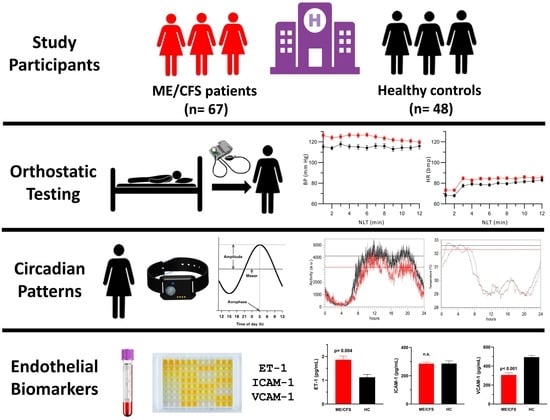
Skin Temperature Circadian Rhythms and Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Role of Endothelin-1 in the Vascular Tone Dysregulation
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Study Population
2.2. Passive Standing Test
2.3. Postural Wrist Temperature Changes
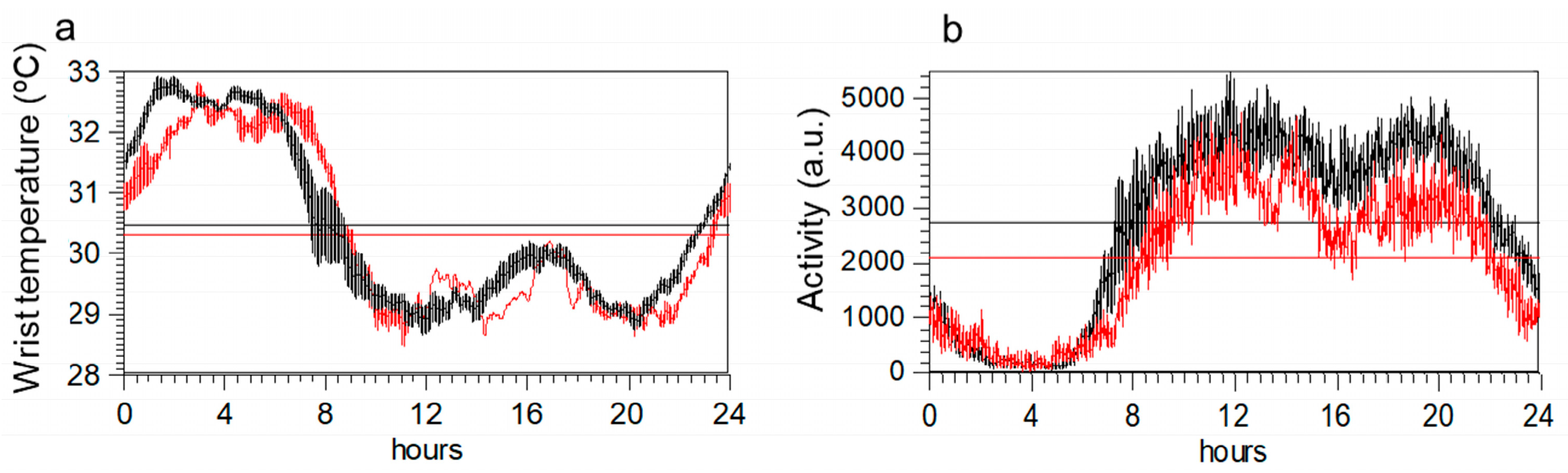
2.4. Wrist Temperature Rhythms and Motor Activity Measured by Actigraphy
2.5. Endothelial Function Biomarkers
- Circadian variables of WT were significant predictors of ET-1 in ME/CFS and healthy controls. As such, the final model was obtained with the stability of the circadian rhythm, amplitude, and nocturnal value (T_M5). Meanwhile, in controls, it was the amplitude of the WT circadian pattern that was the significant predictor of ET-1.
- None of the circadian variables of motor activity were predictors of ET-1 in ME/CFS, nor in healthy controls. As such, no variables remained in the final model.
- NLT variables were significant predictors of ET-1 in healthy controls. In the final model, ET-1 was associated with SBP/DBP, ΔSBP, and ΔDBP in healthy controls. This association was not found in ME/CFS patients.
