In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections
Abstract
:1. Introduction
2. Results
2.1. Characterization of N-Acetylglucosamine
2.1.1. Characterization of GlcNAc in Bulk Form
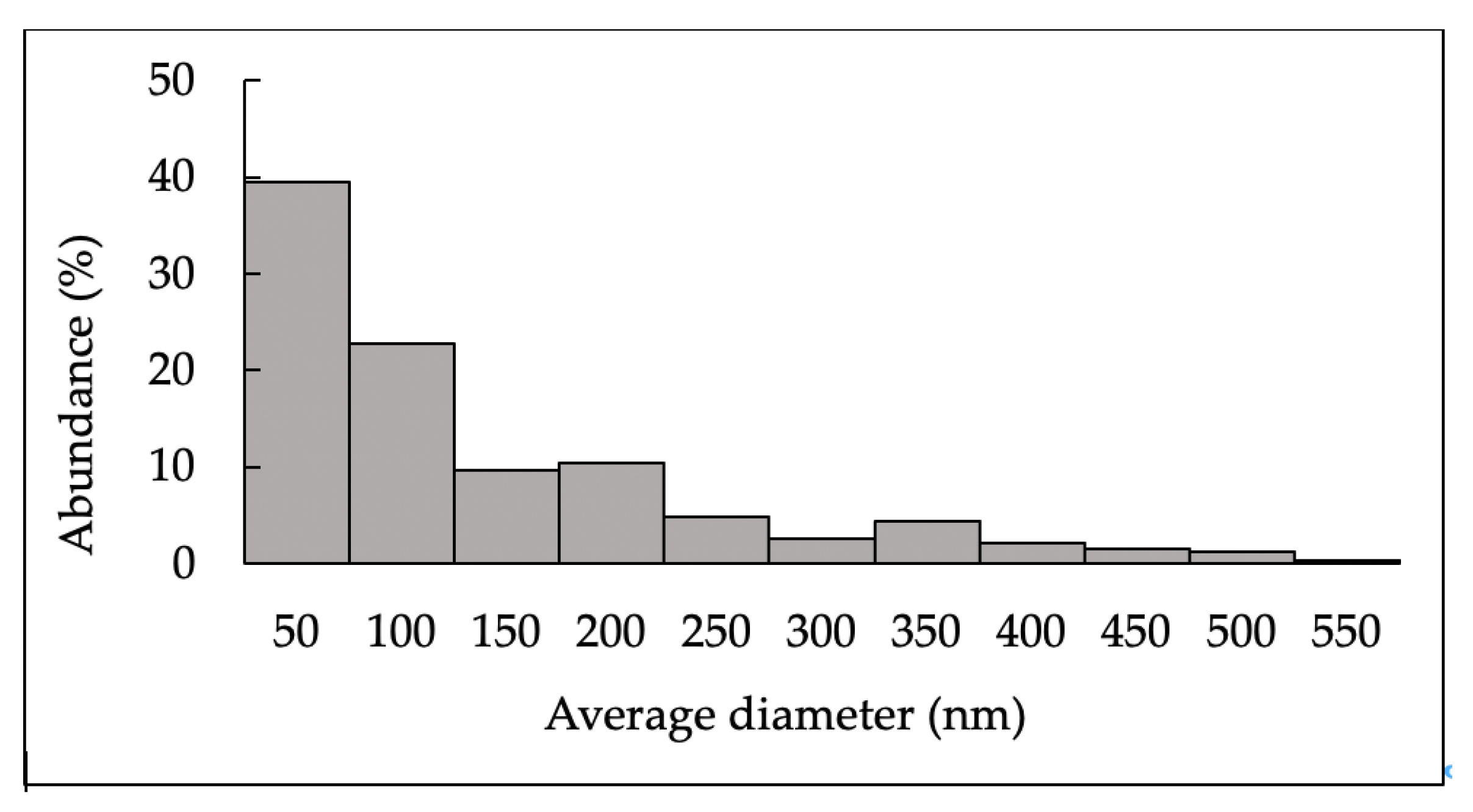
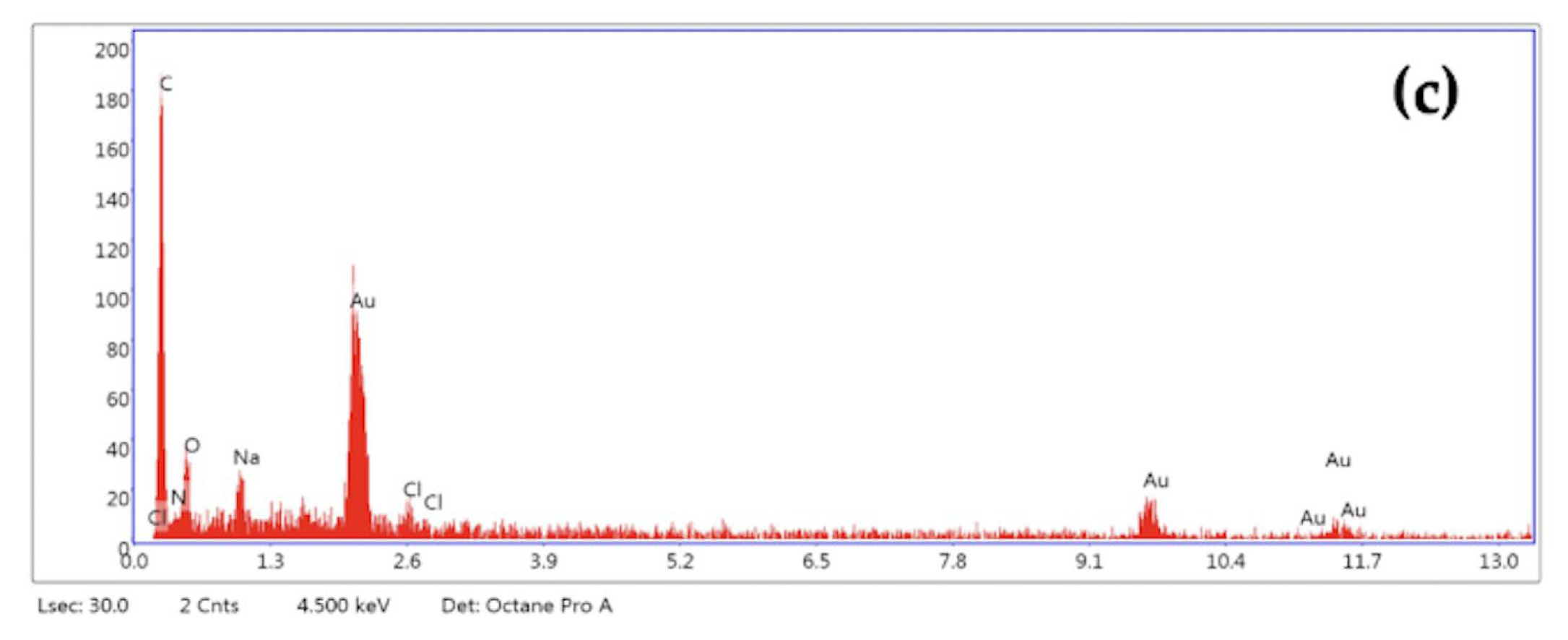
2.1.2. Characterization of N-Acetylglucosamine Nanoparticles (GlcNAc-NPs)
