Small Cellular Particles from European Spruce Needle Homogenate
Abstract
:1. Introduction
2. Results
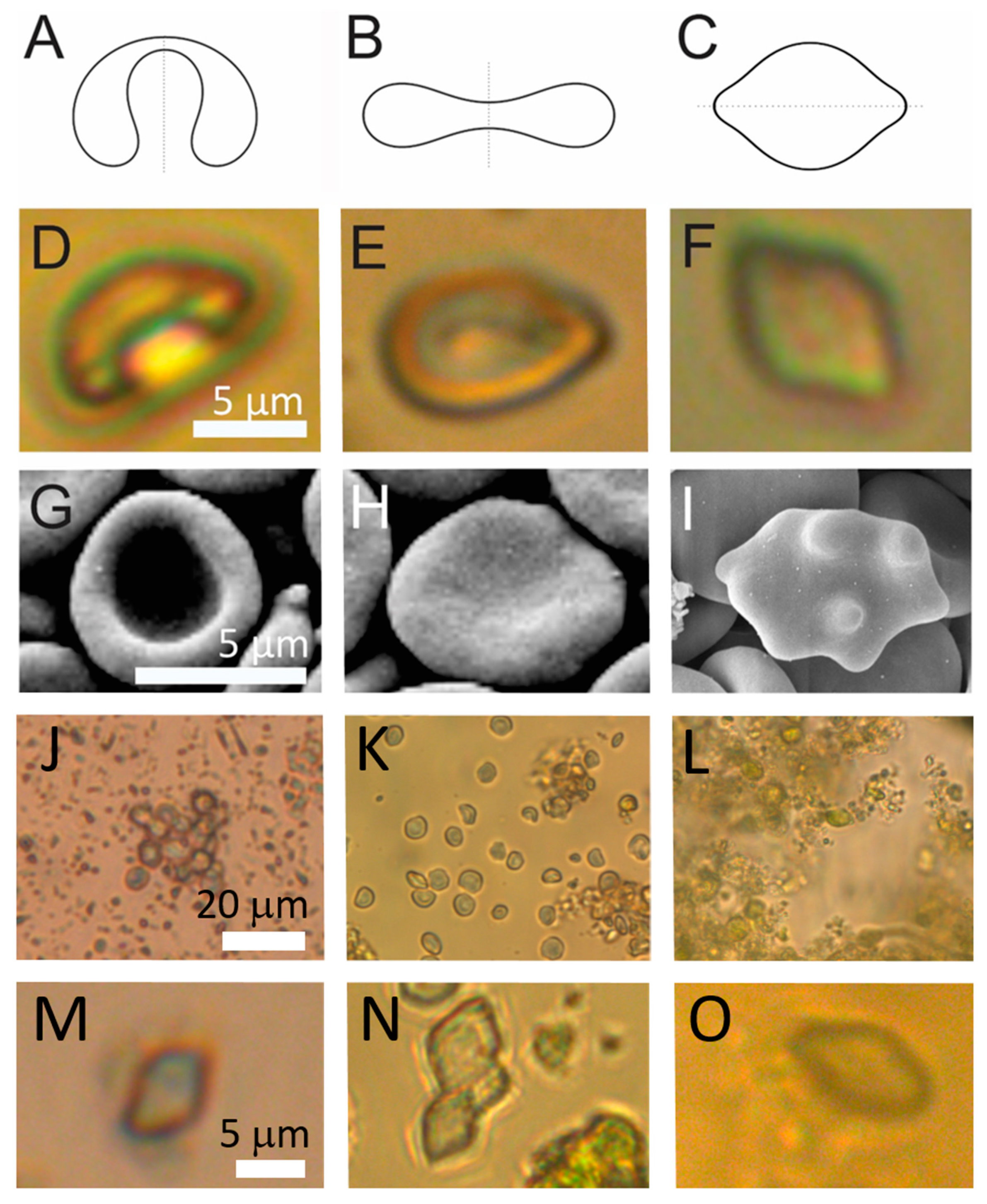
2.1. Visualization of the Samples
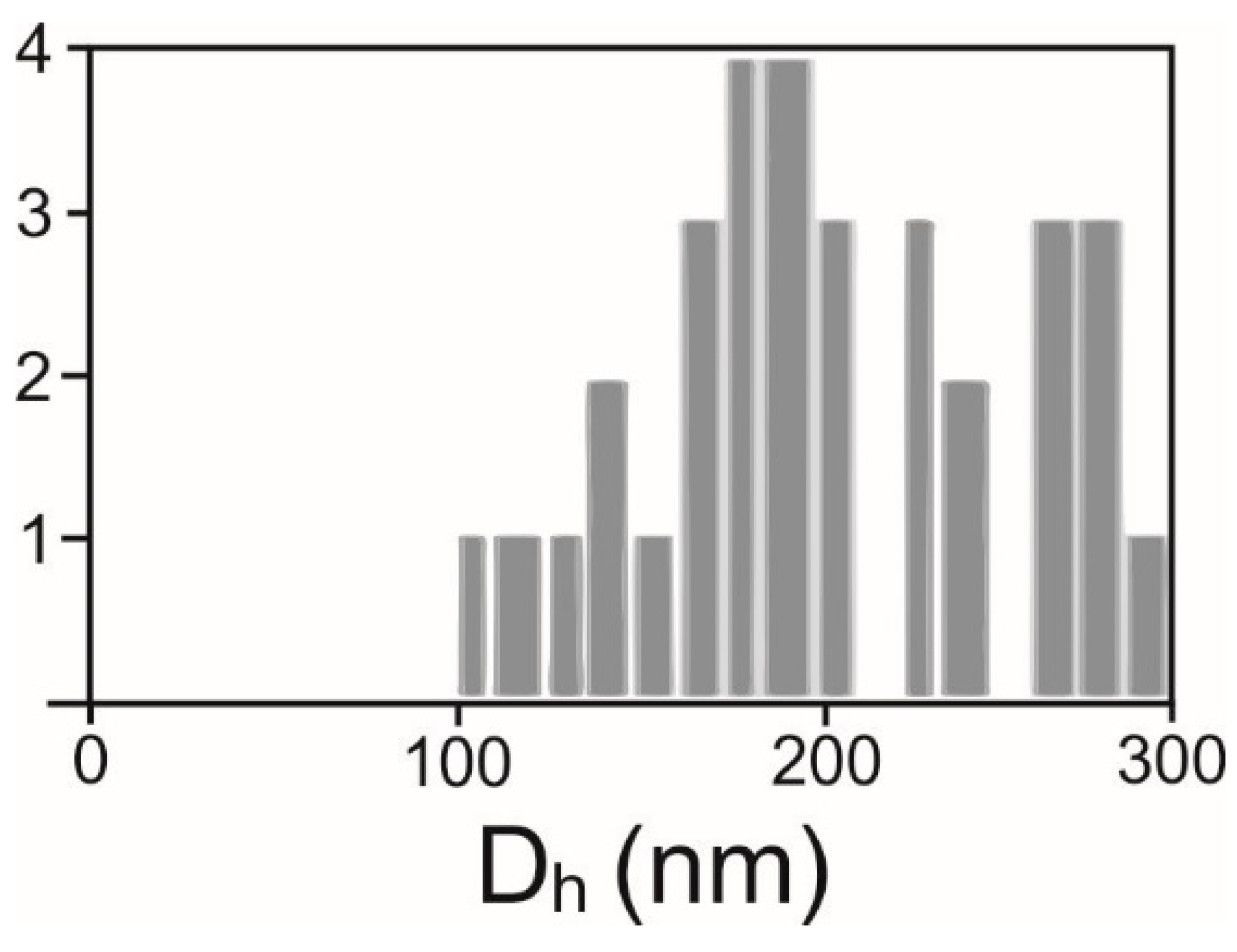
2.2. Determination of Number Density and Size of Particles
2.3. Analysis of the Content of the Samples
2.3.1. Determination of the Ultraviolet-Visual Absorption
2.3.2. Determination of Volatile Terpenoid Content by Gas Chromatography-Mass Spectrometry Analysis
3. Discussion
4. Materials and Methods
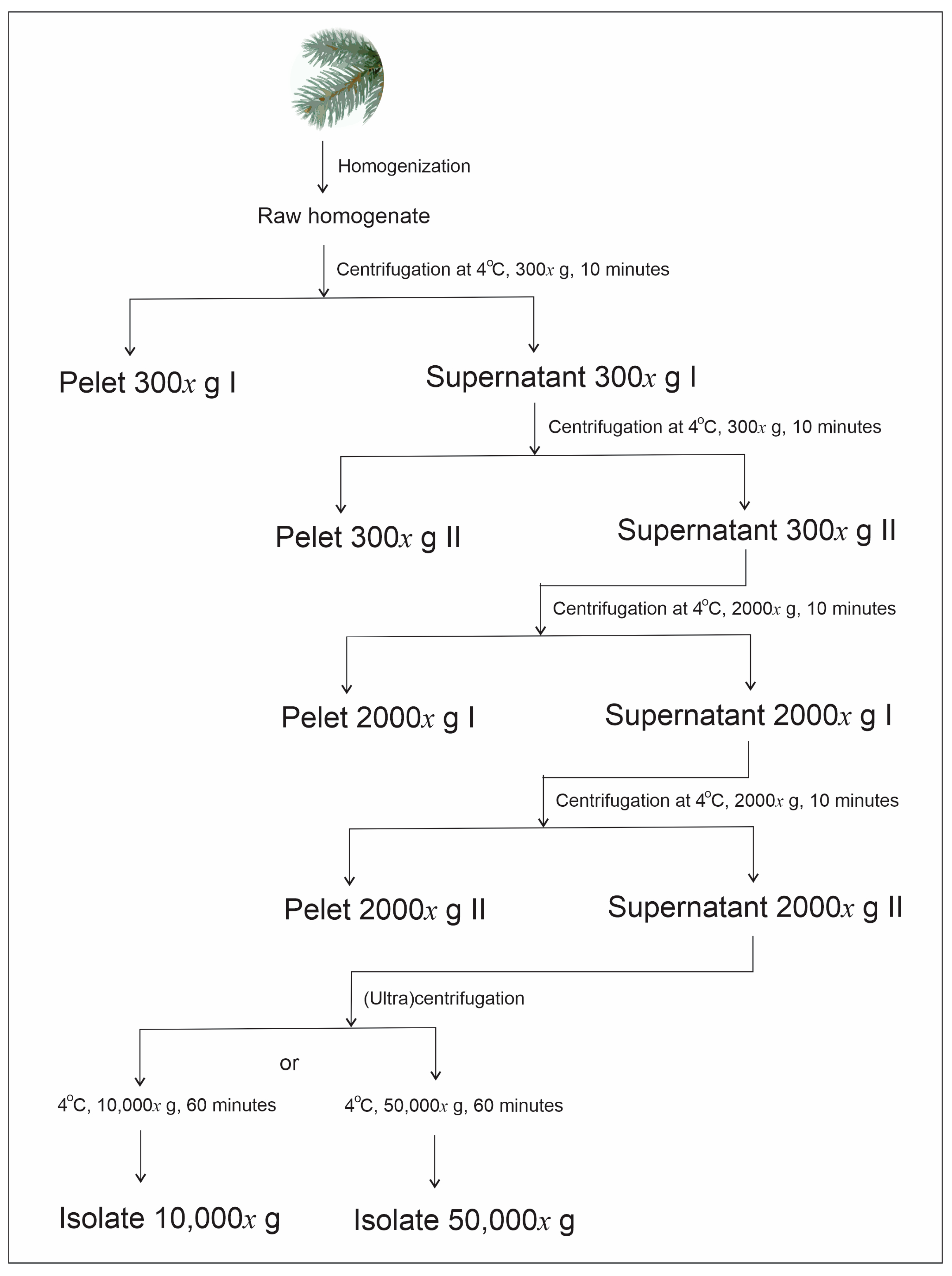
4.1. Preparation of Homogenates
Preparation of Homogenate from Spruce Needles for Isolation of Small Cellular Particles
4.2. Isolation of Small Cellular Particles
4.3. Visualization of Samples
4.3.1. Light Microscopy
4.3.2. Scanning Electron Microscopy
4.3.3. Cryogenic Transmission Electron Microscopy
4.4. Determination of Amount and Size of Particles
4.4.1. Flow Cytometry
4.4.2. Interference Light Microscopy
4.5. Analysis of the Total Phenolic Content of the Samples
4.5.1. Determination of Absorbance of Light at 760 nm
4.5.2. Determination of Volatile Terpenoid Content by Gas Chromatography-Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stotz, H.; Brotherton, D.; Inal, J. Communication Is Key: Extracellular Vesicles as Mediators of Infection and Defence during Host-Microbe Interactions in Animals and Plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [Green Version]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Biller, S.J.; McDaniel, L.D.; Breitbart, M.; Rogers, E.; Paul, J.H.; Chisholm, S.W. Membrane Vesicles in Sea Water: Heterogeneous DNA Content and Implications for Viral Abundance Estimates. ISME J. 2017, 11, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Combarnous, Y.; Nguyen, T.M.D. Cell Communications among Microorganisms, Plants, and Animals: Origin, Evolution, and Interplays. Int. J. Mol. Sci. 2020, 21, 8052. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Duan, P.; Tan, J.; Miao, Y.; Zhang, Q. Potential Role of Exosomes in the Pathophysiology, Diagnosis, and Treatment of Hypoxic Diseases. Am. J. Transl. Res. 2019, 11, 1184–1201. [Google Scholar]
