FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction
Abstract
:1. Introduction
2. Results
2.1. AE Inhibited Liver Injury in Mice with MI
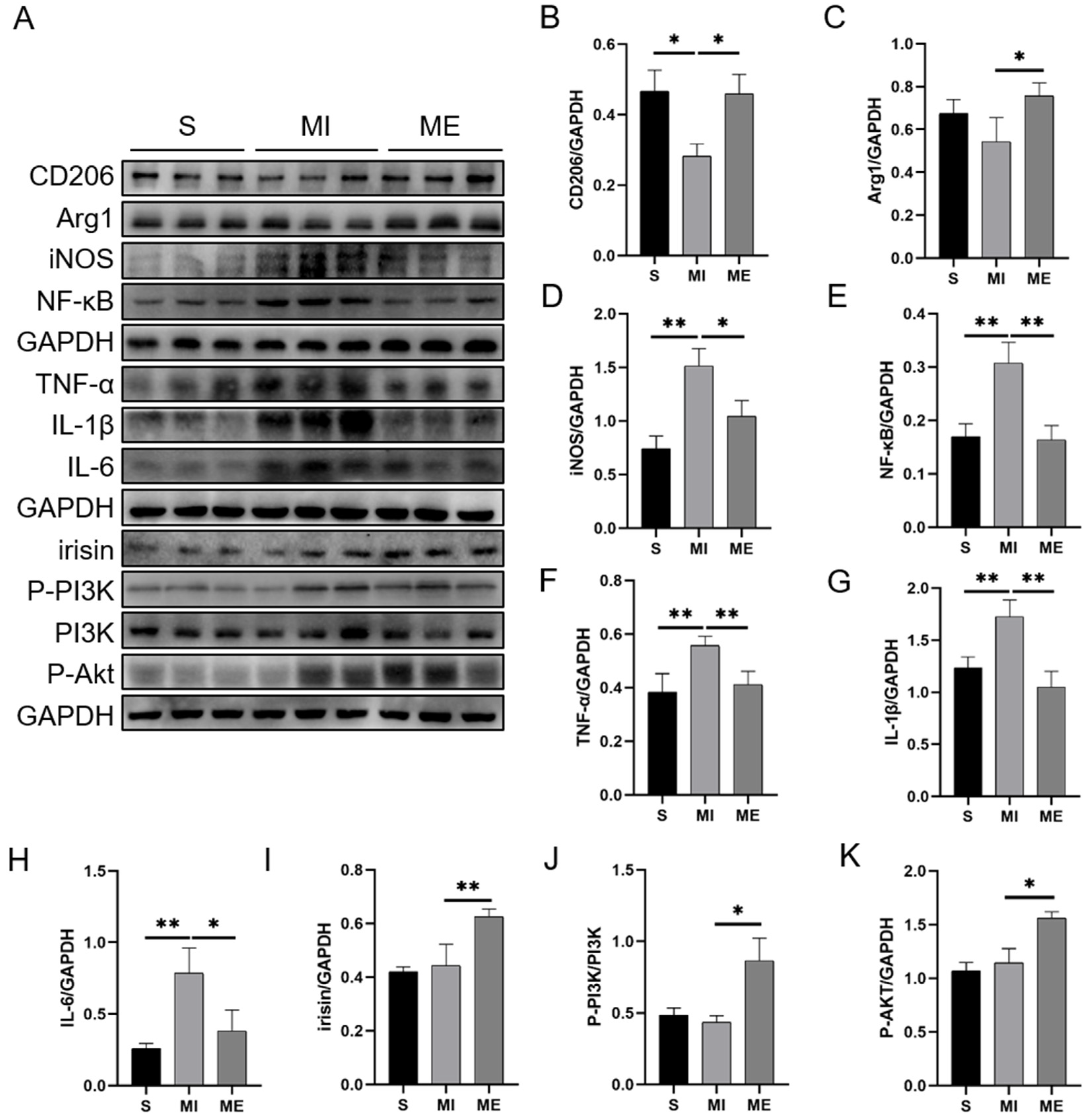
2.2. AE Regulated Macrophage Polarization, Suppressed Inflammatory Response and Activated Irisin/PI3K/Akt Signaling Pathway in the Liver of MI Mice
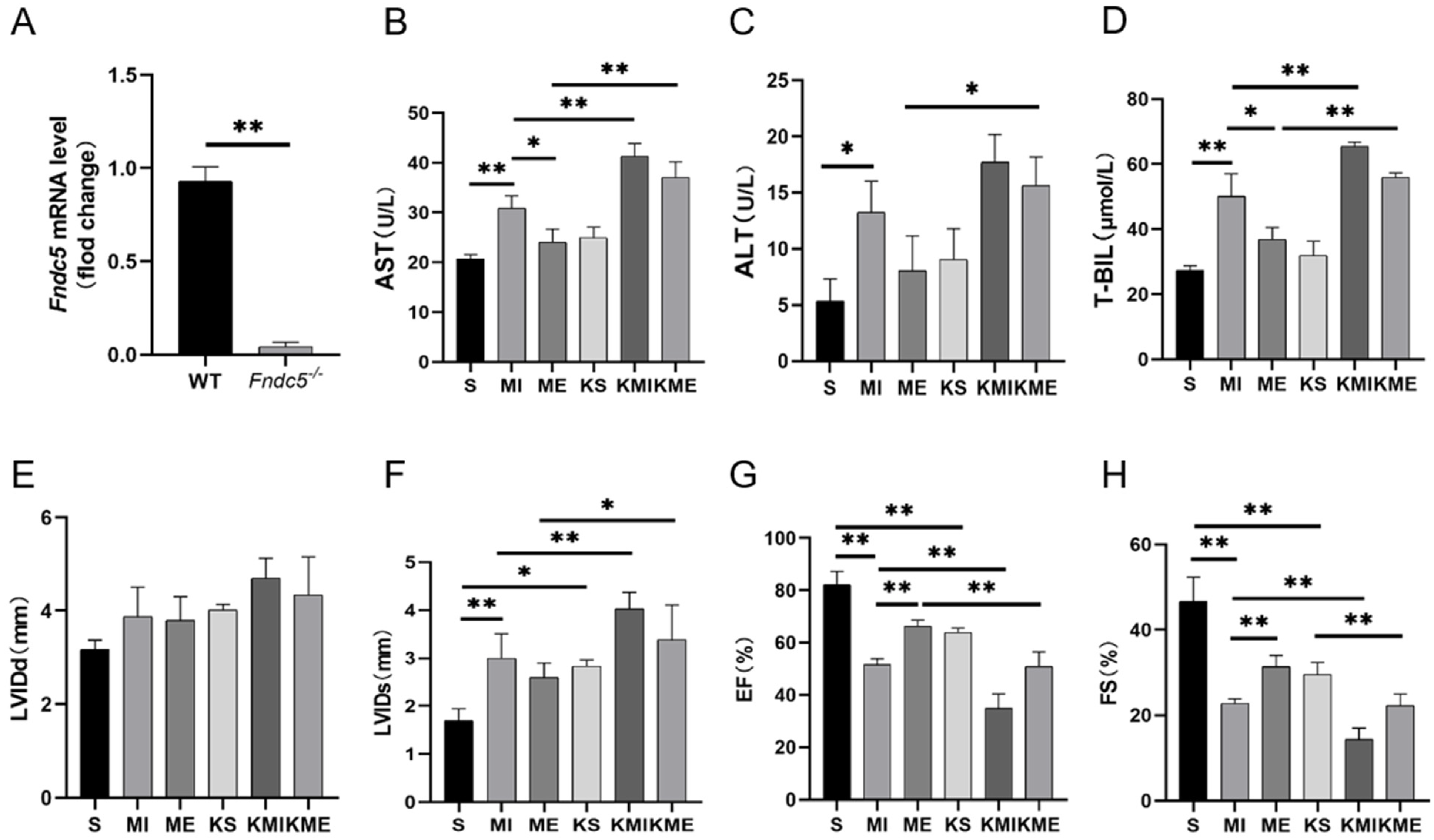
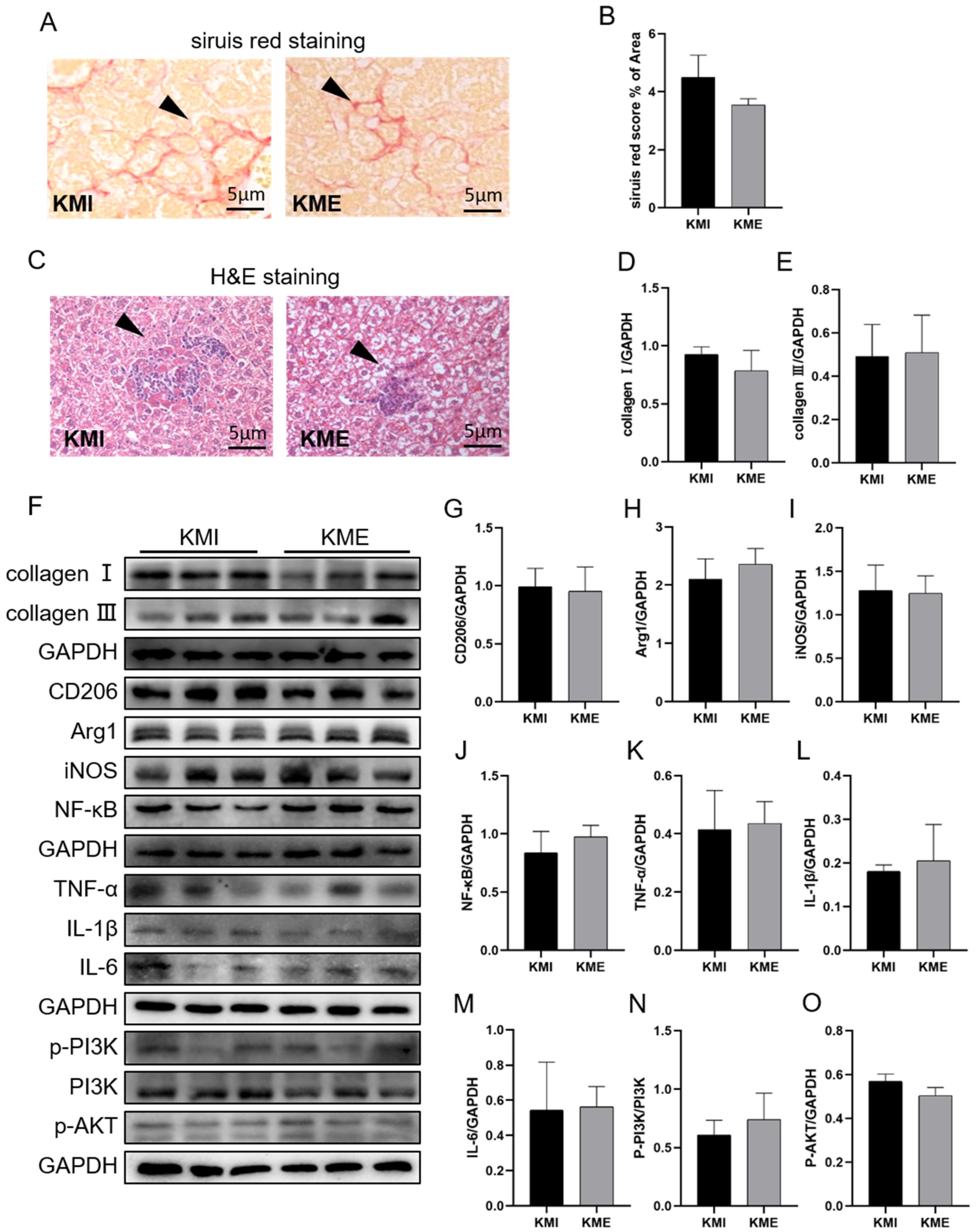
2.3. Knockout of Fndc5 Impaired the Protective Effect of AE on MI-Induced Liver Injury
2.4. Irisin Activated the PI3K/Akt Signaling Pathway and Inhibited Inflammation In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals and Exercise Protocol
4.2. Echocardiographic Measurements
4.3. Histological Staining and Analysis
4.4. Primary Mouse Hepatocyte Isolation and Cell Culture
4.5. Measurement of Liver Function
4.6. RT-qPCR
4.7. Western Blotting
4.8. Statistical Analysis
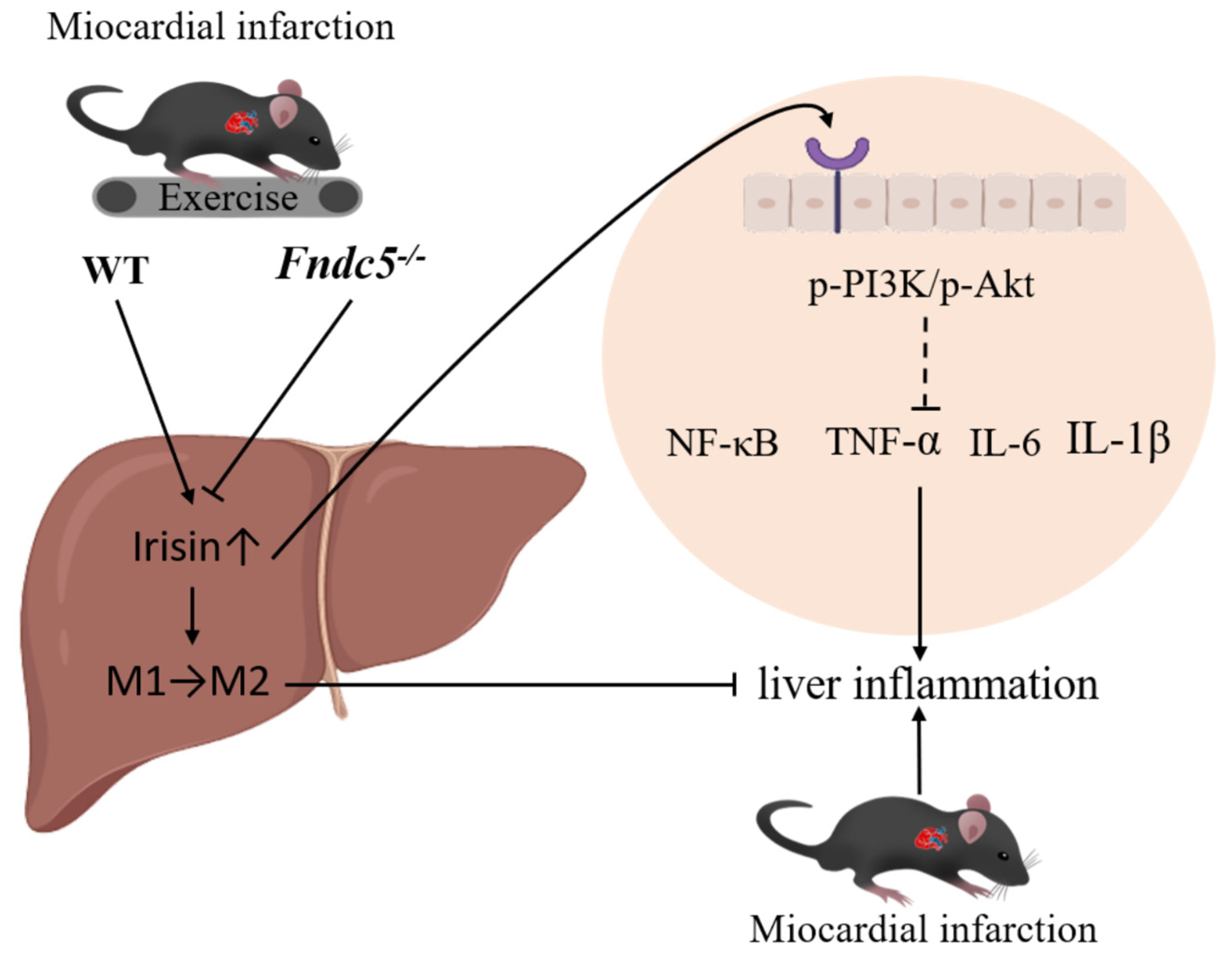
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Reindl, M.; Feistritzer, H.J.; Klug, G.; Mayr, A.; Kofler, M.; Tu, A.M.; Huybrechts, L.; Mair, J.; Franz, W.M.; et al. Prognostic significance of transaminases after acute ST-elevation myocardial infarction: Insights from a cardiac magnetic resonance study. Wien Klin. Wochenschr. 2015, 127, 843–850. [Google Scholar] [CrossRef]
- Lofthus, D.M.; Stevens, S.R.; Armstrong, P.W.; Granger, C.B.; Mahaffey, K.W. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis. 2012, 23, 22–30. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Guzeeva, V.A. Liver pathology in myocardial infarct. Kardiologiia 1977, 17, 119–123. (In Russian) [Google Scholar]
- Ciobanu, A.O.; Gherasim, L. Ischemic Hepatitis—Intercorrelated Pathology. Maedica 2018, 13, 5–11. [Google Scholar] [CrossRef]