2.2. Biological Assays
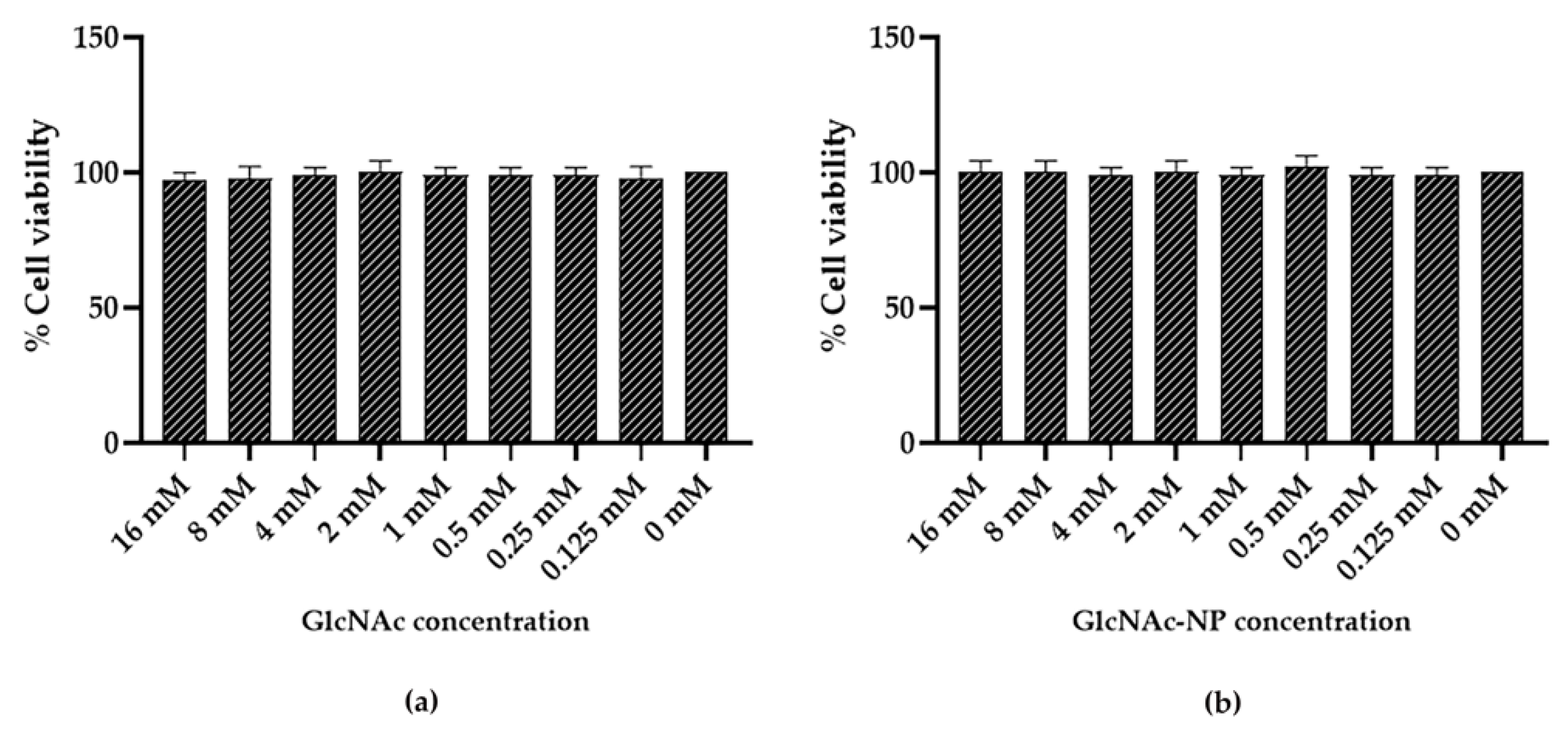
2.2.1. GlcNAc and GlcNAc-NPs Toxicity in Madin-Darby Canine Kidney (MDCK) Cells
2.2.2. GlcNAc and GlcNAc-NPs Toxicity in A549 Cells
