committee; Canadian Cancer Society: Toronto, ON, Canada, 2018. [Google Scholar]
- Fenton, H.M.; Taylor, J.C.; Lodge, J.P.A.; Toogood, G.J.; Finan, P.J.; Young, A.L.; Morris, E.J.A. Variation in the Use of Resection for Colorectal Cancer Liver Metastases. Ann. Surg. 2019, 270, 892–898. [Google Scholar] [CrossRef]
- Ito, K.; Govindarajan, A.; Ito, H.; Fong, Y. Surgical treatment of hepatic colorectal metastasis: Evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010, 16, 103–110. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Choti, M.A.; Helton, W.S. AHPBA/SSO/SSAT Consensus Conference on hepatic colorectal metastases: Rationale and overview of the conference. Ann. Surg. Oncol. 2006, 13, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Dyson, J.; McPherson, S.; Anstee, Q. Non-alcoholic fatty liver disease: Non-invasive investigation and risk stratification. J. Clin. Pathol. 2013, 66, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Mulhall, B.P.; Ong, J.P.; Younossi, Z.M. Non-alcoholic fatty liver disease: An overview. J. Gastroenterol. Hepatol. 2002, 17, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Younossi, Z.M. Epidemiology and natural history of NAFLD and NASH. Clin. Liver Dis. 2007, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in steatohepatitis. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: Stuttgart, Germany, 2001; pp. 057–070. [Google Scholar]
- Laurent, A.; Nicco, C.; Tran Van Nhieu, J.; Borderie, D.; Chéreau, C.; Conti, F.; Jaffray, P.; Soubrane, O.; Calmus, Y.; Weill, B. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology 2004, 39, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Veteläinen, R.; van Vliet, A.; Gouma, D.J.; van Gulik, T.M. Steatosis as a risk factor in liver surgery. Ann. Surg. 2007, 245, 20. [Google Scholar] [CrossRef] [PubMed]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Wilke, M.S.; Perrine, M.; Pawlowicz, M.; Mourtzakis, M.; Lieffers, J.R.; Maneshgar, M.; Bruera, E.; Clandinin, M.T.; Baracos, V.E.; et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 2010, 29, 482–487. [Google Scholar] [CrossRef]
- Xue, H.; Sawyer, M.B.; Field, C.J.; Dieleman, L.A.; Baracos, V.E. Nutritional modulation of antitumor efficacy and diarrhea toxicity related to irinotecan chemotherapy in rats bearing the ward colon tumor. Clin. Cancer Res. 2007, 13, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, A.S.; Anoveros-Barrera, A.; Dunichand-Hoedl, A.; Martins, K.; Bigam, D.; Khadaroo, R.G.; McMullen, T.; Bathe, O.F.; Putman, C.T.; Clandinin, M.T. Lipid is heterogeneously distributed in muscle and associates with low radiodensity in cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Pratt, V.; Watanabe, S.; Bruera, E.; Mackey, J.; Clandinin, M.; Baracos, V.E.; Field, C. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementation. Br. J. Cancer 2002, 87, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Monirujjaman, M.; Pant, A.; Nelson, R.; Bathe, O.; Jacobs, R.; Mazurak, V.C. Alterations in hepatic fatty acids reveal depletion of total polyunsaturated fatty acids following irinotecan plus 5-fluorouracil treatment in an animal model of colorectal cancer. Prostaglandins Leukot. Essent. Fat. Acids 2021, 174, 102359. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsiao, M. Leptin and cancer: Updated functional roles in carcinogenesis, therapeutic niches, and developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef]