- Fortea, J.I.; Puente, Á.; Cuadrado, A.; Huelin, P.; Pellón, R.; González Sánchez, F.J.; Mayorga, M.; Cagigal, M.L.; García Carrera, I.; Cobreros, M.; et al. Congestive Hepatopathy. Int. J. Mol. Sci. 2020, 21, 9420. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, M.; Wang, L.; Chen, W. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Hoyer, F.F.; Naxerova, K.; Schloss, M.J.; Hulsmans, M. Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity 2019, 51, 899–914. [Google Scholar] [CrossRef]
- Jin, G.; Liu, H.P.; Huang, Y.X.; Zeng, Q.Q.; Chen, J.X.; Lan, X.B.; Xin, Z.M.; Xiong, B.J.; Yue, R.C.; Yu, C.X. Koumine regulates macrophage M1/M2 polarization via TSPO, alleviating sepsis-associated liver injury in mice. Phytomedicine 2022, 107, 154484. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Zhao, J.; Xia, L.; Li, J.; Xu, M.; Wang, B.; Guo, H.; Yu, C.; Gao, Y.; et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol. Immunol. 2020, 17, 753–764. [Google Scholar] [CrossRef]
- Xu, F.; Guo, M.; Huang, W.; Feng, L.; Zhu, J.; Luo, K.; Gao, J.; Zheng, B.; Kong, L.D.; Pang, T.; et al. Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 2020, 36, 101634. [Google Scholar] [CrossRef]
- Shang, L.; Ren, H.; Wang, S.; Liu, H.; Hu, A.; Gou, P.; Lin, Y.; Zhou, J.; Zhu, W.; Shi, X. SS-31 Protects Liver from Ischemia-Reperfusion Injury via Modulating Macrophage Polarization. Oxid. Med. Cell. Longev. 2021, 2021, 6662156. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.G.; Wang, F.; Jin, K.; Shen, H.; Zhang, L.; Yuan, X.; Wang, J.; Zhang, H.; Yu, W.; et al. The zinc finger protein Miz1 suppresses liver tumorigenesis by restricting hepatocyte-driven macrophage activation and inflammation. Immunity 2021, 54, 1168–1185. [Google Scholar] [CrossRef]