2.6. Discriminant Analysis
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Participants
4.2. Experimental Procedures
4.3. Measures
4.4. Orthostatic Grading Scale
4.5. Assessment of Cardiovascular Autonomic Function
4.6. Concomitant Medication
4.7. Hemodynamic Definitions Recorded during the Orthostatic Test
4.8. Actigraphy Analysis
4.9. Blood Sampling and Processing
4.10. Measurement of Endothelial Biomarkers
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Saez-Francàs, N.; Santillo, D.; Alegre, J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: All roads lead to Rome. Br. J. Pharmacol. 2017, 174, 345–369. [Google Scholar] [CrossRef] [Green Version]
- Cambras, T.; Castro-Marrero, J.; Zaragoza, M.C.; Diez-Noguera, A.; Alegre, J. Circadian rhythm abnormalities and autonomic dysfunction in patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. PLoS ONE 2018, 13, e0198106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Marrero, J.; Zaragoza, M.C.; Gonzalez-Garcia, S.; Aliste, L.; Saez-Francàs, N.; Romero, O.; Ferré, A.; Fernandez de Sevilla, T.; Alegre, J. Poor self-reported sleep quality and health-related quality of life in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J. Sleep Res. 2018, 27, e12703. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Tajima, S.; Mizuno, K.; Ishii, A.; Konishi, Y.; Mike, T.; Watanabe, Y. Frontier studies on fatigue, autonomic nerve dysfunction, and sleep-rhythm disorder. J. Physiol. Sci. 2015, 65, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, M.J.; Bahl, J.S.; Buckley, J.D.; Thomson, R.L.; Davison, K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e17600. [Google Scholar] [CrossRef]
- Natelson, B.H.; Brunjes, D.L.; Mancini, D. Chronic Fatigue Syndrome and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1056–1067. [Google Scholar] [CrossRef]
- Natelson, B.H.; Lin, J.S.; Blate, M.; Khan, S.; Chen, Y.; Unger, E.R. Physiological assessment of orthostatic intolerance in chronic fatigue syndrome. J. Transl. Med. 2022, 20, 95. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Roerink, M.E.; Lenders, J.W.; Schmits, I.C.; Pistorius, A.M.; Smit, J.W.; Knoop, H.; van der Meer, J.W. Postural orthostatic tachycardia is not a useful diagnostic marker for chronic fatigue syndrome. J. Intern. Med. 2017, 281, 179–188. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.J. Circadian rhythm disruption in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Implications for the post-acute sequelae of COVID-19. Brain Behav. Immun. Health 2022, 20, 100412. [Google Scholar] [CrossRef]
- Buijs, R.M.; Escobar, C.; Swaab, D.F. The circadian system and the balance of the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Prada, V.; Soddu, A.; di Perri, C.; Sannita, W.G. Circadian Rhythms and Measures of CNS/Autonomic Interaction. Int. J. Environ. Res. Public Health 2019, 16, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijs, R.M.; Soto Tinoco, E.C.; Hurtado Alvarado, G.; Escobar, C. The circadian system: From clocks to physiology. Handb. Clin. Neurol. 2021, 179, 233–247. [Google Scholar] [CrossRef]
