Amorphous System of Hesperetin and Piperine—Improvement of Apparent Solubility, Permeability, and Biological Activities
Abstract
:1. Introduction
2. Results
2.1. Solid-State Identification
2.1.1. X-ray Powder Diffraction (XRPD)
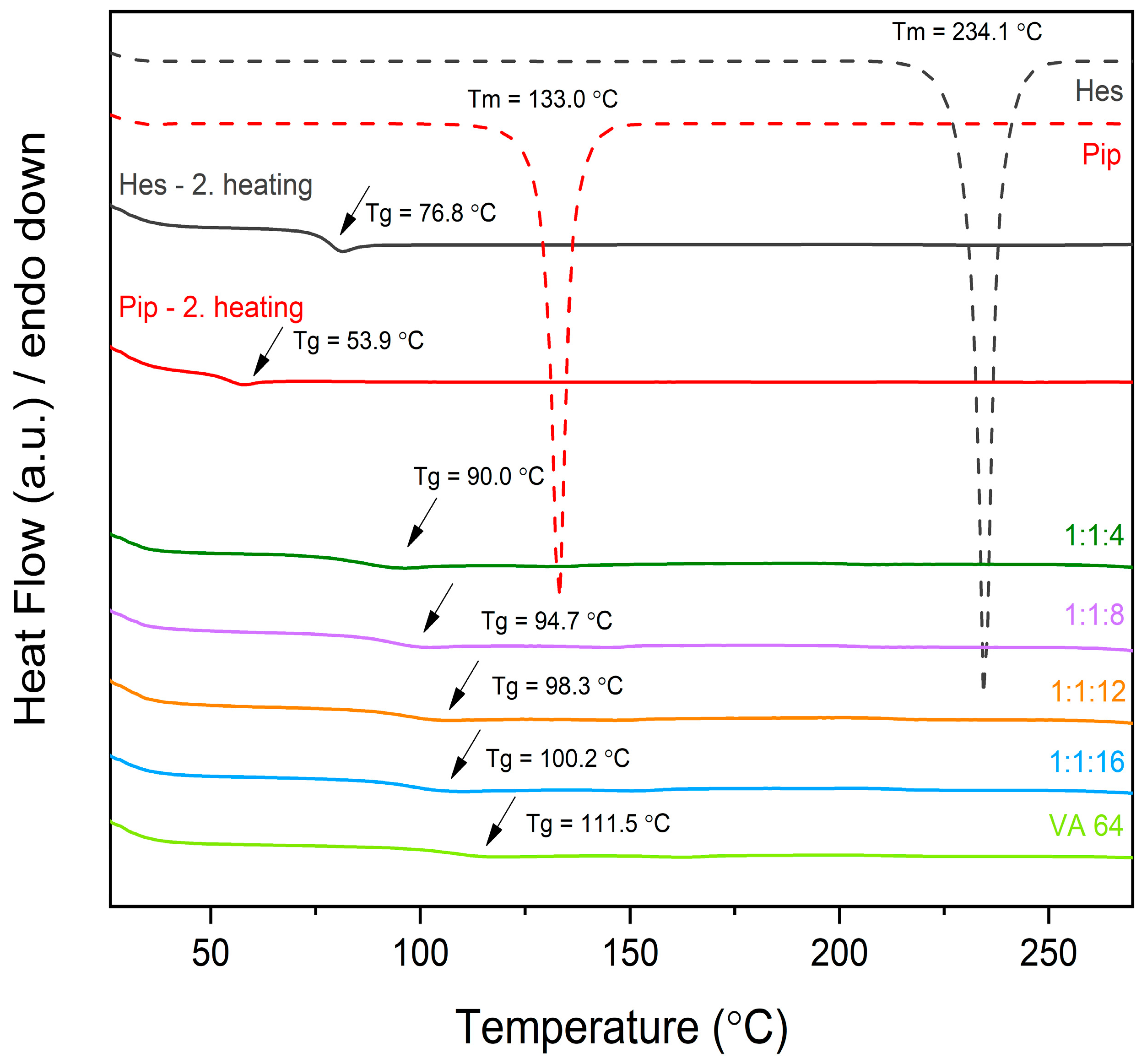
2.1.2. Differential Scanning Calorimetry (DSC)
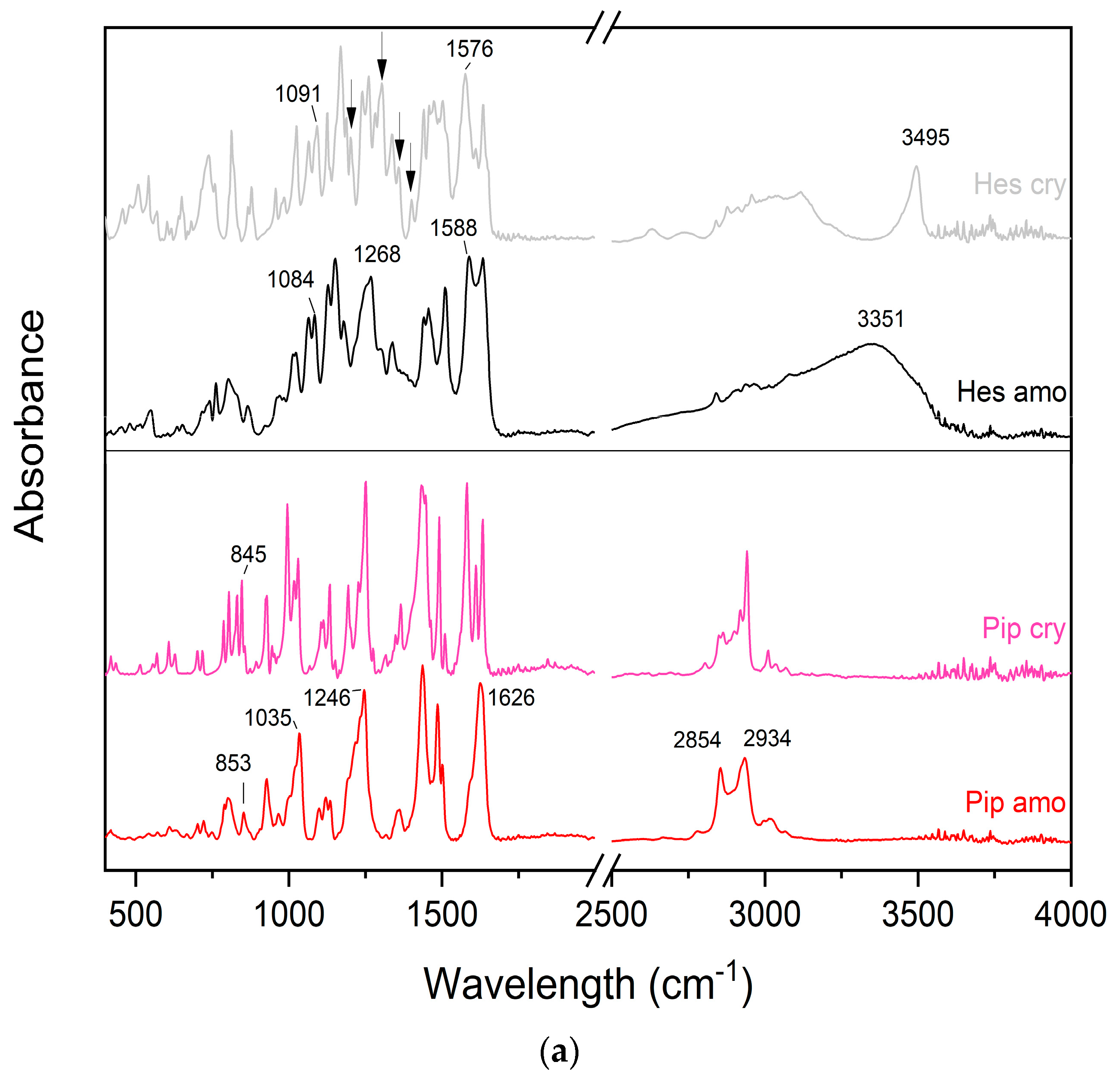
2.1.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.2. Physicochemical Characterization of Amorphous Systems
2.2.1. Dissolution Rate Studies
2.2.2. Solubility Study
2.2.3. Permeability Studies
2.3. Biological Activity Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Systems
4.3. Solid-State Identification
4.3.1. X-ray Powder Diffraction (XRPD)
4.3.2. Differential Scanning Calorimetry (DSC)
4.3.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
4.4. Physicochemical Characterization
4.4.1. HPLC Conditions
4.4.2. Media for Dissolution and Solubility Studies
4.4.3. Dissolution Studies
4.4.4. Solubility Studies
4.4.5. Permeability Studies
4.5. Biological Activities Studies
4.5.1. Antioxidant Activity Assay
4.5.2. Determination of Butyrylcholinesterase (BuChE) Inhibition
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, J.T.; Tian, D.; Tanna, R.S.; Hadi, D.L.; Bansal, S.; Calamia, J.C.; Arian, C.M.; Shireman, L.M.; Molnár, B.; Horváth, M. Assessing transporter-mediated natural product-drug interactions via in vitro-in vivo extrapolation: Clinical evaluation with a probe cocktail. Clin. Pharmacol. Ther. 2021, 109, 1342–1352. [Google Scholar] [CrossRef]
- Salehi, B.; Cruz-Martins, N.; Butnariu, M.; Sarac, I.; Bagiu, I.-C.; Ezzat, S.M.; Wang, J.; Koay, A.; Sheridan, H.; Adetunji, C.O. Hesperetin’s health potential: Moving from preclinical to clinical evidence and bioavailability issues, to upcoming strategies to overcome current limitations. Crit. Rev. Food Sci. Nutr. 2022, 62, 4449–4464. [Google Scholar] [CrossRef]
- Teng, J.; Li, J.; Zhao, Y.; Wang, M. Hesperetin, a dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264. 7 cells. J. Funct. Foods 2021, 81, 104480. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Xu, S.; Ren, J.; Tang, L.; Gong, J.; Lin, Y.; Fang, H.; Su, D. Hesperetin, a Promising Treatment Option for Diabetes and Related Complications: A Literature Review. J. Agric. Food Chem. 2022, 70, 8582–8592. [Google Scholar] [CrossRef]