- Liang, Q.; Cai, M.; Zhang, J.; Song, W.; Zhu, W.; Xi, L.; Tian, Z. Role of Muscle-Specific Histone Methyltransferase (Smyd1) in Exercise-Induced Cardioprotection against Pathological Remodeling after Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 7010. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Kaltenmeier, C.; Morder, K.; Moon, J.; Traczek, M.; Loughran, P.; Zamora, R.; Vodovotz, Y.; Li, F.; Wang, J.; et al. Exercise Training Decreases Hepatic Injury and Metastases through Changes in Immune Response to Liver Ischemia/Reperfusionin Mice. Hepatology 2021, 73, 2494–2509. [Google Scholar] [CrossRef]
- Nikbin, S.; Tajik, A.; Allahyari, P.; Matin, G.; Hoseini Roote, S.S.; Barati, E.; Ayazi, M.; Karimi, L.; Dayani Yazdi, F.; Javadinejad, N.; et al. Aerobic exercise and eugenol supplementation ameliorated liver injury induced by chlorpyrifos via modulation acetylcholinesterase activation and antioxidant defense. Environ. Toxicol. 2020, 35, 783–793. [Google Scholar] [CrossRef]
- Asada, F.; Nomura, T.; Hosui, A.; Kubota, M. Influence of increased physical activity without body weight loss on hepatic inflammation in patients with nonalcoholic fatty liver disease. Environ. Health Prev. Med. 2020, 25, 18. [Google Scholar] [CrossRef]
- Linden, M.A.; Fletcher, J.A.; Morris, E.M.; Meers, G.M.; Laughlin, M.H.; Booth, F.W.; Sowers, J.R.; Ibdah, J.A.; Thyfault, J.P.; Rector, R.S. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med. Sci. Sports Exerc. 2015, 47, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Ai, L.; Luo, W.; Yuan, P.; Liu, L.; Zhou, Y. Liver macrophages mediate effects of downhill running and caloric restriction on nonalcoholic fatty liver disease of high fat diet-fed mice. Life Sci. 2020, 256, 117978. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Sun, S.; Ma, S.; Cai, Y.; Wang, S.; Ren, J.; Yang, Y.; Ping, J.; Wang, X.; Zhang, Y.; Yan, H.; et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation 2023, 4, 100380. [Google Scholar] [CrossRef]