- Krauchi, K.; Wirz-Justice, A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology 2001, 25, S92–S96. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, G.; Spence, V.; Khan, F.; Belch, J.J. Plasma endothelin-1 levels in chronic fatigue syndrome. Rheumatology 2004, 43, 252–253, author reply 253–254. [Google Scholar] [CrossRef] [Green Version]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Paschos, G.K.; FitzGerald, G.A. Circadian clocks and vascular function. Circ. Res. 2010, 106, 833–841. [Google Scholar] [CrossRef]
- Douma, L.G.; Barral, D.; Gumz, M.L. Interplay of the Circadian Clock and Endothelin System. Physiology 2021, 36, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Vernon, S.D.; Jeys, P.; Ali, W.; Campos, A.; Unutmaz, D.; Yellman, B.; Bateman, L. Hemodynamics during the 10-minute NASA Lean Test: Evidence of circulatory decompensation in a subset of ME/CFS patients. J. Transl. Med. 2020, 18, 314. [Google Scholar] [CrossRef] [PubMed]
- Timmers, H.J.; Wieling, W.; Soetekouw, P.M.; Bleijenberg, G.; Van Der Meer, J.W.; Lenders, J.W. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin. Auton. Res. 2002, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Murray, A.; Di Maria, C.; Newton, J.L. Chronic fatigue syndrome and impaired peripheral pulse characteristics on orthostasis--a new potential diagnostic biomarker. Physiol. Meas. 2012, 33, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Slomko, J.; Estevez-Lopez, F.; Kujawski, S.; Zawadka-Kunikowska, M.; Tafil-Klawe, M.; Klawe, J.J.; Morten, K.J.; Szrajda, J.; Murovska, M.; Newton, J.L.; et al. Autonomic Phenotypes in Chronic Fatigue Syndrome (CFS) Are Associated with Illness Severity: A Cluster Analysis. J. Clin. Med. 2020, 9, 2531. [Google Scholar] [CrossRef]
- Shilco, P.; Roitblat, Y.; Buchris, N.; Hanai, J.; Cohensedgh, S.; Frig-Levinson, E.; Burger, J.; Shterenshis, M. Normative surface skin temperature changes due to blood redistribution: A prospective study. J. Therm. Biol. 2019, 80, 82–88. [Google Scholar] [CrossRef]
- Krauchi, K.; Gompper, B.; Hauenstein, D.; Flammer, J.; Pfluger, M.; Studerus, E.; Schotzau, A.; Orgul, S. Diurnal blood pressure variations are associated with changes in distal-proximal skin temperature gradient. Chronobiol. Int. 2012, 29, 1273–1283. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Maloney, E.M.; Boneva, R.S.; Lin, J.M.; Reeves, W.C. Chronic fatigue syndrome is associated with metabolic syndrome: Results from a case-control study in Georgia. Metabolism 2010, 59, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Jarrin, D.C.; Ivers, H.; Lamy, M.; Chen, I.Y.; Harvey, A.G.; Morin, C.M. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J. Sleep Res. 2018, 27, e12663. [Google Scholar] [CrossRef]