- Jayaraman, R.; Subramani, S.; Abdullah, S.H.S.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N. Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: A comprehensive review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Stahr, P.-L.; Grewal, R.; Eckert, G.P.; Keck, C.M. Investigating hesperetin nanocrystals with tailor-made sizes for the prevention and treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 659–674. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Chen, Z.; Qi, X.; Wu, X.; Di, G.; Fan, J.; Guo, C. Improved bioavailability and anticancer efficacy of Hesperetin on breast cancer via a self-assembled rebaudioside A nanomicelles system. Toxicol. Appl. Pharmacol. 2021, 419, 115511. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-F.; Wang, L.-Y.; Tian, Y.-J.; Zhou, Z.-X.; Tang, J.-B.; Liu, X.-R.; Jiang, H.-P.; Shen, Y.-Q. Enhanced water solubility, antioxidant activity, and oral absorption of hesperetin by D-α-tocopheryl polyethylene glycol 1000 succinate and phosphatidylcholine. J. Zhejiang Univ. B 2019, 20, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, I.; Mihály, J.; Momekova, D.; Chimshirova, R.; Lazarova, H.; Momekov, G.; Popova, M. Antioxidant activity and modified release profiles of morin and hesperetin flavonoids loaded in Mg-or Ag-modified SBA-16 carriers. Mater. Today Commun. 2020, 24, 101198. [Google Scholar] [CrossRef]
- Haq, I.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phyther. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–24. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; V. Anil Kumar, N.; Salehi, B.; C. Cho, W.; Sharifi-Rad, J. Piperine-a major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef] [Green Version]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Alsaidan, O.A.; Elkomy, M.H.; Ghoneim, M.M.; Alshehri, S.; Ali, A.M.A.; Alharbi, K.S.; Yasir, M. Development of piperine-loaded solid self-nanoemulsifying drug delivery system: Optimization, in-vitro, ex-vivo, and in-vivo evaluation. Nanomaterials 2021, 11, 2920. [Google Scholar] [CrossRef]