- Ranjbar, K.; Nazem, F.; Sabrinezhad, R.; Nazari, A. Aerobic training and L-arginine supplement attenuates myocardial infarction-induced kidney and liver injury in rats via reduced oxidative stress. Indian Heart J. 2018, 70, 538–543. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Chen, R.R.; Fan, X.H.; Chen, G.; Zeng, G.W.; Xue, Y.G.; Liu, X.T.; Wang, C.Y. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFβ1/Smad2/3 signaling axis. Chem. Biol. Interact. 2019, 302, 11–21. [Google Scholar] [CrossRef]
- Wang, X.; Mao, L.; Li, C.; Hui, Y.; Yu, Z.; Sun, M.; Li, Y.; Guo, G.; Yang, W.; Cui, B.; et al. The potential role of FNDC5/irisin in various liver diseases: Awakening the sleeping beauties. Expert Rev. Mol. Med. 2022, 24, e23. [Google Scholar] [CrossRef]
- Li, D.J.; Sun, S.J.; Fu, J.T.; Ouyang, S.X.; Zhao, Q.J.; Su, L.; Ji, Q.X.; Sun, D.Y.; Zhu, J.H.; Zhang, G.Y.; et al. NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics 2021, 11, 4381–4402. [Google Scholar] [CrossRef]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Ren, W.; Xu, Z.; Pan, S.; Ma, Y.; Li, H.; Wu, F.; Bo, W.; Cai, M.; Tian, Z. Irisin and ALCAT1 mediated aerobic exercise-alleviated oxidative stress and apoptosis in skeletal muscle of mice with myocardial infarction. Free Radic. Biol. Med. 2022, 193 Pt 2, 526–537. [Google Scholar] [CrossRef]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef]
- Baĭdiuk, E.V.; Korshak, O.V.; Karpov, A.A.; Kudriavtsev, B.N.; Sakuta, G.A. Cellular mechanisms of regeneration of rats’ liver after experimental myocardial infarction. Tsitologiia 2012, 54, 873–882. (In Russian) [Google Scholar]
