Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test
Abstract
:1. Introduction
2. Results and Discussion
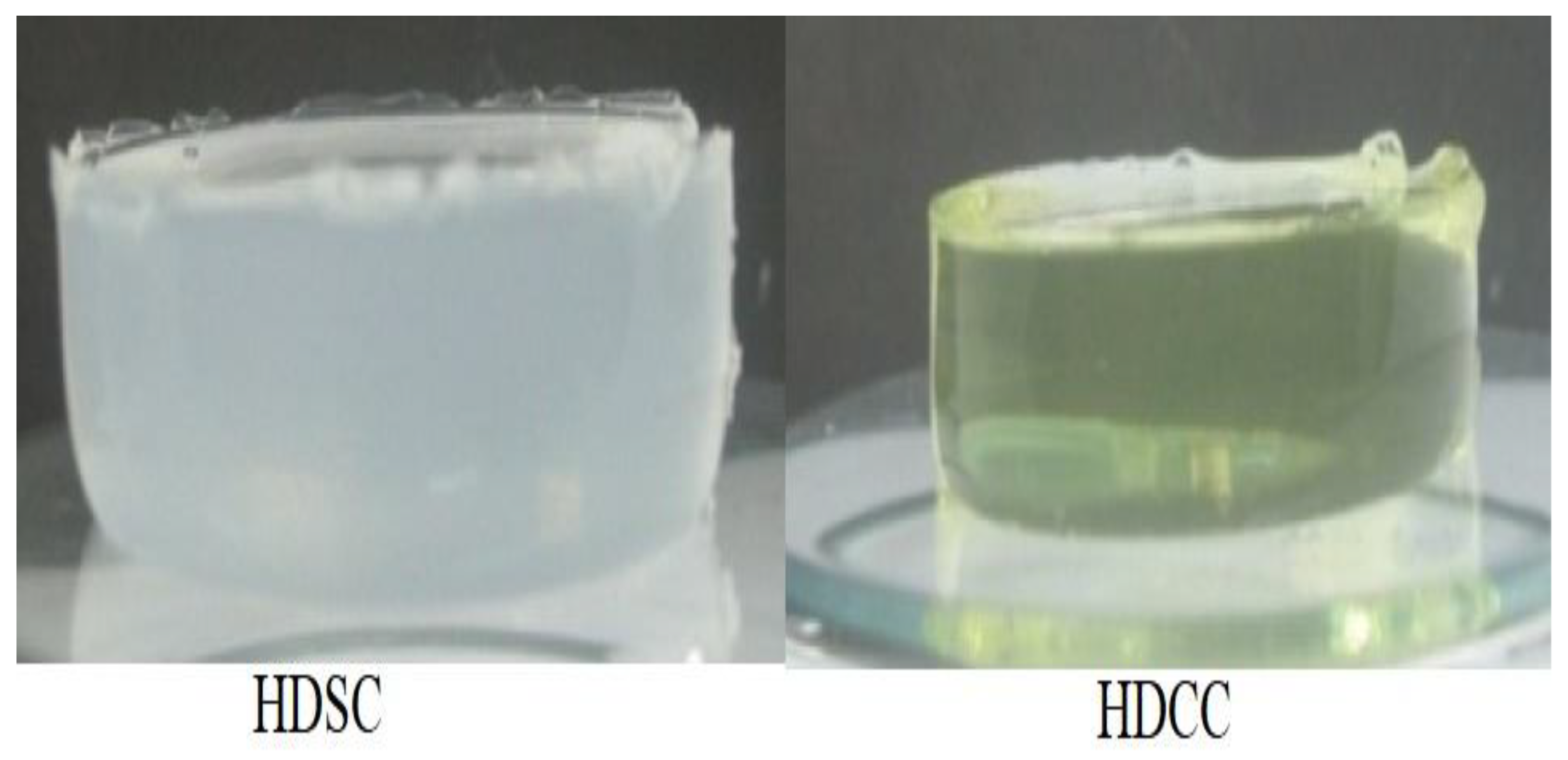
2.1. Preparation of Hydrogel
2.2. Characterization
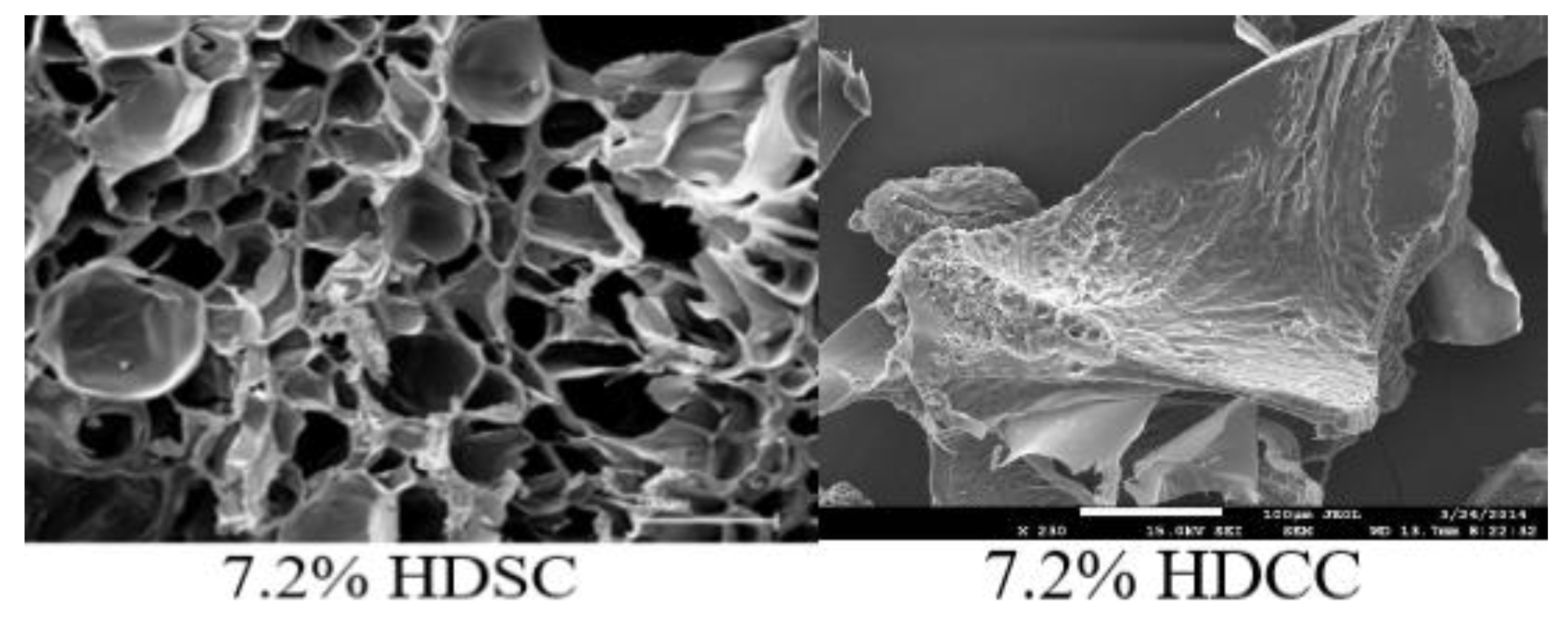
2.2.1. Morphology Analysis
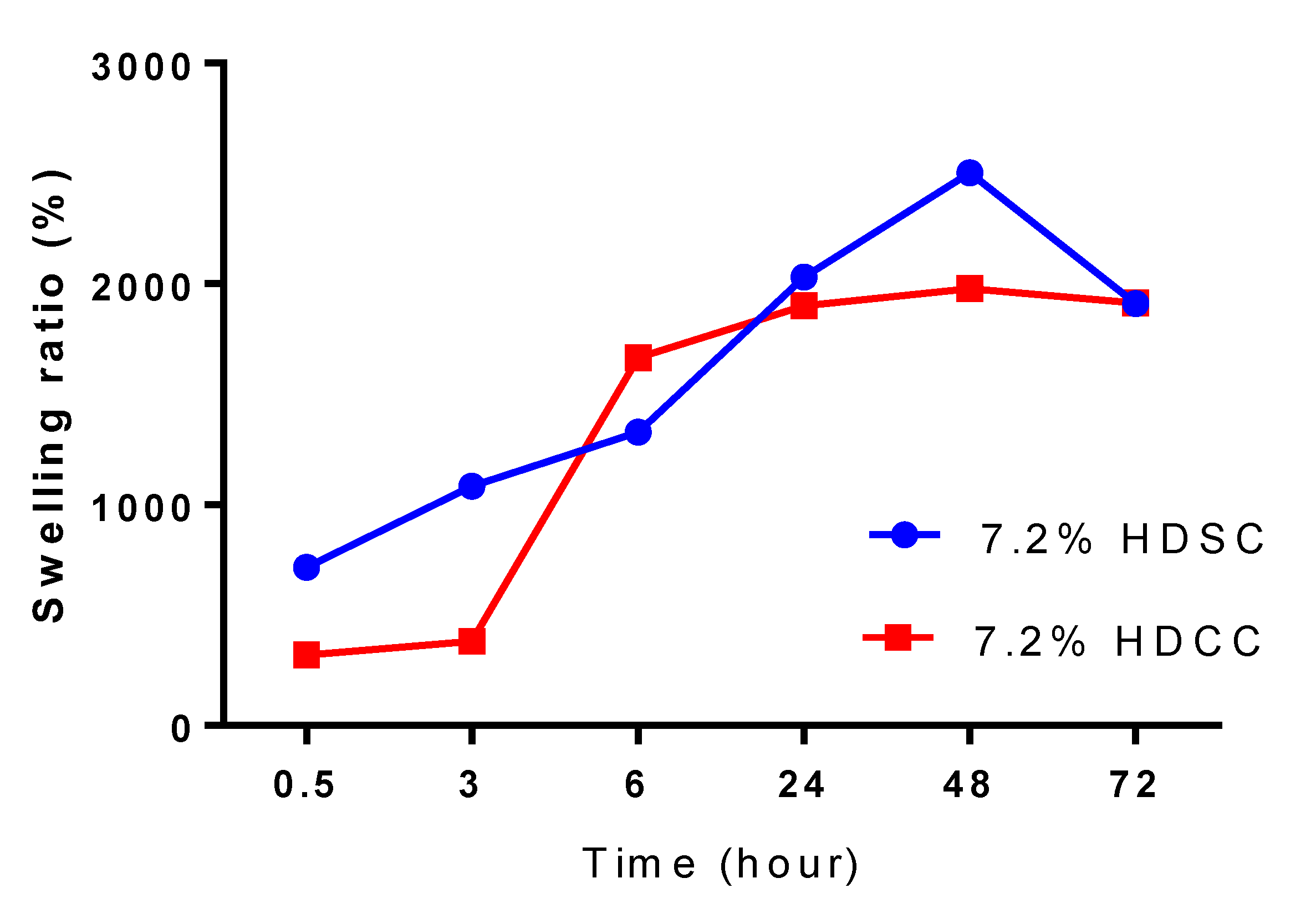
2.2.2. Swelling Studies
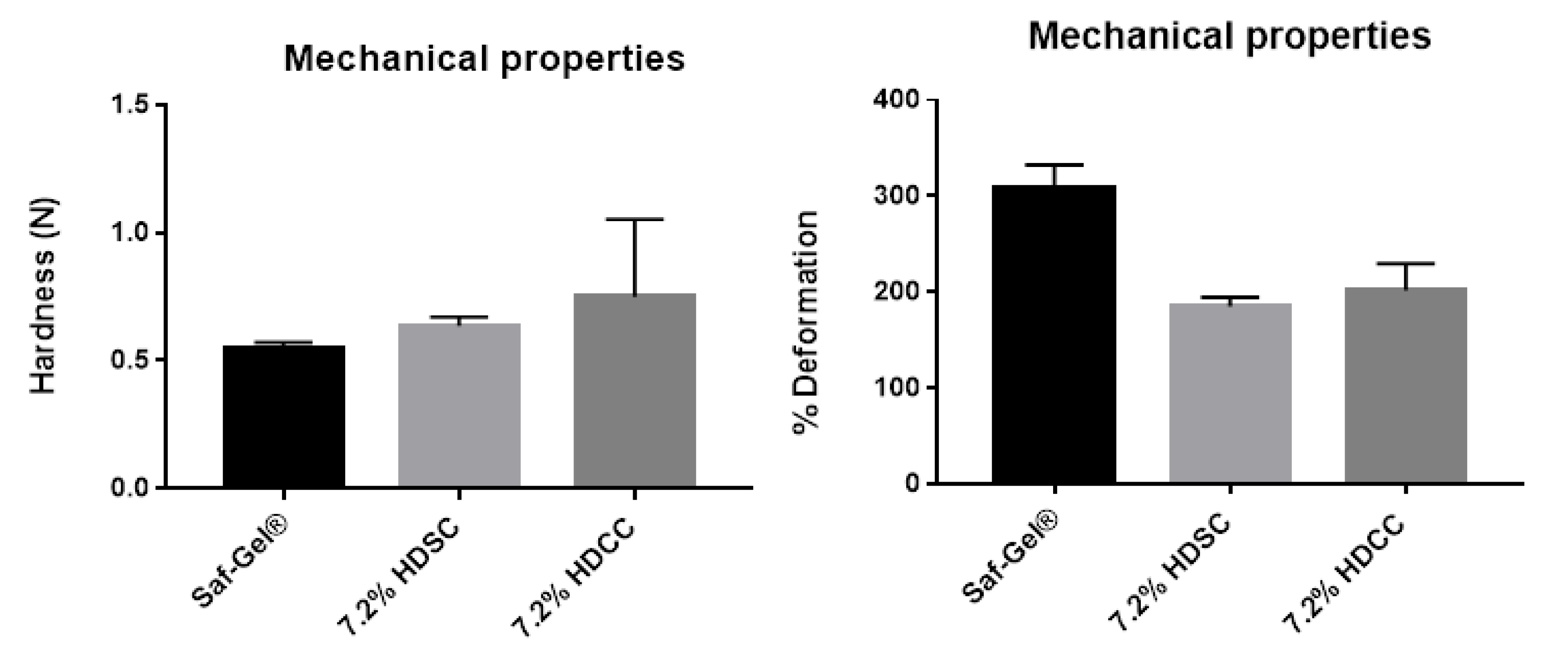
2.2.3. Mechanical Properties by Texturometer
2.3. Hydrogel Biocompatibility Study
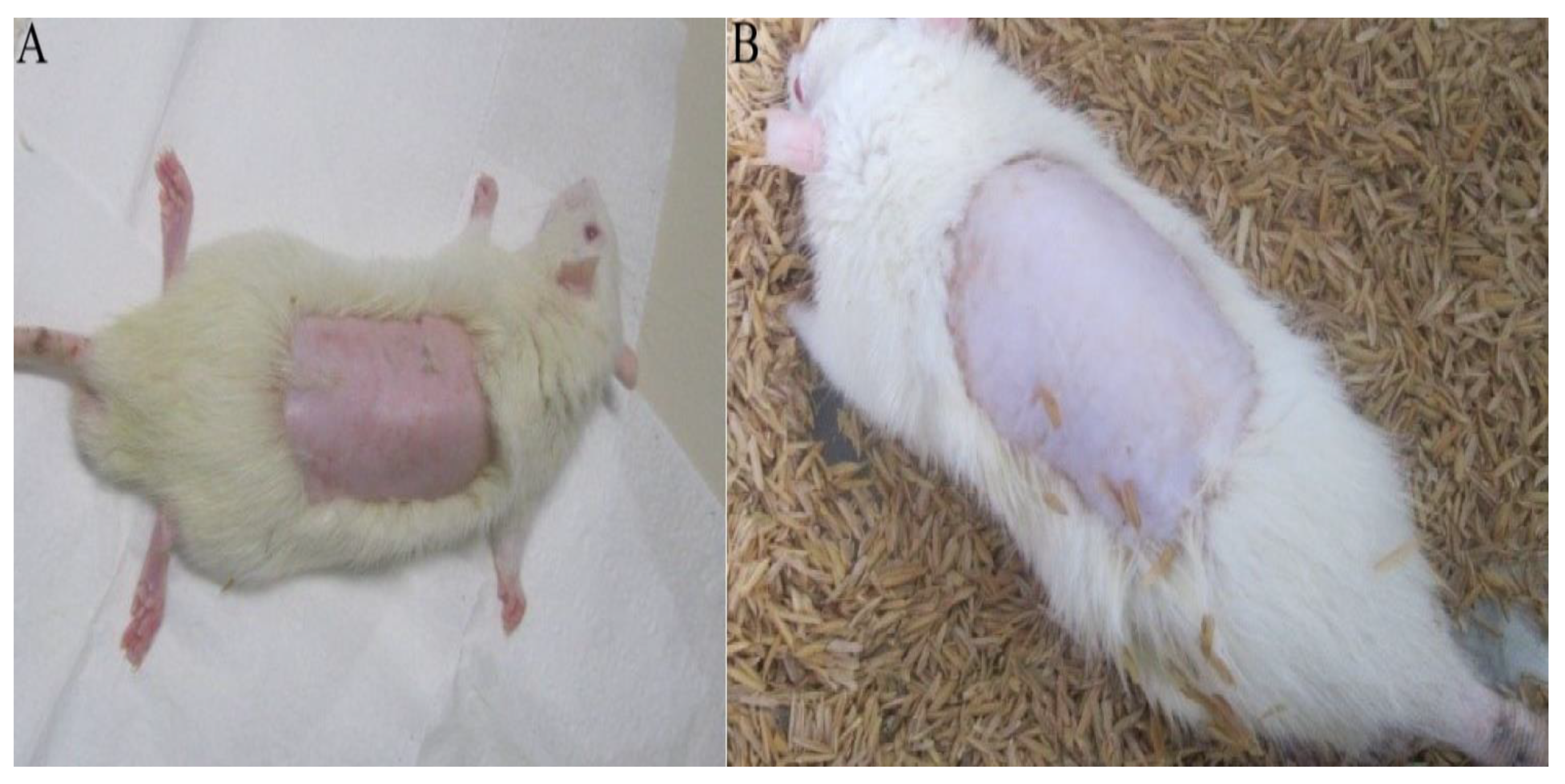
2.3.1. Acute Dermal Toxicity Test
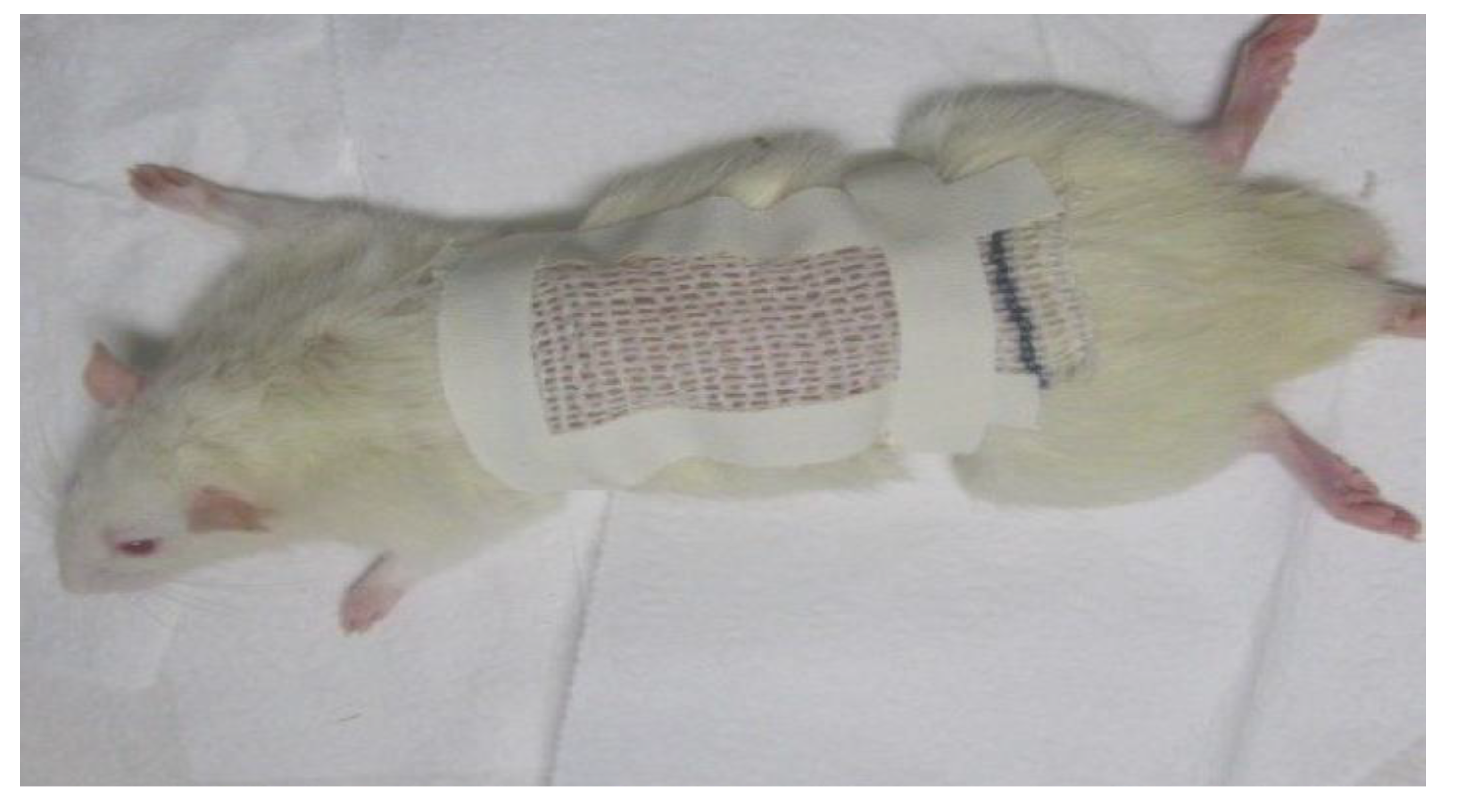
2.3.2. Evaluation of Hydrogel Action in Healing Process
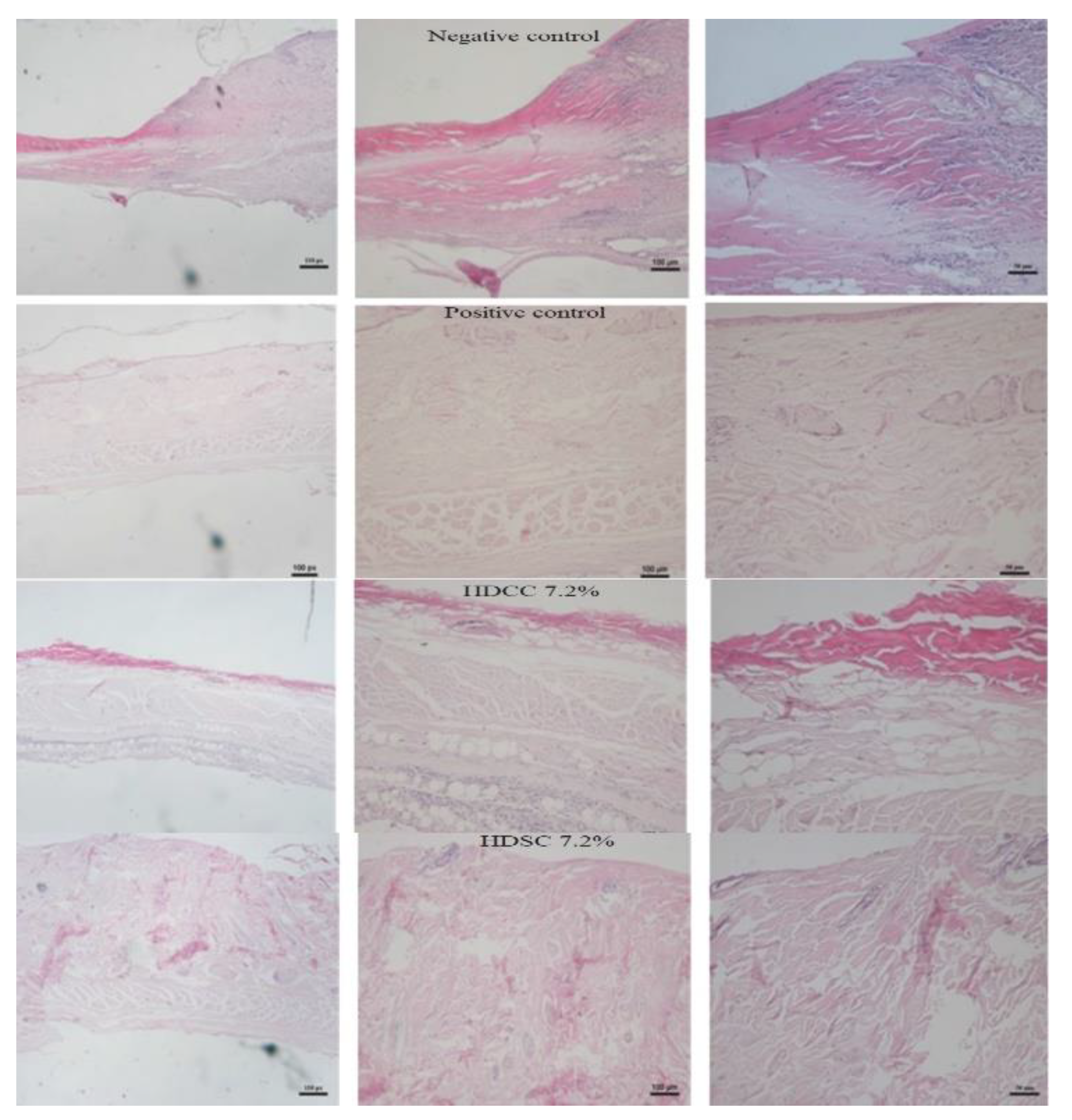
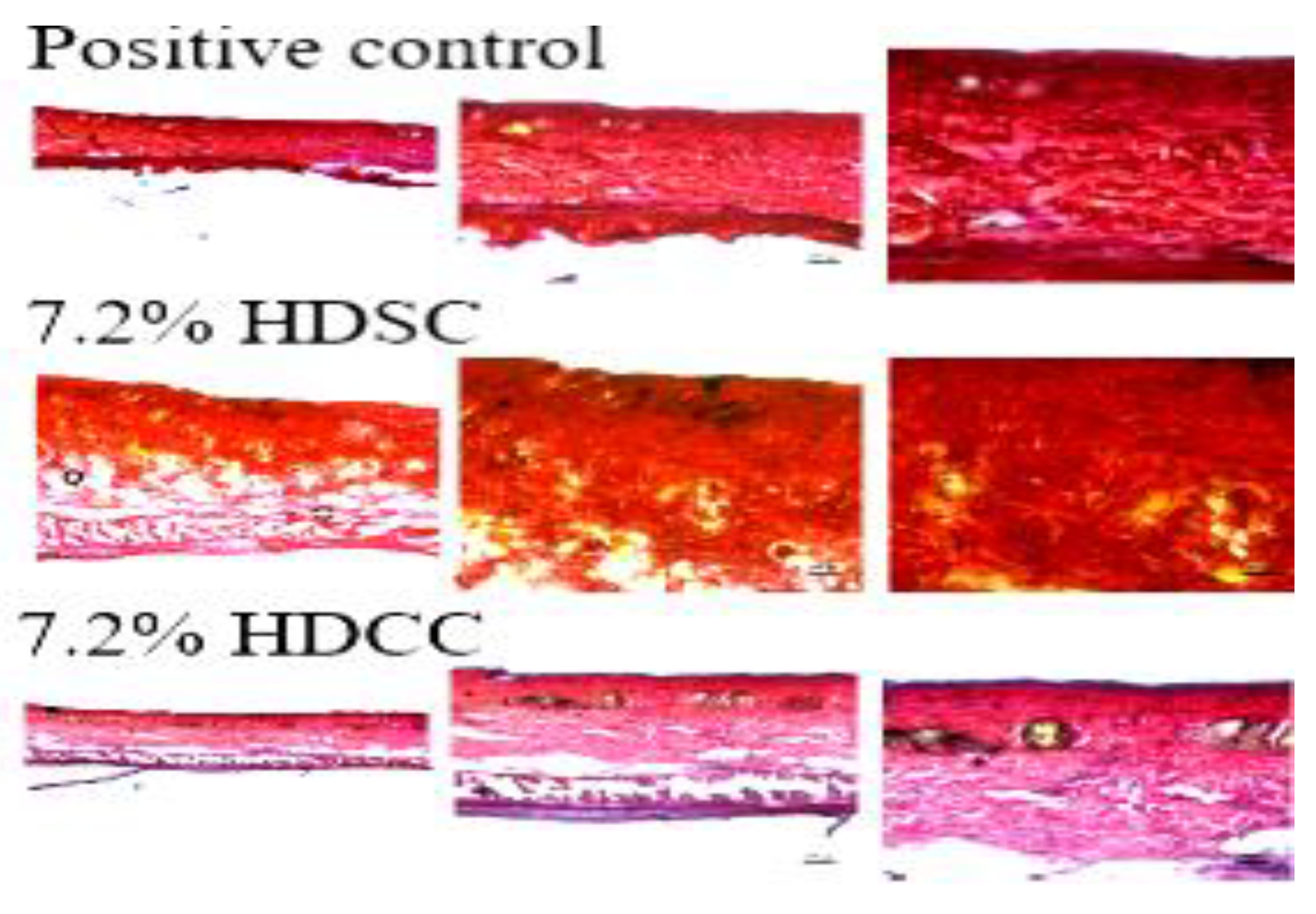
2.3.3. Histopathological Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Calendula Extract
3.3. Synthesis of Hydrogel
3.4. Characterization of Hydrogels
3.4.1. Surface Morphology Analysis
3.4.2. Swelling Assay
3.4.3. Mechanical Properties by Texturometer
3.5. Hydrogel Biocompatibility Study
3.5.1. Animal Test Subjects
3.5.2. Acute Dermal Toxicity Test
3.5.3. Ulcer Formation
Exudate Formation Assessment
Cell Migration Assessment
Necrotic Tissue Area Analysis
Nitric Oxide Activity Evaluation
3.5.4. Histopathological Evaluation
Hematoxylin and Eosin Evaluation
Collagen Evaluation Using Picrosirius Red
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef] [PubMed]
- Edsberg, L.E.; Black, J.M.; Goldberg, M.; McNichol, L.; Moore, L.; Sieggreen, M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System. J. Wound Ostomy Cont. Nurs. 2016, 43, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Nešović, K.; Janković, A.; Radetić, T.; Vukašinović-Sekulić, M.; Kojić, V.; Živković, L.