- Zaini, E.; Afriyani, A.; Fitriani, L.; Ismed, F.; Horikawa, A.; Uekusa, H. Improved Solubility and Dissolution Rates in Novel Multicomponent Crystals of Piperine with Succinic Acid. Sci. Pharm. 2020, 88, 21. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Hu, M.; Cheng, Y.; Shek, T.L.; Xiao, M.; Ho, N.J.; Zhang, C.; Leung, S.S.Y.; Zuo, Z. Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control. Eur. J. Pharm. Sci. 2019, 137, 104988. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Alzahrani, T.A.; Hussain, A.; Altamimi, M.A. Formulation and evaluation of supramolecular food-grade piperine HP β CD and TPGS complex: Dissolution, physicochemical characterization, molecular docking, in vitro antioxidant activity, and antimicrobial assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, F.; Zhao, X.; Wang, S.; Yang, Q.; Zhang, X. Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer. Pharmaceutics 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Sivanandam, M.; Hunday, G.; Pavan, M.S.; Kumaradhas, P. Exploring the different environments effect of piperine via combined crystallographic, QM/MM and molecular dynamics simulation study. J. Mol. Graph. Model. 2019, 92, 280–295. [Google Scholar] [CrossRef]
- Garrido, B.; González, S.; Hermosilla, J.; Millao, S.; Quilaqueo, M.; Guineo, J.; Acevedo, F.; Pesenti, H.; Rolleri, A.; Shene, C. Carbonate-β-cyclodextrin-based nanosponge as a nanoencapsulation system for piperine: Physicochemical characterization. J. Soil Sci. Plant Nutr. 2019, 19, 620–630. [Google Scholar] [CrossRef]
- Akram, A.; Irfan, M.; Abualsunun, W.A.; Bukhary, D.M.; Alissa, M. How to Improve Solubility and Dissolution of Irbesartan by Fabricating Ternary Solid Dispersions: Optimization and In-Vitro Characterization. Pharmaceutics 2022, 14, 2264. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.-T. The biological fate and bioefficacy of citrus flavonoids: Bioavailability, biotransformation, and delivery systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef]
- Linnankoski, J.; Ranta, V.-P.; Yliperttula, M.; Urtti, A. Passive oral drug absorption can be predicted more reliably by experimental than computational models—Fact or myth. Eur. J. Pharm. Sci. 2008, 34, 129–139. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Kurek, M.; Gawlak, K.; Górska, A.; Peralta, S.; Knapik-Kowalczuk, J.; Kramarczyk, D.; Paluch, M.; Jachowicz, R. How does the addition of Kollidon® VA64 inhibit the recrystallization and improve ezetimibe dissolution from amorphous solid dispersions? Pharmaceutics 2021, 13, 147. [Google Scholar] [CrossRef]