- Yang, C.-P.; Shiau, M.-Y.; Lai, Y.-R.; Ho, K.-T.; Hsiao, C.-W.; Chen, C.-J.; Lo, Y.-L.; Chang, Y.-H. Interleukin-4 Boosts Insulin-Induced Energy Deposits by Enhancing Glucose Uptake and Lipogenesis in Hepatocytes. Oxidative Med. Cell. Longev. 2018, 2018, 6923187. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Lombardo, Y.B.; Chicco, A.G. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J. Nutr. Biochem. 2006, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Orang, Z.; Mozaffari-Khosravi, H. Effect of omega-3 supplementation on fatty liver and visceral adiposity indices in diabetic patients with non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Nutr. ESPEN 2021, 44, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Orang, Z.; Mohsenpour, M.A.; Mozaffari-Khosravi, H. Effect of Omega-3 fatty acid supplementation on inflammatory markers and insulin resistance indices in patient with type 2 diabetes and nonalcoholic fatty liver: A randomized double-blind clinical trial. Obes. Med. 2020, 19, 100278. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Gura, K.; Nosé, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Andersson, C.; Zauscher, B.; Gura, K.; Nosé, V.; Puder, M. Omega-3 fatty acids improve hepatic steatosis in a murine model: Potential implications for the marginal steatotic liver donor. Transplantation 2005, 79, 606–608. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef]
- Pawlowicz, M.C. What Happens to Essential Fatty Acids in Cancer and during Chemotherapy Treatment? Master’s Thesis, University of Edmonton, Edmonton, AB, Canada, 2008. [Google Scholar]
- Lee, M.C.; Kachura, J.J.; Vlachou, P.A.; Dzulynsky, R.; Di Tomaso, A.; Samawi, H.; Baxter, N.; Brezden-Masley, C. Evaluation of Adjuvant Chemotherapy-Associated Steatosis (CAS) in Colorectal Cancer. Curr. Oncol. 2021, 28, 3030–3040. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Popescu, L.A.; Virgolici, B.; Lixandru, D.; Miricescu, D.; Condruţ, E.; Timnea, O.; Ranetti, A.; Militaru, M.; Mohora, M.; Zăgrean, L. Effect of diet and omega-3 fatty acids in NAFLD. Rom. J. Morphol. Embryol. 2013, 54, 785–790. [Google Scholar]
- Bargut, T.C.L.; Frantz, E.D.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; Cardoso, J.F.R.; Calder, P.C.; Jordão, A.A.; Vannucchi, H. Fish oil decreases hepatic lipogenic genes in rats fasted and refed on a high fructose diet. Nutrients 2015, 7, 1644–1656. [Google Scholar] [CrossRef]
- Janczyk, W.; Lebensztejn, D.; Wierzbicka-Rucińska, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P.; Socha, P. Omega-3 fatty acids therapy in children with nonalcoholic fatty liver disease: A randomized controlled trial. J. Pediatr. 2015, 166, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, L.; Magliocco, O.; Spampinato, D.; Piro, S.; Oliveri, C.; Alagona, C.; Papa, G.; Rabuazzo, A.; Purrello, F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008, 40, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Yerian, L.; Hawkins, C.; Sargent, R.; McCullough, A.J. Double blind randomized placebo controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2015, 49, 137. [Google Scholar] [CrossRef]