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Synthesis of a novel anti-freezing, non-drying antibacterial hydrogel dressing by one-pot method. Chem. Eng. J. 2019, 372, 216–225. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Tavares, G. Recent Advances in Hydrogel-Mediated Nitric Oxide Delivery Systems Targeted for Wound Healing Applications. Pharmaceutics 2022, 14, 1377. [Google Scholar] [CrossRef]
- Albuquerque Alvim de Paula, V.; Duarte Souza, I.; Lúcia Muniz de Almeida, R.; Santos, K.B. O conhecimento dos enfermeiros assistenciais no tratamento de feridas. HU Rev. 2019, 45, 295–303. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.S.; Khan, F.; Kim, Y.M.; Heo, S.Y.; Oh, G.W.; Kim, N.G.; Jung, W.K. Wound healing properties of triple cross-linked poly (vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.W.; Jang, Y.M.; Ko, S.C.; Park, W.S.; Choi, I.W.; Kim, Y.M.; Jung, W.K. Antimicrobial hydrogels based on PVA and diphlorethohydroxycarmalol (DPHC) derived from brown alga Ishige okamurae: An in vitro and in vivo study for wound dressing application. Mater. Sci. Eng. C 2020, 107, 110352. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Jakfar, S.; Lin, T.; Chen, Z.; Yang, I.; Gani, B.A.; Ningsih, D.S.; Kusuma, H.; Chang, C.; Lin, F. A Polysaccharide Isolated from the Herb Bletilla striata Combined with Methylcellulose to Form a Hydrogel via Self-Assembly as a Wound Dressing. Int. J. Mol. Sci. 2022, 23, 12019. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef]
- Qi, X.; Pan, W.; Tong, X.; Gao, T.; Xiang, Y.; You, S.; Mao, R.; Chi, J.; Hu, R.; Zhang, W.; et al. ε-Polylysine-stabilized agarose/polydopamine hydrogel dressings with robust photothermal property for wound healing. Carbohydr. Polym. 2021, 264, 118046. [Google Scholar] [CrossRef]