- Strindberg, S.; Plum, J.; Stie, M.B.; Christiansen, M.L.; Hagner Nielsen, L.; Rades, T.; Müllertz, A. Effect of supersaturation on absorption of indomethacin and tadalafil in a single pass intestinal perfusion rat model, in the absence and presence of a precipitation inhibitor. Eur. J. Pharm. Biopharm. 2020, 151, 108–115. [Google Scholar] [CrossRef]
- Suys, E.J.A.; Chalmers, D.K.; Pouton, C.W.; Porter, C.J.H. Polymeric precipitation inhibitors promote fenofibrate supersaturation and enhance drug absorption from a type IV lipid-based formulation. Mol. Pharm. 2018, 15, 2355–2371. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, G.; Tian, B.; Huang, H.; Zhang, Y.; Gou, J.; Tang, X.; He, H.; Wang, Y. Supersaturation induced by Itraconazole/Soluplus® micelles provided high GI absorption in vivo. Asian J. Pharm. Sci. 2016, 11, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, S.; Minamisono, T.; Yamashita, T.; Kato, T.; Kushida, I. Supersaturation–nucleation behavior of poorly soluble drugs and its impact on the oral absorption of drugs in thermodynamically high-energy forms. J. Pharm. Sci. 2012, 101, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Higashino, H.; Hasegawa, T.; Yamamoto, M.; Matsui, R.; Masaoka, Y.; Kataoka, M.; Sakuma, S.; Yamashita, S. In vitro-in vivo correlation of the effect of supersaturation on the intestinal absorption of BCS class 2 drugs. Mol. Pharm. 2014, 11, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Knopp, M.M.; Wendelboe, J.; Holm, R.; Rades, T. Effect of amorphous phase separation and crystallization on the in vitro and in vivo performance of an amorphous solid dispersion. Eur. J. Pharm. Biopharm. 2018, 130, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Brand, W.; Van Der Wel, P.A.I.; Rein, M.J.; Barron, D.; Williamson, G.; Van Bladeren, P.J.; Rietjens, I.M.C.M. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab. Dispos. 2008, 36, 1794–1802. [Google Scholar] [CrossRef] [Green Version]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple strategies to improve oral bioavailability by fabricating coamorphous forms of ursolic acid with piperine: Enhancing water-solubility, permeability, and inhibiting cytochrome p450 isozymes. Mol. Pharm. 2020, 17, 4443–4462. [Google Scholar] [CrossRef]