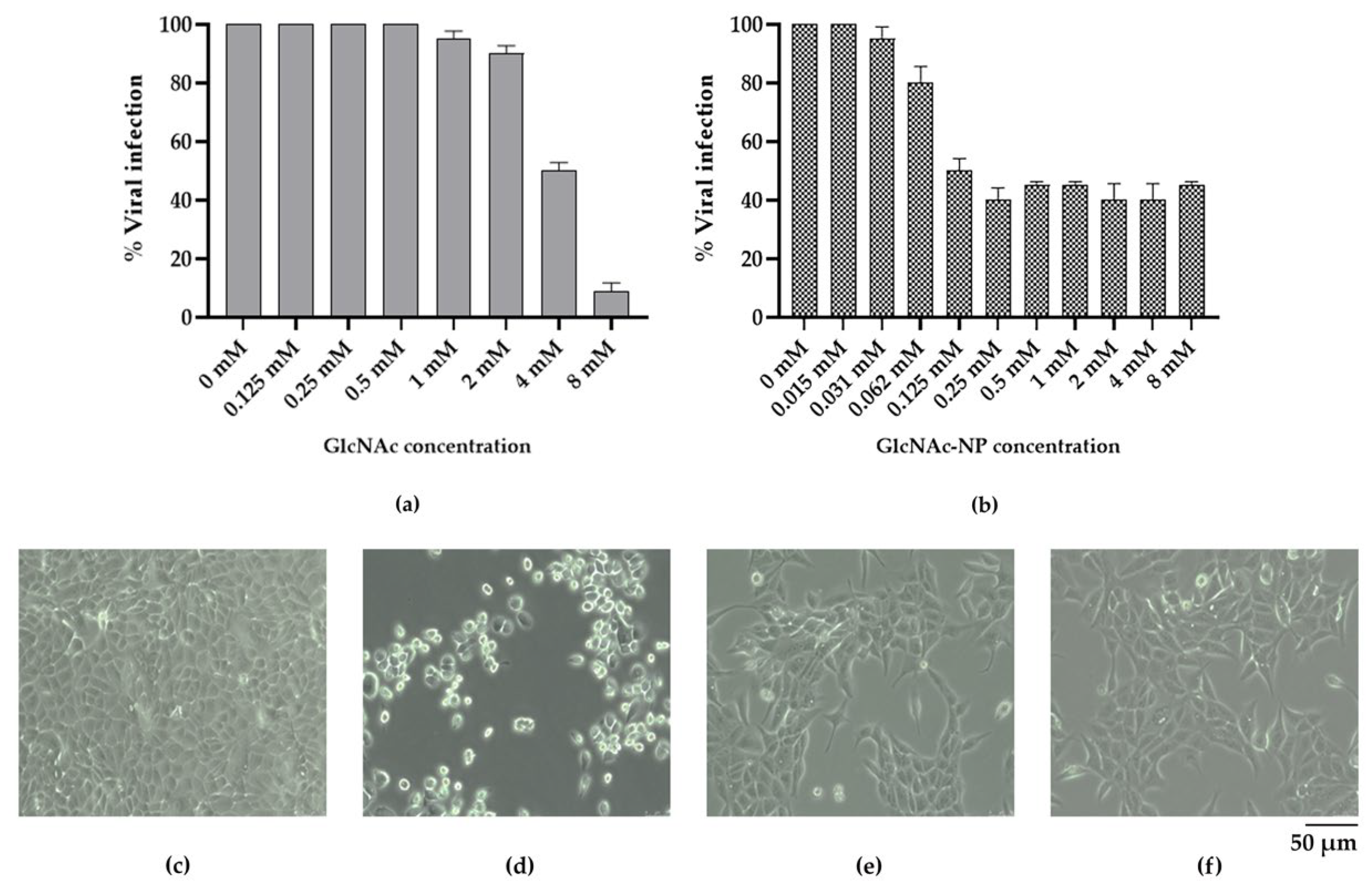
2.2.3. In Vitro Antiviral Activity of GlcNAc and GlcNAc-NPs towards Influenza A Virus (IAV) Infection