- Tabassum, N.; Ahmed, S.; Ali, M.A. Chitooligosaccharides and their structural-functional effect on hydrogels: A review. Carbohydr. Polym. 2021, 261, 117882. [Google Scholar] [CrossRef] [PubMed]
- de Clifford, L.T.; Lowe, J.N.; McKellar, C.D.; Bolwell, C.; David, F. Use of a 2.5% Cross-Linked Polyacrylamide Hydrogel in the Management of Joint Lameness in a Population of Flat Racing Thoroughbreds: A Pilot Study. J. Equine Vet. Sci. 2019, 77, 57–62. [Google Scholar] [CrossRef]
- Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. Int. J. Mol. Sci. 2022, 23, 11618. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.; Macocinschi, D.; Zaltariov, M.; Ciubotaru, B.; Bargan, A.; Varganici, C.; Vasiliu, A.; Peptanariu, D.; Balan-porcarasu, M. Hydroxypropyl Cellulose/Pluronic-Based Composite Hydrogels as Biodegradable Mucoadhesive Scaffolds for Tissue Engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Mehta, S.K. Hydrogels: From Simple Networks to Smart Materials-Advances and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128136898. [Google Scholar]
- Kang-Mieler, J.J.; Mieler, W.F. Thermo-responsive hydrogels for ocular drug delivery. Dev. Ophthalmol. 2016, 55, 104–111. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256, 117590. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Gyles, D.A.; Pereira, A.D.; Castro, L.D.; Brigida, A.S.; Nobre Lamarão, M.L.; Ramos Barbosa, W.L.; Carréra Silva, J.O.; Ribeiro-Costa, R.M. Polyacrylamide-metilcellulose hydrogels containing aloe barbadensis extract as dressing for treatment of chronic cutaneous skin lesions. Polymers 2020, 12, 690. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Ma, H.; Yu, J.; Liu, L.; Fan, Y. An optimized preparation of nanofiber hydrogels derived from natural carbohydrate polymers and their drug release capacity under different pH surroundings. Carbohydr. Polym. 2021, 265, 118008. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Lauritzen, E.; Krammer, C.W. Breastfeeding difficulty after polyacrylamide hydrogel (PAAG) mediated breast augmentation. Int. J. Surg. Case Rep. 2018, 47, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, P.; Huang, C.; Zeng, R.; Yang, L.; Han, Z.; Qu, Y.; Zhang, C. Photothermal-promoted multi-functional dual network polysaccharide hydrogel adhesive for infected and susceptible wound healing. Carbohydr. Polym. 2021, 273, 118557. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.V.G.; Tavares, E.J.M.; Aouada, F.A.; Negrão, C.A.B.; Oliveira, M.E.C.; Duarte Júnior, A.P.; Ferreira Da Costa, C.E.; Silva Júnior, J.O.C.; Ribeiro Costa, R.M. Thermal analysis characterization of PAAm-co-MC hydrogels. J. Therm. Anal. Calorim. 2011, 106, 717–724. [Google Scholar] [CrossRef]
- Hua, J.; Liu, C.; Ng, P.F.; Fei, B. Bacterial cellulose reinforced double-network hydrogels for shape memory strand. Carbohydr. Polym. 2021, 259, 117737. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, flexible, and multifunctional starch-based double-network hydrogels as high-performance wearable electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 572, 118779. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larrañeta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef]