- Fink, A.M.; Bronas, U.G.; Calik, M.W. Autonomic regulation during sleep and wakefulness: A review with implications for defining the pathophysiology of neurological disorders. Clin. Auton. Res. 2018, 28, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18 (Suppl. S1), S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo. Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Alonso, J.; Prieto, L.; Antó, J.M. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Schrezenmaier, C.; Gehrking, J.A.; Hines, S.M.; Low, P.A.; Benrud-Larson, L.M.; Sandroni, P. Evaluation of orthostatic hypotension: Relationship of a new self-report instrument to laboratory-based measures. Mayo. Clin. Proc. 2005, 80, 330–334. [Google Scholar] [CrossRef]
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R.; et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. 2021, 235, 102828. [Google Scholar] [CrossRef]
- Raj, S.R.; Bourne, K.M.; Stiles, L.E.; Miglis, M.G.; Cortez, M.M.; Miller, A.J.; Freeman, R.; Biaggioni, I.; Rowe, P.C.; Sheldon, R.S.; et al. Postural orthostatic tachycardia syndrome (POTS): Priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting—Part 2. Auton. Neurosci. 2021, 235, 102836. [Google Scholar] [CrossRef]
- Van Someren, E.J.; Mirmiran, M.; Swaab, D.F. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer’s disease: Chronobiological perspectives. Behav. Brain Res. 1993, 57, 235–253. [Google Scholar] [CrossRef] [Green Version]

| Variables a | ME/CFS | HCs | Adjusted p-Value b |
|---|---|---|---|
| (n = 67) | (n = 48) | ||
| Age, years | 49.0 ± 1.04 | 44.3 ± 1.90 | 0.016 |
| BMI, kg/m2 | 27.6 ± 5.50 | 24.1 ± 0.60 | <0.001 |
| Supine SBP, mmHg | 124.18 ± 1.9 | 112.95 ± 1.6 | <0.05 |
| Supine DBP, mmHg | 78.97 ± 1.10 | 70.97 ± 1.06 | <0.01 |
| Supine HR, bpm | 74.47 ± 1.44 | 67.5 ± 1.18 | <0.01 |
| Duration of illness, years | 6.21 ± 0.50 | n/a | n/a |
| Routine blood tests § | |||
| Hematocrit, % | 42.8 ± 0.82 | 42.4 ± 0.63 | 0.667 |
| Glucose, mg/dL | 89.1 ± 1.86 | 85.30 ± 1.58 | 0.122 |
| Urea, mg/dL | 31.2 ± 1.48 | 29.5 ± 1.47 | 0.406 |
| Creatinine, mg/dL | 0.69 ± 0.03 | 0.73 ± 0.02 | 0.310 |
| Urate, mg/dL | 4.69 ± 0.24 | 4.48 ± 0.19 | 0.493 |
| Cholesterol, mg/dL | 227.07 ± 7.87 | 200.33 ± 7.30 | 0.016 |
| Triglycerides, mg/dL | 113.90 ± 10.67 | 71.78 ± 4.86 | 0.001 |
| HDL, mg/dL | 66.86 ± 2.87 | 69.24 ± 3.19 | 0.582 |
| LDL, mg/dL | 141.61 ± 6.42 | 118.76 ± 6.05 | 0.012 |
| eGFR, mL/min/1.73 m2 | 72.6 ± 7.22 | 76.0 ± 2.00 | 0.790 |
| Sodium, mmol/L | 139.75 ± 0.31 | 138.95 ± 0.29 | 0.060 |
| Potassium, mmol/L | 4.17 ± 0.06 | 4.22 ± 0.05 | 0.503 |
| Phosphate, mg/dL | 3.59 ± 0.12 | 3.49 ± 0.08 | 0.478 |
| Calcium, mg/dL | 9.66 ± 0.07 | 9.65 ± 0.05 | 0.973 |
| Albumin, g/dL | 4.35 ± 0.05 | 4.46 ± 0.05 | 0.137 |
| Total protein, g/dL | 7.14 ± 0.07 | 7.26 ± 0.08 | 0.284 |
| TSH, mUI/L | 2.12 ± 0.20 | 2.01 ± 0.23 | 0.715 |
| Free T4, ng/dL | 1.10 ± 0.20 | 1.18 ± 0.03 | 0.071 |
| 25-hydroxy-vitamin D, ng/mL | 20.74 ± 2.08 | 23.50 ± 1.59 | 0.293 |
| Cortisol, μg/dL | 14.96 ± 1.18 | 12.61 ± 1.28 | 0.183 |
| 17β-estradiol, pg/mL | 118.23 ± 27.65 | 52.07 ± 16.42 | 0.044 |
| Progesterone, ng/mL | 0.54 ± 0.25 | 0.72 ± 0.51 | 0.847 |
| Aldosterone, ng/dL | 10.35 ± 0.98 | 10.03 ± 0.51 | 0.772 |
| Prolactin, ng/mL | 8.53 ± 0.62 | 8.38 ± 0.58 | 0.864 |
