Amyloid Fibrils of Stefin B Show Anisotropic Properties
Abstract
:1. Introduction
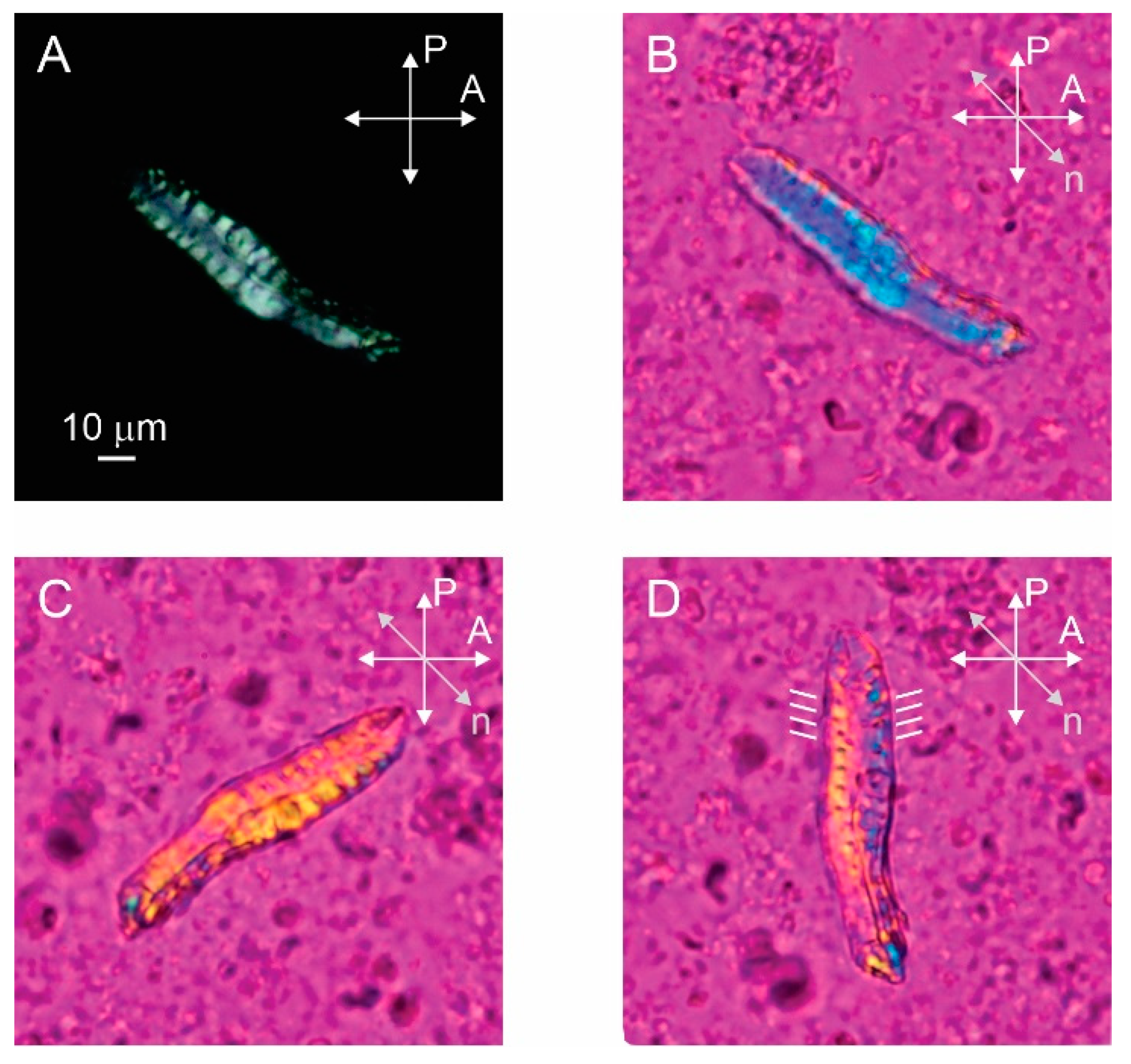
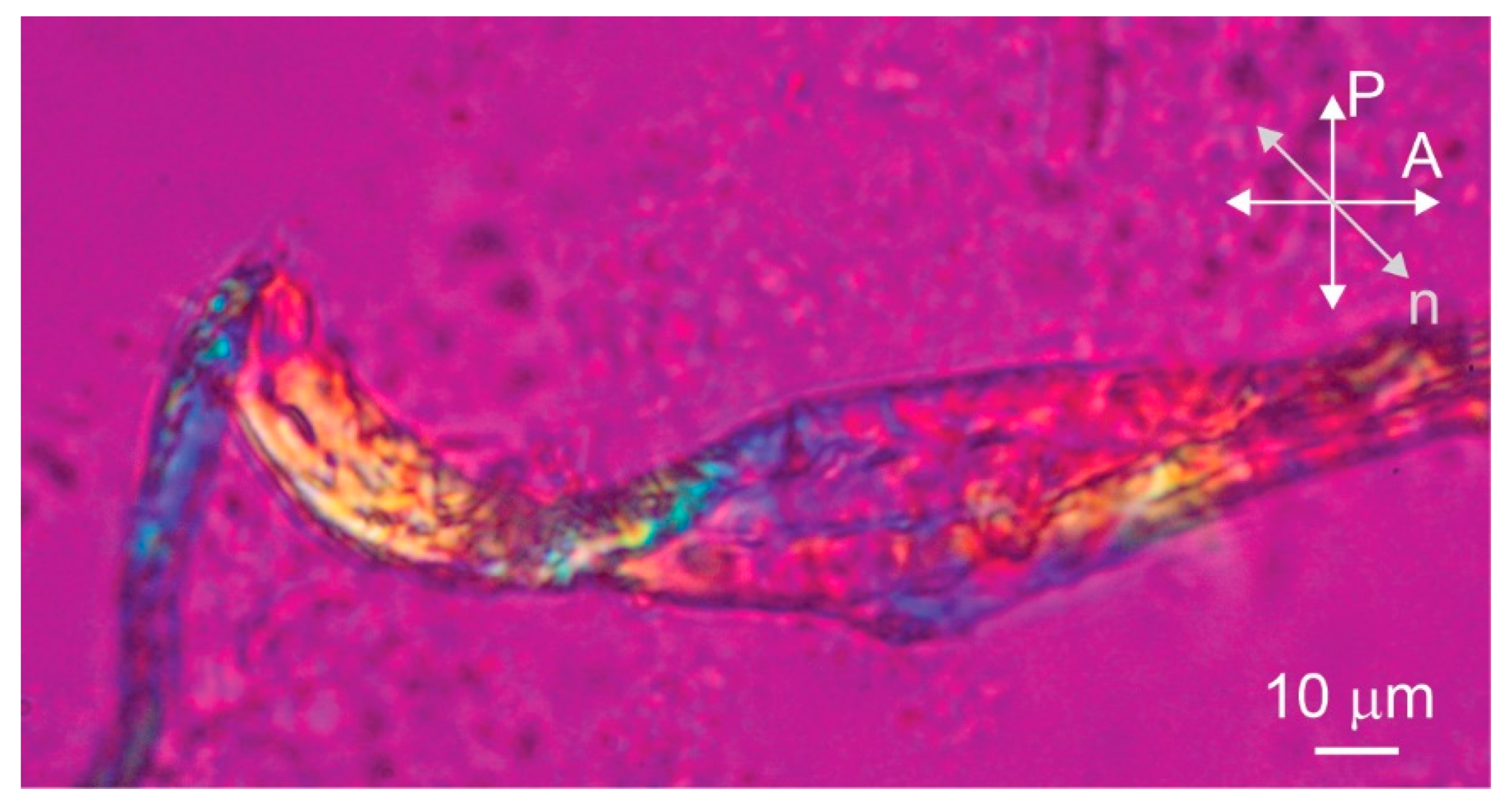
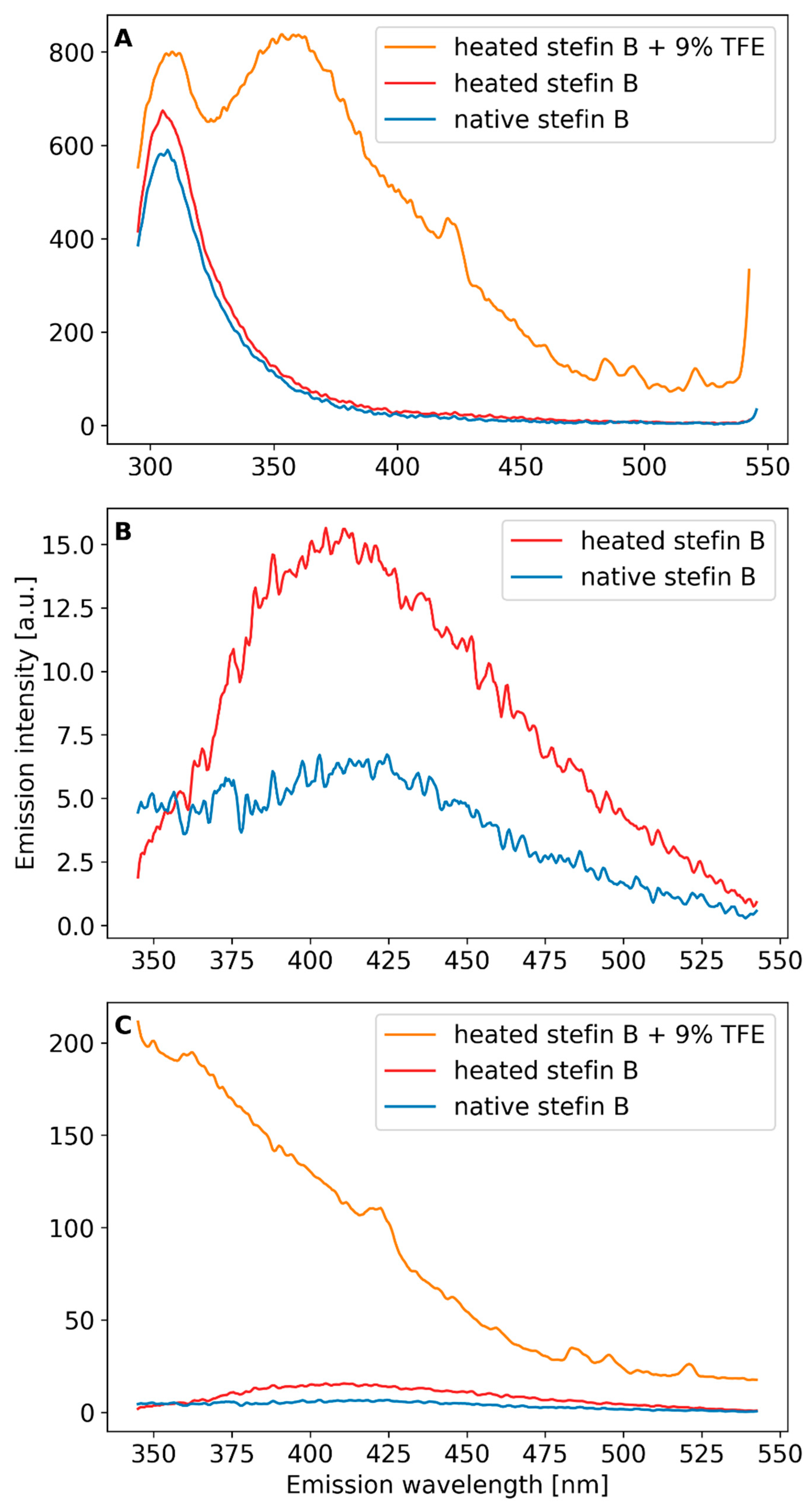
2. Results
3. Discussion
4. Methods
4.1. Protein Production and Purification
4.2. Amyloid Fibrillation, ThT Fluorescence
4.3. Intrinsic Fluorescence
4.4. Atomic Force Microscopy (AFM)
4.5. Polarized Optical Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunde, M.; Blake, C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv. Protein. Chem. 1997, 50, 123–159. [Google Scholar] [PubMed]
- Serpell, L.C.; Sunde, M.; Benson, M.D.; Tennent, G.A.; Pepys, M.B.; Fraser, P.E. The protofilament substructure of amyloid fibrils. J. Mol. Biol. 2000, 300, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Guijarro, J.L.; Orlova, E.; Zurdo, J.; Dobson, C.M.; Sunde, M.; Saibil, H.R. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999, 18, 815–821. [Google Scholar] [CrossRef]
- Marsagishvili, L.G.; Shpagina, M.D.; Emel’ianenko, V.I.; Podlubnaia, Z.A. Sarcomeric proteins of the titin family form amyloids. Biofizika 2005, 50, 803–809. [Google Scholar] [PubMed]
- Taylor, A.I.P.; Staniforth, R.A. General Principles Underpinning Amyloid Structure. Front. Neurosci. 2022, 16, 878869. [Google Scholar] [CrossRef]
- Demirel, G.; Malvadkar, N.; Demirel, M.C. Control of protein adsorption onto core-shell tubular and vesicular structures of diphenylalanine/parylene. Langmuir 2010, 26, 1460–1463. [Google Scholar] [CrossRef]