- Naschitz, J.E.; Slobodin, G.; Lewis, R.J.; Zuckerman, E.; Yeshurun, D. Heart diseases affecting the liver and liver diseases affecting the heart. Am. Heart J. 2000, 140, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Correale, M.; Tarantino, N.; Petrucci, R.; Tricarico, L.; Laonigro, I.; Di Biase, M.; Brunetti, N.D. Liver disease and heart failure: Back and forth. Eur. J. Intern. Med. 2018, 48, 25–34. [Google Scholar] [CrossRef]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Tang, T.; Li, Y.; Li, J.; Wang, K. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction. Theranostics 2018, 8, 4552–4562. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhou, Z.; Pan, C.; Jiang, L.; Zhou, Z.; Meng, Y.; Charugundla, S.; Li, T.; Allayee, H.; et al. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science 2022, 377, 1399–1406. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.S.M.H.; Baniyas, M.M.Y.H.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Zhao, J.; Qiao, L.; Dong, J.; Wu, R. Antioxidant Effects of Irisin in Liver Diseases: Mechanistic Insights. Oxid. Med. Cell. Longev. 2022, 2022, 3563518. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 2020, 10, e173. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef]
- Jiang, Q.; Pan, D.; Yang, Y.; Hu, Y.; Fang, L.; Shang, P.; Xia, Y.; Li, D. Luteolin Regulates Macrophage Polarization via the PI3K/Akt Pathway to Inhibit the Apoptosis Stimulated by Angiotensin II. Curr. Pharm. Biotechnol. 2018, 19, 428–437. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xie, L.; Liu, C.; Zhang, Q.; Sun, S. PTEN/PI3k/AKT Regulates Macrophage Polarization in Emphysematous mice. Scand. J. Immunol. 