| Measures a | |||
| FIS-40 | |||
| Global score (0–160) | 130.3 ± 2.7 | 18.9 ± 3.0 | <0.001 |
| Physical | 33.9 ± 0.6 | 82.6 ± 0.8 | <0.001 |
| Cognitive | 61.9 ± 1.5 | 8.6 ± 1.5 | <0.001 |
| Psychosocial | 34 ± 0.8 | 4.5 ± 0.8 | <0.001 |
| PSQI | |||
| Global score (0–21) | 15.5 ± 0.5 | 5.9 ± 0.5 | <0.001 |
| Subjective sleep quality | 2.2 ± 0.11 | 0.7 ± 0.11 | <0.001 |
| Sleep latency | 2.1 ± 0.31 | 0.6 ± 0.16 | <0.001 |
| Sleep duration | 1.9 ± 0.11 | 1.1 ± 0.09 | <0.001 |
| Habitual sleep efficiency | 2.1 ± 0.14 | 0.6 ± 0.14 | <0.001 |
| Sleep disturbances | 2.3 ± 0.18 | 1.1 ± 0.05 | <0.001 |
| Use of sleeping medication | 2.1 ± 0.15 | 0.3 ± 0.11 | <0.001 |
| Daytime dysfunction | 2.4 ± 0.08 | 0.6 ± 0.97 | <0.001 |
| HADS | |||
| Global score (0–42) | 24.5 ± 0.9 | 6.9 ± 0.7 | <0.001 |
| Anxiety | 12.8 ± 0.5 | 5.3 ± 0.5 | <0.001 |
| Depression | 11.7 ± 0.5 | 1.7 ± 0.32 | <0.001 |
| COMPASS-31 | |||
| Global score (0–100) | 59.5 ± 1.68 | 13.9 ± 1.53 | <0.001 |
| Orthostatic intolerance | 25.4 ± 0.9 | 5.9 ± 0.9 | <0.001 |
| Vasomotor | 1.9 ± 0.18 | 0.1 ± 0.07 | <0.001 |
| Secretomotor | 10.3 ± 0.32 | 1.4 ± 0.37 | <0.001 |
| Gastrointestinal | 13.5 ± 0.50 | 4.8 ± 0.57 | <0.001 |
| Bladder | 4.4 ± 0.35 | 0.6 ± 0.17 | <0.001 |
| Pupillomotor | 3.8 ± 0.12 | 1.06 ± 0.13 | <0.001 |
| SF-36 | |||
| Global score (0–100) | 26.0 ± 3.1 | 85.5 ± 2.5 | <0.001 |
| Physical functioning | 30.3 ± 2.5 | 96.9 ± 0.8 | <0.001 |
| Physical role functioning | 1.8 ± 0.9 | 90.5 ± 3.3 | <0.001 |
| Bodily pain | 20.9 ± 2.9 | 85.4 ± 2.9 | <0.001 |
| General health perception | 23.1 ± 1.9 | 85.6 ± 1.7 | <0.001 |
| Vitality | 13.5 ± 1.9 | 70.6 ± 2.7 | <0.001 |
| Social role functioning | 34.4 ± 3.1 | 92.7 ± 2.2 | <0.001 |
| Emotional role functioning | 39.4 ± 5.6 | 87.4 ± 4.1 | <0.001 |
| Mental health | 43.9 ± 2.8 | 75.2 ± 2.5 | <0.001 |
| OGS † | |||
| Global score (0–20) | 12.82 ± 4.39 | 0.87 ± 1.81 | <0.001 |
| Frequency of orthostatic symptoms | 2.68 ± 1.04 | 0.26 ± 0.59 | <0.001 |
| Severity of orthostatic symptoms | 2.59 ± 1.01 | 0.22 ± 0.47 | <0.001 |
| Conditions under which orthostatic symptoms occur | 2.73 ± 1.12 | 0.24 ± 0.48 | <0.001 |
| Interference with activities of daily living | 2.50 ± 1.06 | 0.02 ± 0.15 | <0.001 |
| Standing time | 2.32 ± 1.21 | 0.12 ± 0.55 | <0.001 |
| Variables | ME/CFS (n = 67) | HCs (n = 48) | Adjusted p-Value a |
|---|---|---|---|
| Cardiovascular variables | |||
| Supine SBP, mmHg | 124.18 ± 1.99 | 112.95 ± 1.6 | <0.012 |
| ΔSBP_3F, mmHg | 1.06 ± 1.14 | 1.79 ± 1.01 | 0.979 |
| Supine DBP, mmHg | 78.97 ± 1.13 | 70.97 ± 1.06 | <0.001 |
| ΔDBP_3F, mmHg | 6.69 ± 0.84 | 6.66 ± 0.87 | 0.959 |
| Supine HR, bpm | 74.47 ± 1.44 | 67.5 ± 1.18 | <0.001 |
| ΔHR_3L, bpm | 14.65 ± 1.23 | 17.28 ± 1.64 | 0.961 |
| Wrist temperature (WT) during the NLT † | |||
| Supine WT, °C | 30.72 ± 0.32 | 29.67 ± 0.39 | 0.474 |
| ΔWT_3L, °C | 1.08 ± 0.25 | 1.97 ± 0.28 | 0.173 |
| Temperature rhythm variables | |||
| T_Mesor, °C | 30.35 ± 0.10 | 30.54 ± 0.12 | 0.745 |
| T_Acrophase, min | 318.77 ± 33.19 | 190.55 ± 12.91 | 0.391 |