- Anjana, R.; Vaishnavi, M.K.; Sherlin, D.; Kumar, S.P.; Naveen, K.; Kanth, P.S.; Sekar, K. Aromatic-aromatic interactions in structures of proteins and protein-DNA complexes: A study based on orientation and distance. Bioinformation 2012, 8, 1220–1224. [Google Scholar] [CrossRef]
- Marshall, K.E.; Morris, K.L.; Charlton, D.; O’Reilly, N.; Lewis, L.; Walden, H.; Serpell, L.C. Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry 2011, 50, 2061–2071. [Google Scholar] [CrossRef]
- Gazit, E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 77–83. [Google Scholar]
- Stanković, I.M.; Božinovski, D.M.; Brothers, E.N.; Belić, M.R.; Hall, M.B.; Zarić, S.D. Interactions of Aromatic Residues in Amyloids: A Survey of Protein Data Bank Crystallographic Data. Cryst. Growth Des. 2017, 17, 6353–6362. [Google Scholar] [CrossRef]
- Cukalevski, R.; Boland, B.; Frohm, B.; Thulin, E.; Walsh, D.; Linse, S. Role of Aromatic Side Chains in Amyloid β-Protein Aggregation. ACS Chem. Neurosci. 2012, 3, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanković, I.M.; Niu, S.; Hall, M.B.; Zarić, S.D. Role of aromatic amino acids in amyloid self-assembly. Int. J. Biol. Macromol. 2020, 156, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zganec, M.; Zerovnik, E. Amyloid fibrils compared to peptide nanotubes. Biochim. Biophys. Acta 2014, 1840, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.M.; Muller, C.; Krebs, M.R.H. The formation of nematic liquid crystal phases by hen lysozyme amyloid fibrils. J. Am. Chem. Soc. 2006, 128, 14740–14741. [Google Scholar] [CrossRef] [PubMed]