- Su, H.; Zheng, R.; Jiang, L.; Zeng, N.; Yu, K.; Zhi, Y.; Shan, S. Dextran hydrogels via disulfide-containing Schiff base formation: Synthesis, stimuli-sensitive degradation and release behaviors. Carbohydr. Polym. 2021, 265, 118085. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, J.; Liu, Y.; Kang, H.; Xie, M.; Wu, F.; Qiu, H. Copper sulfide-macroporous polyacrylamide hydrogel for solar steam generation. Chem. Eng. Sci. 2019, 207, 516–526. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 143, 235–242. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Vázquez, M.; Miguel, P.; Rodríguez, S.; Madeline, J.; Montero, V.; Álvarez, M. Calendula officinalis L. en el tratamiento tópico de la candidiasis vaginal recurrente. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2010, 9, 343–352. [Google Scholar]
- Zaki, A.A.; Qiu, L. Machaerinic acid 3-O-β-D-glucuronopyranoside from Calendula officinalis. Nat. Prod. Res. 2019, 34, 2938–2944. [Google Scholar] [CrossRef]
- Kozlowska, J.; Stachowiak, N.; Prus, W. Stability studies of collagen-based microspheres with Calendula officinalis flower extract. Polym. Degrad. Stab. 2019, 163, 214–219. [Google Scholar] [CrossRef]
- Marinescu, M.; Tecuceanu, V.; Bercu, V. Antioxidant capacity of some calendula extracts by epr spectroscopy. Rom. Rep. Phys. 2019, 706, 4–7. [Google Scholar]
- Baghizadeh, A.; Ranjbar, S.; Gupta, V.K.; Asif, M.; Pourseyedi, S.; Karimi, M.J.; Mohammadinejad, R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. [Google Scholar] [CrossRef]
- Mubashar Sabir, S.; Khan, M.F.; Rocha, J.B.T.; Boligon, A.A.; Athayde, M.L. Phenolic Profile, Antioxidant Activities and Genotoxic Evaluations of Calendula officinalis. J. Food Biochem. 2015, 39, 316–324. [Google Scholar] [CrossRef]