- Omidfar, F.; Gheybi, F.; Davoodi, J.; Amirinejad, M.; Badiee, A. Nanophytosomes of Hesperidin and of Hesperetin: Preparation, characterization and in vivo evaluation. Biotechnol. Appl. Biochem. 2022. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Huang, A.-L.; Zhang, Y.-L.; Li, Z.; Ding, H.-W.; Huang, C.; Meng, X.-M.; Li, J. Design, synthesis and evaluation of hesperetin derivatives as potential multifunctional anti-Alzheimer agents. Molecules 2017, 22, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Manap, A.S.; Wei Tan, A.C.; Leong, W.H.; Yin Chia, A.Y.; Vijayabalan, S.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Synergistic effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front. Aging Neurosci. 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasiecki, J.; Wasąg, B. Butyrylcholinesterase protein ends in the pathogenesis of Alzheimer’s disease—Could BCHE genotyping be helpful in Alzheimer’s therapy? Biomolecules 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Jasiecki, J.; Targońska, M.; Wasąg, B. The role of butyrylcholinesterase and iron in the regulation of cholinergic network and cognitive dysfunction in Alzheimer’s disease pathogenesis. Int. J. Mol. Sci. 2021, 22, 2033. [Google Scholar] [CrossRef]
- De la Rubia Ortí, J.E.; Platero, J.L.; Yang, I.H.; Ceron, J.J.; Tvarijonaviciute, A.; Sabater, P.S.; Benlloch, M.; Sancho-Cantus, D.; Sancho, S. Possible Role of Butyrylcholinesterase in Fat Loss and Decreases in Inflammatory Levels in Patients with Multiple Sclerosis after Treatment with Epigallocatechin Gallate and Coconut Oil: A Pilot Study. Nutrients 2021, 13, 3230. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Lu, S.; Fang, Z.; Tu, K.; Zheng, M. Solubility improvement of hesperetin by using different octenyl succinic anhydride modified starches. LWT 2018, 95, 255–261. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential microwave-ultrasound-assisted extraction for isolation of piperine from black pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Školáková, T.; Slámová, M.; Školáková, A.; Kadeřábková, A.; Patera, J.; Zámostný, P. Investigation of dissolution mechanism and release kinetics of poorly water-soluble tadalafil from amorphous solid dispersions prepared by various methods. Pharmaceutics 2019, 11, 383. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, Q.A.; Latif, S.; Rashid, M.; Arshad, M.S.; Hussain, A.; Bukhari, N.I.; Riaz, S.; Abbas, N. Preparation and Characterization of pH-Independent Sustained-Release Tablets Containing Hot Melt Extruded Solid Dispersions of Clarithromycin. AAPS PharmSciTech 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Fu, Q.; Fang, M.; Hou, Y.; Yang, W.; Shao, J.; Guo, M.; Li, M.; Li, J.; Wang, Y.; He, Z. A physically stabilized amorphous solid dispersion of nisoldipine obtained by hot melt extrusion. Powder Technol. 2016, 301, 342–348. [Google Scholar] [CrossRef]
- Liu, K.; Liu, H.; Li, Z.; Li, W.; Li, L. In vitro dissolution study on inclusion complex of piperine with ethylenediamine-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 233–243. [Google Scholar] [CrossRef]




| System (Hes:Pip:VA 64) | Compound | |||
|---|---|---|---|---|
| Hesperetin | Piperine | |||
| Conc. [mg/mL] | Improv. [-fold] | Conc. [mg/mL] | Improv. [-fold] | |
| Raw | 0.005 ± 0.001 e | - | 0.006 ± 0.001 e | - |
| 1:1:4 | 0.269 ± 0.001 d | 58 | 0.167 ± 0.001 d | 27 |
| 1:1:8 | 0.312 ± 0.002 c | 67 | 0.237 ± 0.001 c | 38 |
| 1:1:12 | 0.509 ± 0.021 b | 110 | 0.674 ± 0.044 b | 109 |
| 1:1:16 | 1.136 ± 0.100 a | 245 | 1.134 ± 0.075 a | 183 |
| System (Hes:Pip:VA 64) | Assay | |
|---|---|---|
| DPPH | BChE | |
| % of Inhibition | % of Inhibition | |
| Raw | 2.51 ± 1.05 e | 2.12 ± 0.53 e |
| 1:1:4 | 15.55 ± 1.15 d | 10.17 ± 1.24 d |
| 1:1:8 | 32.06 ± 1.22 c | 29.48 ± 1.87 c |
| 1:1:12 | 80.22 ± 0.54 b | 78.16 ± 2.89 b |
| 1:1:16 | 90.62 ± 0.58 a | 87.53 ± 1.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Miklaszewski, A.; Pietrzak, R.; Cielecka-Piontek, J. Amorphous System of Hesperetin and Piperine—Improvement of Apparent Solubility, Permeability, and Biological Activities. Int. J. Mol. Sci. 2023, 24, 4859. https://doi.org/10.3390/ijms24054859
Wdowiak K, Miklaszewski A, Pietrzak R, Cielecka-Piontek J. Amorphous System of Hesperetin and Piperine—Improvement of Apparent Solubility, Permeability, and Biological Activities. International Journal of Molecular Sciences. 2023; 24(5):4859. https://doi.org/10.3390/ijms24054859
Chicago/Turabian StyleWdowiak, Kamil, Andrzej Miklaszewski, Robert Pietrzak, and Judyta Cielecka-Piontek. 2023. "Amorphous System of Hesperetin and Piperine—Improvement of Apparent Solubility, Permeability, and Biological Activities" International Journal of Molecular Sciences 24, no. 5: 4859. https://doi.org/10.3390/ijms24054859