- Song, B.-J.; Moon, K.-H.; Olsson, N.U.; Salem, N., Jr. Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J. Hepatol. 2008, 49, 262–273. [Google Scholar] [CrossRef]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.I.; Yamada, N.; Shimano, H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar] [CrossRef]
- Wada, S.; Yamazaki, T.; Kawano, Y.; Miura, S.; Ezaki, O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol. 2008, 49, 441–450. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.-J.; Feng, K.; He, C.; Li, P.; Hu, Y.-J.; Su, H.; Wan, J.-B. Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef]
- Huang, W.; Wang, B.; Li, X.; Kang, J.X. Endogenously elevated n-3 polyunsaturated fatty acids alleviate acute ethanol-induced liver steatosis. Biofactors 2015, 41, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-L.; Wan, J.-B.; Wang, B.; He, C.-W.; Ma, H.; Li, T.-W.; Kang, J.X. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.-Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Williams, J.; Bell-Anderson, K.S.; Farrell, G.C. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J. Hepatol. 2008, 49, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Hernandez-Ono, A.; Siri, P.; Weisberg, S.; Conlon, D.; Graham, M.J.; Crooke, R.M.; Huang, L.-S.; Ginsberg, H.N. Aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 2006, 281, 37603–37615. [Google Scholar] [CrossRef] [PubMed]
- Skat-Rørdam, J.; Højland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef]
- Lee, J.J.; Lambert, J.E.; Hovhannisyan, Y.; Ramos-Roman, M.A.; Trombold, J.R.; Wagner, D.A.; Parks, E.J. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am. J. Clin. Nutr. 2015, 101, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Lytle, K.A.; Depner, C.M.; Tripathy, S. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol. Ther. 2018, 181, 108–125. [Google Scholar] [CrossRef]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepaa, A.-L.; Oresic, M.; Yki-Jarvinen, H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 2009, 58, 203–208. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Cao, B.; Liu, C.; Zhang, Q.; Dong, Y. Maternal high-fat diet leads to non-alcoholic fatty liver disease through upregulating hepatic SCD1 expression in neonate rats. Front. Nutr. 2020, 7, 581723. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221. [Google Scholar] [PubMed]
- Wei, Q.; Zhou, B.; Yang, G.; Hu, W.; Zhang, L.; Liu, R.; Li, M.; Wang, K.; Gu, H.F.; Guan, Y. JAZF1 ameliorates age and diet-associated hepatic steatosis through SREBP-1c-dependent mechanism. Cell Death Dis. 2018, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zeng, R.; Cao, G.; Song, Z.; Zhang, Y.; Liu, C. Vibration training triggers brown adipocyte relative protein expression in rat white adipose tissue. BioMed Res. Int. 2015, 2015, 919401. [Google Scholar] [CrossRef]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwarkanath, A.; Dwivedi, U.N.; Kakkar, P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. 2021, 12, 892–909. [Google Scholar] [CrossRef]
- García-Villafranca, J.; Guillén, A.; Castro, J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie 2008, 90, 460–466. [Google Scholar] [CrossRef]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef]