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Pant, S.; Hilton, H.; Burczynski, M.E. The Multifaceted Exosome: Biogenesis, Role in Normal and Aberrant Cellular Function, and Frontiers for Pharmacological and Biomarker Opportunities. Biochem. Pharmacol. 2012, 83, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal. CCS 2022, 20, 69. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral Administration of Ginger-Derived Nanolipids Loaded with SiRNA as a Novel Approach for Efficient SiRNA Drug Delivery to Treat Ulcerative Colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Pérez-Jiménez, M.I.; Martínez-Esparza, M.; Teruel, J.A.; Ruiz-Alcaraz, A.J.; García-Peñarrubia, P.; Carvajal, M. Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model. Int. J. Mol. Sci. 2022, 23, 1940. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.-H.; Jang, H.; Park, S.-Y.; Seol, J.-W. Naringenin Exerts Anticancer Effects by Inducing Tumor Cell Death and Inhibiting Angiogenesis in Malignant Melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant Extracellular Vesicles and Nanovesicles: Focus on Secondary Metabolites, Proteins and Lipids with Perspectives on Their Potential and Sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Oberkofler, L.; Robatzek, S.; Weiberg, A. Spotlight on Plant RNA-Containing Extracellular Vesicles. Curr. Opin. Plant Biol. 2022, 69, 102272. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Mofikoya, O.O.; Mäkinen, M.; Jänis, J. Compositional Analysis of Essential Oil and Solvent Extracts of Norway Spruce Sprouts by Ultrahigh-Resolution Mass Spectrometry. Phytochem. Anal. 2022, 33, 392–401. [Google Scholar] [CrossRef]