- Hernández-Rosas, N.A.; García-Zebadúa, J.C.; Hernández-Delgado, N.; Torres-Castillo, S.; Figueroa-Arredondo, P.; Mora-Escobedo, R. Perfil de polifenoles, capacidad antioxidante y efecto citotóxico in vitro en líneas celulares humanas de un extracto hidroalcohólico de pétalos de Calendula officinalis L. TIP Rev. Espec. Cienc. Químico-Biológicas 2018, 21, 54–64. [Google Scholar] [CrossRef]
- Okuma, C.H.; Andrade, T.A.M.; Caetano, G.F.; Finci, L.I.; Maciel, N.R.; Topan, J.F.; Cefali, L.C.; Polizello, A.C.M.; Carlo, T.; Rogerio, A.P.; et al. Development of lamellar gel phase emulsion containing marigold oil (Calendula officinalis) as a potential modern wound dressing. Eur. J. Pharm. Sci. 2015, 71, 62–72. [Google Scholar] [CrossRef]
- Pawłowicz, K.; Paczkowska-walendowska, M.; Osmałek, T.; Cielecka-piontek, J. Towards the Preparation of a Hydrogel from Lyophilisates of the Aloe arborescens Aqueous Extract. Pharmaceutics 2022, 14, 1489. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A novel high-strength poly(ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dhiman, A. Acacia gum polysaccharide based hydrogel wound dressings: Synthesis, characterization, drug delivery and biomedical properties. Carbohydr. Polym. 2017, 165, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Q.; Zhang, M.; Lv, X.; Li, Z.; Mohammadniaei, M.; Zhou, N.; Sun, Y. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr. Polym. 2021, 265, 118065. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, T.; Xing, C.; Chang, J.; Li, M. A blend hydrogel based on polyoxometalate for long-term and repeatedly localized antibacterial application study. Int. J. Pharm. 2020, 591, 119990. [Google Scholar] [CrossRef]
- Palem, R.R.; Madhusudana Rao, K.; Kang, T.J. Self-healable and dual-functional guar gum-grafted-polyacrylamidoglycolic acid-based hydrogels with nano-silver for wound dressings. Carbohydr. Polym. 2019, 223, 115074. [Google Scholar] [CrossRef]