| T_Amplitude, °C | 1.79 ± 0.10 | 1.73 ± 0.11 | 0.381 |
| T_IV, a.u. | 0.01 ± 0 | 0.01 ± 0 | 0.116 |
| T_R, a.u. | 0.83 ± 0.03 | 0.88 ± 0.02 | 0.423 |
| T_ PV, % | 41.15 ± 2.03 | 42.07 ± 1.56 | 0.887 |
| T_M5, °C | 32.52 ± 0.15 | 32.82 ± 0.12 | 0.621 |
| T_L10, °C | 28.96 ± 0.11 | 29.28 ± 0.17 | 0.259 |
| Activity rhythm variables | |||
| A_ Mesor, a.u. | 2058 ± 95 | 2746 ± 82 | <0.001 |
| A_Acrophase, min | 858 ± 11.4 | 856 ± 11 | 0.986 |
| A_ Amplitude, °C | 1744 ± 80 | 2116 ± 72 | <0.009 |
| A_ IV, a.u. | 0.42 ± 0.008 | 0.37 ± 0.01 | 0.102 |
| A_ R, a.u. | 0.93 ± 0.009 | 0.92 ± 0.009 | 0.745 |
| A_ PV, % | 32.73 ± 0.97 | 31.02 ± 0.88 | 0.107 |
| A_ M10, a.u. | 3390 ± 149 | 4308 ± 127 | <0.001 |
| A_L5, a.u. | 199 ± 26 | 175 ± 20 | 0.911 |
| Endothelial biomarkers § | |||
| ET-1, pg/mL | 1.84 ± 0.14 | 1.12 ± 0.63 | 0.004 |
| VCAM-1, ng/mL | 739 ± 33 | 494 ± 17 | < 0.001 |
| ICAM-1, ng/mL | 284 ± 10 | 285 ± 19 | 0.611 |
| ET-1, pg/mL | VCAM-1, ng/mL | ICAM-1, ng/mL | ||||
|---|---|---|---|---|---|---|
| Variables | ME/CFS (n = 32) | HCs (n = 29) | ME/CFS (n = 32) | HCs (n = 29) | ME/CFS (n = 32) | HCs (n = 29) |
| B a | B a | B a | B a | B a | B a | |
| Temperature | ||||||
| Step 1 (initial model) | ||||||
| Amplitude, °C | 0.063 | 0.553 | −60.484 | 44.436 | −27.118 | 102.581 |
| T_ IV, a.u. | −33.770 | 15.620 | −1171.484 | −8496.904 | 40.607 | −18,658.229 |
| T_R, a.u. | −0.552 | 0.416 | 542.522 | 138.721 | 66.280 | 429.192 |
| T_PV, % | −0.034 | −0.024 | −2.726 | −5.450 | 0.571 | −7.151 |
| T_M5, °C | 0.537 | 0.013 | −17.247 | −0.443 | 2.547 | −80.401 |
| T_L10, °C | 0.040 | 0.035 | −49.908 | 8.894 | −16.097 | 47.057 |
| Final model | ||||||
| T_ PV, % | −0.034 * | - | - | - | - | - |
| T_M5, °C | 0.564 ** | - | - | - | - | - |
| Amplitude, °C | - | 0.486 * | - | - | - | - |
| Motor activity | ||||||
| Step 1 (initial model) | ||||||
| A_Mesor, a.u. | −0.002 | 0.002 | 0.107 | −0.058 | 0.195 | 0.076 |
| A_Amplitude, °C | 0.000 | 0.002 | −0.167 | −0.203 | 0.205 | −0.075 |
| A_IV, a.u | 0.524 | −3.459 | 1082.393 | 136.465 | 171.076 | 170.145 |
| A_R, a.u. | −0.287 | −1.141 | 152.340 | −352.068 | 22.964 | 203.005 |
| A_PV, % | −0.054 | −0.023 | 0.436 | 1.572 | 2.958 | −1.292 |
| A_M10, a.u. | 0.001 | −0.002 | 0.034 | 0.165 | −0.245 | −0.006 |
| A_L5, a.u. | −0.001 | 0.002 | 0.564 | −0.360 | 0.165 | −0.119 |
| Final model | ||||||
| A_L10, a.u. | - | - | 0.665 * | - | - | - |
| Passive standing test (NLT) | ||||||
| Step 1 (initial model) | ||||||
| Supine SBP, mmHg | −0.004 | 0.015 | −2.117 | −1.809 | 0.535 | 0.855 |
| Supine DBP, mmHg | 0.011 | −0.033 | −0.713 | 3.620 | −0.352 | 7.752 |
| Supine HR, bpm | 0.014 | −0.012 | −2.286 | 5.209 | 1.605 | 5.633 |
| ΔSBP_3F, mmHg | 0.010 | 0.033 | −4.509 | 6.352 | −0.588 | −0.913 |
| ΔDBP_3F, mmHg | −0.069 | −0.063 | 0.742 | −2.246 | −2.822 | −5.600 |
| ΔHR_3L, bpm | 0.051 | −0.011 | −10.802 | 1.260 | −2.959 | 2.758 |
| ΔWT_3L, °C | −0.454 | −0.053 | −34.913 | 8.589 | −11.224 | 26.378 |
| Final model | ||||||
| Supine SBP, mmHg | - | 0.022 ** | −3.348 * | - | - | - |