- Rautio, M.; Sipponen, A.; Peltola, R.; Lohi, J.; Jokinen, J.J.; Papp, A.; Carlson, P.; Sipponen, P. Antibacterial Effects of Home-Made Resin Salve from Norway Spruce (Picea abies). APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 335–340. [Google Scholar] [CrossRef]
- Kamaitytė-Bukelskienė, L.; Ložienė, K.; Labokas, J. Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season. Molecules 2021, 26, 2138. [Google Scholar] [CrossRef]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic Resistance Modulation and Modes of Action of (-)-α-Pinene in Campylobacter Jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [Green Version]
- Aydin, E.; Türkez, H.; Geyikoğlu, F. Antioxidative, Anticancer and Genotoxic Properties of α-Pinene on N2a Neuroblastoma Cells. Biologia 2013, 68, 1004–1009. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic Antitumor Effect of α-Pinene and β-Pinene with Paclitaxel against Non-Small-Cell Lung Carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Canham, P.B. The Minimum Energy of Bending as a Possible Explanation of the Biconcave Shape of the Human Red Blood Cell. J. Theor. Biol. 1970, 26, 61–81. [Google Scholar] [CrossRef]
- Lipowsky, R. The Conformation of Membranes. Nature 1991, 349, 475–481. [Google Scholar] [CrossRef]
- Seifert, U. Configurations of Fluid Membranes and Vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Kralj-Iglič, V.; Pocsfalvi, G.; Mesarec, L.; Šuštar, V.; Hägerstrand, H.; Iglič, A. Minimizing Isotropic and Deviatoric Membrane Energy—An Unifying Formation Mechanism of Different Cellular Membrane Nanovesicle Types. PLoS ONE 2020, 15, e0244796. [Google Scholar] [CrossRef]