- Afrin, S.; Haque, P.; Islam, S.; Hossain, S. Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug. Gels 2022, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, W.; Zhang, S.; Hu, X.; Sun, S.; Gao, H.; Kong, J. Poloxam Thermosensitive Hydrogels Loaded with hFGF2-Linked Camelina Lipid Droplets Accelerate Skin Regeneration in Deep Second-Degree Burns. Int. J. Mol. Sci. 2022, 23, 12716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, Y.; Xue, Y.; Zhu, Z.; Wu, Y.; Zeng, Q.; Wang, Y.; Shen, C.; Shen, Q.; Jiang, C.; et al. Log P Determines Licorice Flavonoids Release Behaviors and Classification from CARBOMER Cross-Linked Hydrogel. Pharmaceutics 2022, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Eakwaropas, P.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Nuntharatanapong, N. Formulation and Optimal Design of Dioscorea bulbifera and Honey-Loaded Gantrez®/Xyloglucan Hydrogel as Wound Healing Patches. Pharmaceutics 2022, 14, 1302. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Damdimopoulou, P.; Acharya, G.; Škalko-Basnet, N. Toxicity Assessment of Resveratrol Liposomes-in-Hydrogel Delivery System by EpiVaginalTM Tissue Model. Pharmaceutics 2022, 14, 1295. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA) Farmacopeia Brasileira; ANVISA: Brasília, Brazil, 2010; Volume 2, ISBN 9788588233416.
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, M.T.S.; Amaral, R.H.; Rogero, S.O.; Ditchfield, C.; Tadini, C.C. Propriedades Mecânicas Da Blenda De Poli (Vinilpirrolidona)/Carboximetil Cellulose (Pvp/Cmc). Micro 2007, 9, 1–8. [Google Scholar]