- Berriot-Varoqueaux, N.; Aggerbeck, L.; Samson-Bouma, M.-E.; Wetterau, J. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000, 20, 663–697. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kobayashi, H.; Ashakumary, L.; Rouyer, I.A.; Takahashi, Y.; Aoyama, T.; Hashimoto, T.; Mizugaki, M. Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1485, 23–35. [Google Scholar] [CrossRef]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Hui, T.Y.; Young, S.G.; Davis, R.A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the, E.R. J. Lipid Res. 2003, 44, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.; Beigneux, A.; Bergo, M.O.; Maher, J.J.; Young, S.G. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J. Biol. Chem. 2002, 277, 5476–5483. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.V.; Stefano, J.T.; Oliveira, C.P. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 245–251. [Google Scholar] [CrossRef]
- Moon, H.-S.; Dalamaga, M.; Kim, S.-Y.; Polyzos, S.A.; Hamnvik, O.-P.; Magkos, F.; Paruthi, J.; Mantzoros, C.S. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr. Rev. 2013, 34, 377–412. [Google Scholar] [CrossRef]
- Denechaud, P.-D.; Dentin, R.; Girard, J.; Postic, C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett. 2008, 582, 68–73. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; García-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of leptin in non-alcoholic fatty liver disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, L.; Clark, G.O.; Lee, Y.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Charron, M.J.; Newgard, C.B. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 4813–4819. [Google Scholar] [CrossRef]
- Asilmaz, E.; Cohen, P.; Miyazaki, M.; Dobrzyn, P.; Ueki, K.; Fayzikhodjaeva, G.; Soukas, A.A.; Kahn, C.R.; Ntambi, J.M.; Socci, N.D. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J. Clin. Investig. 2004, 113, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef]
- Cao, S.; Rustum, Y.M. Synergistic antitumor activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: Role of drug sequence and dose. Cancer Res. 2000, 60, 3717–3721. [Google Scholar] [PubMed]
- Xue, H.; Le Roy, S.; Sawyer, M.B.; Field, C.J.; Dieleman, L.A.; Baracos, V.E. Single and combined supplementation of glutamine and n-3 polyunsaturated fatty acids on host tolerance and tumour response to 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothecin (CPT-11)/5-fluorouracil chemotherapy in rats bearing Ward colon tumour. Br. J. Nutr. 2009, 102, 434–442. [Google Scholar]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Almasud, A.A.; Giles, K.H.; Miklavcic, J.J.; Martins, K.J.; Baracos, V.E.; Putman, C.T.; Guan, L.L.; Mazurak, V.C. Fish oil mitigates myosteatosis and improves chemotherapy efficacy in a preclinical model of colon cancer. PLoS ONE 2017, 12, e0183576. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Galloway, S.D.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fat. Acids 2014, 90, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]


| (a) | |||||
| Fatty Acid (%) | Reference | Tumor | Chemo | Chemo + Fish Oil | p-Value |
|---|---|---|---|---|---|
| C16:0 | 19.6 ± 0.4 b | 22.0 ± 1.4 a | 18.6 ± 1.3 b | 17.6 ± 1.8 b | <0.001 |
| C18:0 | 7.1 ± 0.1 | 6.9 ± 0.5 | 7.9 ± 0.1 | 7.95 ± 1.1 | 0.058 |
| C18:1n-9 | 47.6 ± 1.7 a | 46.7 ± 2.31 a | 49.9 ± 2.9 a | 37.2 ± 1.2 b | 0.001 |
| C18:2n-6 | 16.1 ± 1.0 | 15.0 ± 1.2 | 14.1 ± 2.5 | 13.8 ± 3.0 | 0.270 |