- Mohler, W.; Millard, A.C.; Campagnola, P.J. Second harmonic generation imaging of endogenous structural proteins. Methods 2003, 29, 97–109. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef]
- Handelman, A.; Lavrov, S.; Kudryavtsev, A.; Khatchatouriants, A.; Rosenberg, Y.; Mishina, E.; Rosenman, G. Nonlinear Optical Bioinspired Peptide Nanostructures. Adv. Opt. Mater. 2013, 1, 875–884. [Google Scholar] [CrossRef]
- Handelman, A.; Natan, A.; Rosenman, G. Structural and optical properties of short peptides: Nanotubes-to-nanofibers phase transformation. J. Pept. Sci. 2014, 20, 487–493. [Google Scholar] [CrossRef]
- Amdursky, N.; Molotskii, M.; Aronov, D.; Adler-Abramovich, L.; Gazit, E.; Rosenman, G. Blue Luminescence Based on Quantum Confinement at Peptide Nanotubes. Nano Lett. 2009, 9, 3111–3115. [Google Scholar] [CrossRef]
- Chan, F.T.; Kaminski Schierle, G.S.; Kumita, J.R.; Bertoncini, C.W.; Dobson, C.M.; Kaminski, C.F. Protein amyloids develop an intrinsic fluorescence signature during aggregation. Analyst 2013, 138, 2156–2162. [Google Scholar] [CrossRef]
- Shukla, A.; Mukherjee, S.; Sharma, S.; Agrawal, V.; Kishan, K.V.R.; Guptasarma, P. A novel UV laser-induced visible blue radiation from protein crystals and aggregates: Scattering artifacts or fluorescence transitions of peptide electrons delocalized through hydrogen bonding? Arch Biochem. Biophys. 