- Campese, G.M.; Tambourgi, E.B.; Guilherme, M.R.; De Moura, M.R.; Muniz, E.C.; Youssef, E.Y. Resistência mecânica de hidrogéis termo-sensíveis constituídos de alginato-Ca2+/PNIPAAm, tipo semi-IPN. Quim. Nova 2007, 30, 1649–1652. [Google Scholar] [CrossRef] [Green Version]
- Boztas, N. Effects of Midazolam, Propofol and Thiopental on Gastric Ulcer in Rats Midazolam. Haydarpasa Numune Train. Res. Hosp. Med. J. 2019, 61, 24–30. [Google Scholar] [CrossRef]
- Tsutakawa, S.; Kobayashi, D.; Kusama, M.; Moriya, T.; Nakahata, N. Nicotine enhances skin necrosis and expression of inflammatory mediators in a rat pressure ulcer model. Br. J. Dermatol. 2009, 161, 1020–1027. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Aziz, S.J.; Zeman-Pocrnich, C.E. Tissue Processing; Elsevier: New York, NY, USA, 2022; Volume 2422, ISBN 9780702068645. [Google Scholar]
- Wan, X.; Liu, S.; Xin, X.; Li, P.; Dou, J.; Han, X.; Kang, I.K.; Yuan, J.; Chi, B.; Shen, J. S-nitrosated keratin composite mats with NO release capacity for wound healing. Chem. Eng. J. 2020, 400, 125964. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. Int. J. Mol. Sci. 2023, 24, 3806. https://doi.org/10.3390/ijms24043806
Ferreira LMdMC, Bandeira EdS, Gomes MF, Lynch DG, Bastos GNT, Silva-Júnior JOC, Ribeiro-Costa RM. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. International Journal of Molecular Sciences. 2023; 24(4):3806. https://doi.org/10.3390/ijms24043806
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Elanne de Sousa Bandeira, Maurício Ferreira Gomes, Desireé Gyles Lynch, Gilmara Nazareth Tavares Bastos, José Otávio Carréra Silva-Júnior, and Roseane Maria Ribeiro-Costa. 2023. "Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test" International Journal of Molecular Sciences 24, no. 4: 3806. https://doi.org/10.3390/ijms24043806