| Supine DBP, mmHg | - | −0.033 ** | - | - | - | 5.290 * |
| ΔSBP_3F, mmHg | - | 0.043 * | - | - | - | - |
| ΔDBP_3F, mmHg | - | −0.065 ** | - | - | - | - |
| ΔWT_3L, °C | −0.374 * | - | - | - | - | 31.939 * |
| ME/CFS (n = 32) | Healthy Controls (n = 29) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | ET-1, pg/mL | VCAM-1, ng/mL | ICAM-1, ng/mL | ET-1, pg/mL | VCAM-1, ng/mL | ICAM-1, ng/mL | ||||||
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| FIS-40 | 0.529 | 0.005 | −0.098 | 0.626 | 0.180 | 0.369 | −0.165 | 0.430 | −0.149 | 0.477 | −0.322 | 0.117 |
| HADS | 0.407 | 0.035 | −0.148 | 0.462 | 0.136 | 0.500 | 0.011 | 0.957 | 0.233 | 0.263 | 0.232 | 0.265 |
| COMPASS-31 | 0.452 | 0.018 | −0.104 | 0.607 | 0.042 | 0.834 | 0.063 | 0.764 | 0.046 | 0.826 | −0.009 | 0.965 |
| SF-36 | −0.501 | 0.008 | 0.090 | 0.654 | −0.205 | 0.304 | 0.140 | 0.503 | −0.142 | 0.498 | −0.052 | 0.805 |
| OGS | −0.021 | 0.947 | −0.166 | 0.587 | 0.178 | 0.561 | 0.127 | 0.529 | −0.183 | 0.360 | −0.049 | 0.810 |
| PSQI | 0.468 | 0.014 | −0.296 | 0.134 | −0.055 | 0.783 | 0.038 | 0.861 | 0.357 | 0.086 | 0.494 | 0.014 |
| COMPASS-31 | ||
|---|---|---|
| B a | p-Value | |
| Step 1 (initial model) | ||
| Supine SBP, mmHg | −0.184 | 0.539 |
| Supine DBP, mmHg | 0.787 | 0.103 |
| Supine HR, bpm | 0.089 | 0.725 |
| ΔSBP_3L, mmHg | −0.456 | 0.219 |
| ΔDBP_3L, mmHg | 0.527 | 0.338 |
| ΔHR_3L, bpm | −0.320 | 0.287 |
| ET-1, pg/mL | 15.012 | 0.000 |
| VCAM-1, ng/mL | 0.064 | 0.000 |
| T_Amplitude, °C | 3.709 | 0.420 |
| T_PV, % | 0.610 | 0.062 |
| T_M5; °C | −10.565 | 0.009 |
| Age, years | −0.177 | 0.460 |
| BMI, kg/m2 | −0.132 | 0.804 |
| Final model | ||
| Supine DBP, mmHg | 0.559 | 0.028 |
| ET-1, pg/mL | 14.727 | 0.000 |
| VCAM-1, ng/mL | 0.059 | 0.000 |
| T_PV, % | 0.657 | 0.009 |
| T_M5, °C | −9.263 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambras, T.; Zerón-Rugerio, M.F.; Díez-Noguera, A.; Zaragozá, M.C.; Domingo, J.C.; Sanmartin-Sentañes, R.; Alegre-Martin, J.; Castro-Marrero, J. Skin Temperature Circadian Rhythms and Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Role of Endothelin-1 in the Vascular Tone Dysregulation. Int. J. Mol. Sci. 2023, 24, 4835. https://doi.org/10.3390/ijms24054835
Cambras T, Zerón-Rugerio MF, Díez-Noguera A, Zaragozá MC, Domingo JC, Sanmartin-Sentañes R, Alegre-Martin J, Castro-Marrero J. Skin Temperature Circadian Rhythms and Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Role of Endothelin-1 in the Vascular Tone Dysregulation. International Journal of Molecular Sciences. 2023; 24(5):4835. https://doi.org/10.3390/ijms24054835
Chicago/Turabian StyleCambras, Trinitat, Maria Fernanda Zerón-Rugerio, Antoni Díez-Noguera, Maria Cleofé Zaragozá, Joan Carles Domingo, Ramon Sanmartin-Sentañes, Jose Alegre-Martin, and Jesus Castro-Marrero. 2023. "Skin Temperature Circadian Rhythms and Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Role of Endothelin-1 in the Vascular Tone Dysregulation" International Journal of Molecular Sciences 24, no. 5: 4835. https://doi.org/10.3390/ijms24054835