- Kralj-Iglič, V.; Pocsfalvi, G.; Iglič, A. Morphology and Formation Mechanisms of Cellular Vesicles Harvested from Blood. Physiology 2022. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Mantile, F.; Kisovec, M.; Adamo, G.; Romancino, D.P.; Hočevar, M.; Božič, D.; Bedina Zavec, A.; Podobnik, M.; Stoppelli, M.P.; Kisslinger, A.; et al. A Novel Localization in Human Large Extracellular Vesicles for the EGF-CFC Founder Member CRIPTO and Its Biological and Therapeutic Implications. Cancers 2022, 14, 3700. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sharma, N.K.; Kaur, N.; Chunduri, V.; Chawla, M.; Sharma, S.; Singh, K.; Garg, M. Soft and Hard Textured Wheat Differ in Starch Properties as Indicated by Trimodal Distribution, Morphology, Thermal and Crystalline Properties. PLoS ONE 2016, 11, e0147622. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Gerald Lim, H.W.; Wortis, M. Echinocyte Shapes: Bending, Stretching, and Shear Determine Spicule Shape and Spacing. Biophys. J. 2002, 82, 1756–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular Microvesicles/Exosomes: Discovery, Disbelief, Acceptance, and the Future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Božič, D.; Hočevar, M.; Kononenko, V.; Jeran, M.; Štibler, U.; Fiume, I.; Pajnič, M.; Pađjen, L.; Kogej, K.; Drobne, D. Pursuing Mechanisms of Extracellular Vesicle Formation. Effects of Sample Processing. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2020; Volume 32, pp. 113–155. [Google Scholar] [CrossRef]
- Šuštar, V.; Bedina-Zavec, A.; Štukelj, R.; Frank, M.; Bobojević, G.; Janša, R.; Ogorevc, E.; Kruljc, P.; Mam, K.; Šimunič, B. Nanoparticles Isolated from Blood: A Reflection of Vesiculability of Blood Cells during the Isolation Process. Int. J. Nanomed. 2011, 6, 2737–2748. [Google Scholar] [CrossRef] [Green Version]
- Škufca, D.; Božič, D.; Hočevar, M.; Jeran, M.; Bedina Zavec, A.; Kisovec, M.; Podobnik, M.; Matos, T.; Tomazin, R.; Iglič, A.; et al. Interaction between Microalgae P. Tricornutum and Bacteria Thalassospira Sp. for Removal of Bisphenols from Conditioned Media. Int. J. Mol. Sci. 2022, 23, 8447. [Google Scholar] [CrossRef]