| C18:3n-3 | 1.2 ± 0.4 | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.060 |
| C20:4n-6 | 2.5 ± 0.7 | 2.5 ± 0.6 | 2.4 ± 0.7 | 2.2 ± 0.3 | 0.873 |
| C20:5n-3 | 0.3 ± 0.1 bc | 0.3 ± 0.1 b | 0.2 ± 0.1 c | 6.0 ± 0.8 a | 0.003 |
| C22:5n-3 | 0.4 ± 0.1 b | 0.4 ± 0.1 b | 0.4 ± 0.1 b | 3.6 ± 0.8 a | 0.008 |
| C22:6n-3 | 1.5 ± 0.5 b | 1.3 ± 0.2 b | 1.1 ± 0.3 bc | 8.1 ± 1.9 a | 0.002 |
| ∑n-6 FA | 18.8 ± 1.8 | 17.7 ± 1.8 | 16.8 ± 3.3 | 16.2 ± 3.4 | 0.248 |
| ∑n-3 FA | 3.6 ± 1.2 b | 3.0 ± 0.5 bc | 2.7 ± 0.8 bcd | 18.8 ± 3.5 a | 0.001 |
| n-6/n-3 | 5.2 ± 1.5 a | 5.8 ± 3.3 a | 6.2 ± 4.0 a | 0.9 ± 0.9 b | 0.002 |
| ∑SFA | 27.0 ± 1.5 b | 29.1 ± 2.0 a | 26.8 ± 1.6 b | 25.7 ± 2.9 b | 0.004 |
| ∑MUFA | 49.8 ± 2.6 a | 48.5 ± 2.6 a | 51.2 ± 3.2 a | 38.4 ± 1.3 b | 0.002 |
| ∑Total FA (μg/g) | 2293.5 ± 909.9 ab | 1683.4 ± 642.7 b | 3243.8 ± 848.0 a | 2624.1 ± 654.5 ab | 0.024 |
| (b) | |||||
| Fatty Acid (%) | Reference | Tumor | Chemo | Chemo + Fish Oil | p-Value |
| C16:0 | 8.1 ± 0.1 | 8.8 ± 0.4 | 9.5 ± 2.3 | 9.4 ± 1.9 | 0.104 |
| C18:0 | 37.1 ± 0.8 ab | 38.3 ± 1.1 a | 35.6 ± 2.2 b | 36.1 ± 1.2 b | 0.017 |
| C18:1n-9 | 4.5 ± 0.5 b | 4.8 ± 0.5 b | 5.6 ± 0.5 a | 5.3 ± 0.5 ab | 0.007 |
| C18:2n-6 | 8.1 ± 0.5 | 8.6 ± 0.4 | 8.7 ± 1.6 | 8.4 ± 1.3 | 0.344 |
| C18:3n-3 | 0.06 ± 0.05 | 0.02 ± 0.03 | 0.03 ± 0.04 | 0.05 ± 0.06 | 0.533 |
| C20:4n-6 | 29.9 ± 0.6 a | 26.8 ± 1.2 bc | 28.1 ± 2.5 b | 20.2 ± 0.9 d | <0.001 |
| C20:5n-3 | 0.5 ± 0.1 c | 0.7 ± 0.1 b | 0.5 ± 0.1 c | 6.7 ± 0.7 a | <0.001 |
| C22:5n-3 | 0.6 ± 0.1 b | 0.6 ± 0.1 b | 0.5 ± 0.3 bc | 1.5 ± 0.1 a | 0.006 |
| C22:6n-3 | 9.3 ± 1.2 b | 9.5 ± 1.1 b | 9.4 ± 0.5 b | 11.6 ± 0.9 a | <0.001 |
| ∑n-6 FA | 39.0 ± 0.8 a | 36.5 ± 1.2 bc | 37.6 ± 1.4 b | 29.3 ± 2.1 d | <0.001 |
| ∑n-3 FA | 10.4 ± 1.4 b | 10.8 ± 1.1 b | 10.4 ± 0.79 b | 19.8 ± 1.3 a | 0.004 |
| n-6/n-3 | 3.8 ± 0.6 a | 3.4 ± 0.4 a | 3.6 ± 0.3 a | 1.5 ± 0.2 b | 0.003 |
| ∑SFA | 45.6 ± 0.7 | 47.2 ± 1.4 | 45.4 ± 1.7 | 45.9 ± 1.3 | 0.124 |
| ∑MUFA | 4.8 ± 0.5 b | 5.2 ± 0.5 ab | 5.9 ± 0.6 a | 5.6 ± 0.5 a | 0.007 |
| ∑Total FA (μg/g) | 27.9 ± 2.9 ab | 24.0 ± 2.2 b | 33.6 ± 4.0 a | 32.0 ± 6.3 a | 0.003 |
| Control Diet | Fish Oil Diet | |
|---|---|---|
| Saturated fatty acids | 58.7 | 59.9 |
| Monounsaturated fatty acids | 17.3 | 14.3 |
| Polyunsaturated fatty acids | 20.6 | 22.5 |
| Total n-6 | 18.6 | 13.6 |
| Total n-3 | 2.00 | 8.90 |
| EPA | Nil | 5.10 |
| DHA | Nil | 2.10 |
| Other fatty acids | 3.40 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monirujjaman, M.; Renani, L.B.; Isesele, P.; Dunichand-Hoedl, A.R.; Mazurak, V.C. Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil. Int. J. Mol. Sci. 2023, 24, 3547. https://doi.org/10.3390/ijms24043547
Monirujjaman M, Renani LB, Isesele P, Dunichand-Hoedl AR, Mazurak VC. Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil. International Journal of Molecular Sciences. 2023; 24(4):3547. https://doi.org/10.3390/ijms24043547
Chicago/Turabian StyleMonirujjaman, Md, Leila Baghersad Renani, Peter Isesele, Abha R. Dunichand-Hoedl, and Vera C. Mazurak. 2023. "Increased Expression of Hepatic Stearoyl-CoA Desaturase (SCD)-1 and Depletion of Eicosapentaenoic Acid (EPA) Content following Cytotoxic Cancer Therapy Are Reversed by Dietary Fish Oil" International Journal of Molecular Sciences 24, no. 4: 3547. https://doi.org/10.3390/ijms24043547