2017, 85, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Zhao, Q.; Qin, Y.; Tong, H.; Wang, Q.; Yu, M.; Mao, C.; Lu, T.; Qiu, J.; Jiang, C. Germacrone improves liver fibrosis by regulating the PI3K/AKT/mTOR signalling pathway. Cell Biol. Int. 2021, 45, 1866–1875. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, J.; Sun, K.; Li, Q.; Kuang, B.; Wang, M.M.Z.; Hou, S.; Gong, N. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int. Immunopharmacol. 2021, 99, 108023. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Jung, K.; Kim, M.; So, J.; Lee, S.H.; Ko, S.; Shin, D. Farnesoid X Receptor Activation Impairs Liver Progenitor Cell-Mediated Liver Regeneration via the PTEN-PI3K-AKT-mTOR Axis in Zebrafish. Hepatology 2021, 74, 397–410. [Google Scholar] [CrossRef]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, X.; Cheng, Y.; Liu, Y. Myricitrin pretreatment ameliorates mouse liver ischemia reperfusion injury. Int. Immunopharmacol. 2020, 89 Pt A, 107005. [Google Scholar] [CrossRef]
- Xiao, Q.; Ye, Q.; Wang, W.; Xiao, J.; Fu, B.; Xia, Z.; Zhang, X.; Liu, Z.; Zeng, X. Mild hypothermia pretreatment protects against liver ischemia reperfusion injury via the PI3K/AKT/FOXO3a pathway. Mol. Med. Rep. 2017, 16, 7520–7526. [Google Scholar] [CrossRef] [Green Version]
- Guha, M.; Mackman, N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002, 277, 32124–32132. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Guerra, M.J.; Castrillo, A.; Martín-Sanz, P.; Boscá, L. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J. Immunol. 1999, 162, 6184–6190. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, W.; Gong, J.; Cheng, Y. Pre-conditioning with tanshinone IIA attenuates the ischemia/reperfusion injury caused by liver grafts via regulation of HMGB1 in rat Kupffer cells. Biomed. Pharmacother. 2017, 89, 1392–1400. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Yang, Y.; Bai, Y.; Wei, Y.; Zhang, H.; Tian, Y.; Liu, J.; Bai, L. Mechanical Stimulation Protects Against Chondrocyte Pyroptosis through Irisin-Induced Suppression of PI3K/Akt/NF-κB Signal Pathway in Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 797855. [Google Scholar] [CrossRef]
- Jiang, H.; Jia, D.; Zhang, B.; Yang, W.; Dong, Z.; Sun, X.; Cui, X.; Ma, L.; Wu, J.; Hu, K.; et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res. Cardiol. 2020, 115, 28. [Google Scholar] [CrossRef]
- Høydal, M.A.; Wisløff, U.; Kemi, O.J.; Ellingsen, O. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 753–760. [Google Scholar] [CrossRef]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]
- Charni-Natan, M.; Goldstein, I. Protocol for Primary Mouse Hepatocyte Isolation. STAR Protoc. 2020, 1, 100086. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yu, M.; Li, H.; Qin, S.; Ren, W.; Ma, Y.; Bo, W.; Xi, Y.; Cai, M.; Tian, Z. FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4159. https://doi.org/10.3390/ijms24044159
Wang T, Yu M, Li H, Qin S, Ren W, Ma Y, Bo W, Xi Y, Cai M, Tian Z. FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(4):4159. https://doi.org/10.3390/ijms24044159
Chicago/Turabian StyleWang, Tao, Mengyuan Yu, Hangzhuo Li, Shuguang Qin, Wujing Ren, Yixuan Ma, Wenyan Bo, Yue Xi, Mengxin Cai, and Zhenjun Tian. 2023. "FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction" International Journal of Molecular Sciences 24, no. 4: 4159. https://doi.org/10.3390/ijms24044159
APA StyleWang, T., Yu, M., Li, H., Qin, S., Ren, W., Ma, Y., Bo, W., Xi, Y., Cai, M., & Tian, Z. (2023). FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction. International Journal of Molecular Sciences, 24(4), 4159. https://doi.org/10.3390/ijms24044159