2004, 428, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.K.; Koelsch, P. Label-free imaging of amyloids using their intrinsic linear and nonlinear optical properties. Biomed. Opt. Express 2017, 8, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Jenko, S.; Skarabot, M.; Kenig, M.; Guncar, G.; Musevic, I.; Turk, D.; Zerovnik, E. Different propensity to form amyloid fibrils by two homologous proteins-Human stefins A and B: Searching for an explanation. Proteins 2004, 55, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zerovnik, E.; Pompe-Novak, M.; Skarabot, M.; Ravnikar, M.; Musevic, I.; Turk, V. Human stefin B readily forms amyloid fibrils in vitro. Biochim. Biophys. Acta 2002, 1594, 1–5. [Google Scholar] [CrossRef]
- Taler-Vercic, A.; Kirsipuu, T.; Friedemann, M.; Noormagi, A.; Polajnar, M.; Smirnova, J.; Znidaric, M.T.; Zganec, M.; Skarabot, M.; Vilfan, A.; et al. The role of initial oligomers in amyloid fibril formation by human stefin B. Int. J. Mol. Sci. 2013, 14, 18362–18384. [Google Scholar] [CrossRef]
- Skerget, K.; Vilfan, A.; Pompe-Novak, M.; Turk, V.; Waltho, J.P.; Turk, D.; Zerovnik, E. The mechanism of amyloid-fibril formation by stefin B: Temperature and protein concentration dependence of the rates. Proteins 2009, 74, 425–436. [Google Scholar] [CrossRef]
- Zerovnik, E.; Skarabot, M.; Skerget, K.; Giannini, S.; Stoka, V.; Jenko-Kokalj, S.; Staniforth, R.A. Amyloid fibril formation by human stefin B: Influence of pH and TFE on fibril growth and morphology. Amyloid-J. Protein Fold. Disord. 2007, 14, 237–247. [Google Scholar] [CrossRef]
- Niyangoda, C.; Miti, T.; Breydo, L.; Uversky, V.; Muschol, M. Carbonyl-based blue autofluorescence of proteins and amino acids. PLoS ONE 2017, 12, e0176983. [Google Scholar] [CrossRef]