- Romolo, A.; Jan, Z.; Bedina Zavec, A.; Kisovec, M.; Arrigler, V.; Spasovski, V.; Podobnik, M.; Iglič, A.; Pocsfalvi, G.; Kogej, K.; et al. Assessment of Small Cellular Particles from Four Different Natural Sources and Liposomes by Interferometric Light Microscopy. Int. J. Mol. Sci. 2022, 23, 15801. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Wang, Y.; Wu, D.; Xu, M. Phenolic Extracts from Acacia Mangium Bark and Their Antioxidant Activities. Molecules 2010, 15, 3567–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blainski, A.; Lopes, G.; de Mello, J. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium rrasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Publishing Corporation: Carol Steam, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]


| SCPs | MSPs | CSPs | |||
|---|---|---|---|---|---|
| Sample | Number Density (/mL) | Dh (nm) | Number of Particles Tracked | Number Density (/mL) | Number Density (/mL) |
| Supernatant 300× g I | (7.82 ± 1.85) × 1010 | 162 ± 114 | 2892 | (11.0 ± 2.74) × 106 | (3.20 ± 0.45) × 106 |
| Supernatant 2000× g I | (6.86 ± 1.42) × 1010 | 159 ± 134 | 2413 | (10.1 ± 5.28) × 106 | (0.77 ± 0.33) × 106 |
| Isolate 10,000× g | (11.9 ± 2.03) × 1010 | 172 ± 101 | 2913 | (10.7 ± 5.39) × 106 | (2.12 ± 0.75) × 106 |
| Isolate 50,000× g | (3.64 ± 0.61) × 1010 | 152 ± 107 | 1811 | (11.4 ± 7.45) × 106 | (1.68 ± 0.70) × 106 |
| Sample | UV-Vis Light Absorbance (mg Gallic Acid/ g Spruce Needles) of Fresh Samples | UV-Vis Light Absorbance (mg Gallic Acid/ g Spruce Needles) of Samples Aged 5 Days |
|---|---|---|
| Supernatant 300× g I | 38.47 ± 1.73 | 23.50 ± 3.22 |
| Supernatant 2000× g I | 41.78 ± 3.42 | 19.55 ± 6.25 |
| Isolate 10,000× g | 45.50 ± 1.36 | 20.93 ± 5.21 |
| Isolate 50,000× g | 42.60 ± 5.22 | 20.38 ± 1.68 |
| Pellet 300× g I 1 | Pellet 300× g I 2 | Pellet 300× g I 3 | Average ± SD | ||
|---|---|---|---|---|---|
| Compound | tR (minutes) | Peak area (mV/s) | |||
| α-Pinene | 2.30 | 3,553,050 | 4,463,328 | 3,964,389 | 3,993,589 ± 455,841 |
| Camphene | 3.46 | ||||
| (-)-β-Pinene | 4.07 | 975,290 | 2,401,657 | 1,911,749 | 1,762,899 ± 724,740 |
| β-Myrcene | 4.29 | ||||
| 3-Carene | 4.56 | ||||
| α-Terpinene | 4.89 | ||||
| D-Limonene | 5.00 | 3,759,264 | 528,512 | 4,610,539 | 2,966,105 ± 2,153,500 |
| Eucalyptol | 5.27 | 806,207 | 256,749 | 1,102,675 | 721,877 ± 429,222 |
| p-cymene | 5.35 | ||||
| o-Cymene | 5.29 | ||||
| γ-Terpinene | 5.63 | ||||
| Terpinolen | 6.10 | ||||
| Linalool | 6.52 | ||||
| (-)-Isopulegol | 7.53 | ||||
| Geraniol | 9.32 | ||||
| β-Caryophyllene | 11.18 | 618,754 | 4,965,629 | 7,195,659 | 4,260,014 ± 3,344,748 |
| α-Humulene | 11.79 | ||||
| Nerolidol | 13.37 | ||||
| Caryophyllene oxide | 14.1 | ||||
| Guaiol | 14.1 | ||||
| α-Bisabolol | 15.26 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeran, M.; Romolo, A.; Spasovski, V.; Hočevar, M.; Novak, U.; Štukelj, R.; Šuštar, V.; Kisovec, M.; Bedina Zavec, A.; Kogej, K.; et al. Small Cellular Particles from European Spruce Needle Homogenate. Int. J. Mol. Sci. 2023, 24, 4349. https://doi.org/10.3390/ijms24054349
Jeran M, Romolo A, Spasovski V, Hočevar M, Novak U, Štukelj R, Šuštar V, Kisovec M, Bedina Zavec A, Kogej K, et al. Small Cellular Particles from European Spruce Needle Homogenate. International Journal of Molecular Sciences. 2023; 24(5):4349. https://doi.org/10.3390/ijms24054349
Chicago/Turabian StyleJeran, Marko, Anna Romolo, Vesna Spasovski, Matej Hočevar, Urban Novak, Roman Štukelj, Vid Šuštar, Matic Kisovec, Apolonija Bedina Zavec, Ksenija Kogej, and et al. 2023. "Small Cellular Particles from European Spruce Needle Homogenate" International Journal of Molecular Sciences 24, no. 5: 4349. https://doi.org/10.3390/ijms24054349