- Bagnani, M.; Azzari, P.; Assenza, S.; Mezzenga, R. Six-fold director field configuration in amyloid nematic and cholesteric phases. Sci. Rep. 2019, 9, 12654. [Google Scholar] [CrossRef]
- Mankar, S.; Anoop, A.; Sen, S.; Maji, S.K. Nanomaterials: Amyloids reflect their brighter side. Nano Rev. 2011, 2. [Google Scholar] [CrossRef]
- Tikhonova, T.N.; Rovnyagina, N.R.; Zherebker, A.Y.; Sluchanko, N.N.; Rubekina, A.A.; Orekhov, A.S.; Nikolaev, E.N.; Fadeev, V.V.; Uversky, V.N.; Shirshin, E.A. Dissection of the deep-blue autofluorescence changes accompanying amyloid fibrillation. Arch. Biochem. Biophys. 2018, 651, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ziaunys, M.; Sneideris, T.; Smirnovas, V. Exploring the potential of deep-blue autofluorescence for monitoring amyloid fibril formation and dissociation. PeerJ 2019, 7, e7554. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žganec, M.; Taler Verčič, A.; Muševič, I.; Škarabot, M.; Žerovnik, E. Amyloid Fibrils of Stefin B Show Anisotropic Properties. Int. J. Mol. Sci. 2023, 24, 3737. https://doi.org/10.3390/ijms24043737
Žganec M, Taler Verčič A, Muševič I, Škarabot M, Žerovnik E. Amyloid Fibrils of Stefin B Show Anisotropic Properties. International Journal of Molecular Sciences. 2023; 24(4):3737. https://doi.org/10.3390/ijms24043737
Chicago/Turabian StyleŽganec, Matjaž, Ajda Taler Verčič, Igor Muševič, Miha Škarabot, and Eva Žerovnik. 2023. "Amyloid Fibrils of Stefin B Show Anisotropic Properties" International Journal of Molecular Sciences 24, no. 4: 3737. https://doi.org/10.3390/ijms24043737