2.2.4. In Vitro Antiviral Activity of GlcNAc and GlcNAc-NPs towards Adenovirus 2 (Adv) Infection
2.2.5. Selectivity Index of Different GlcNAc Forms
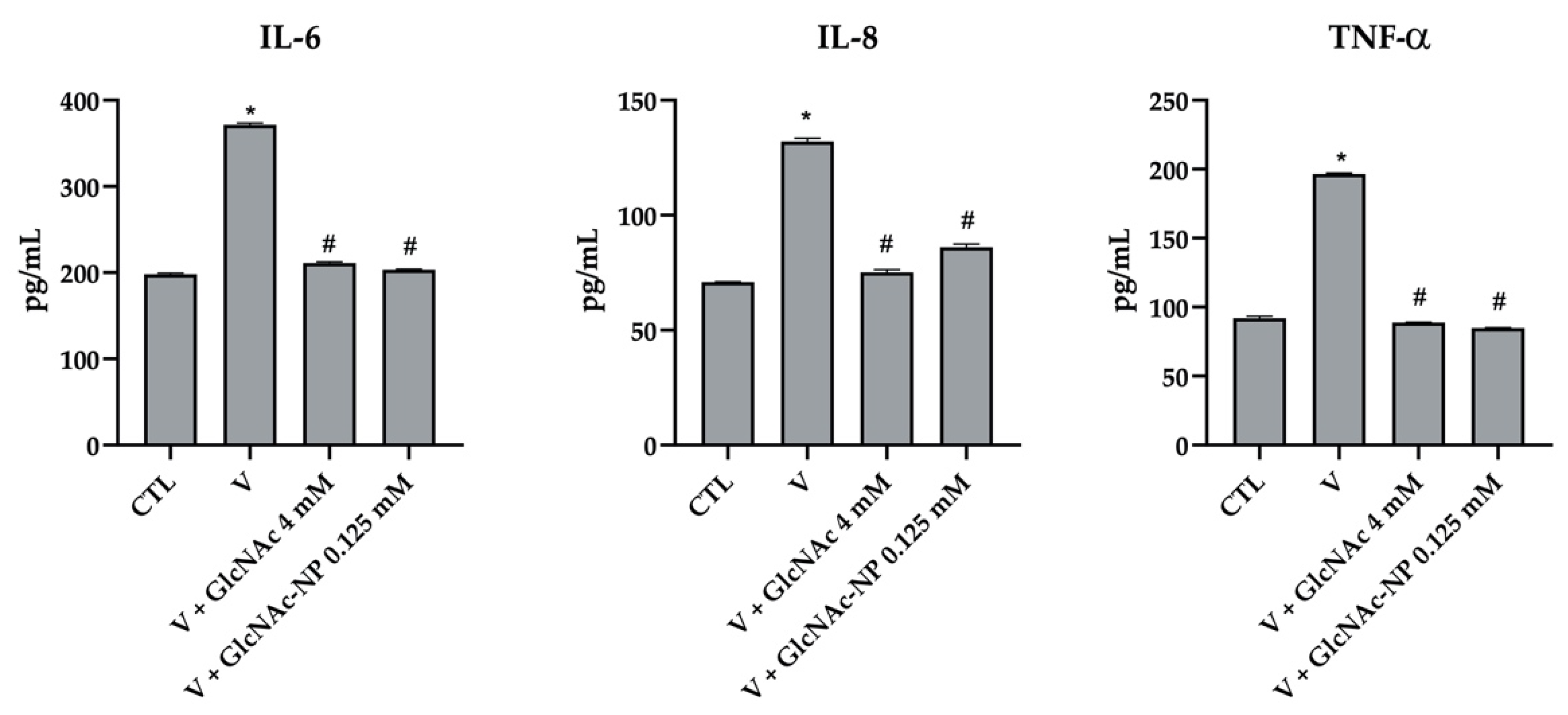
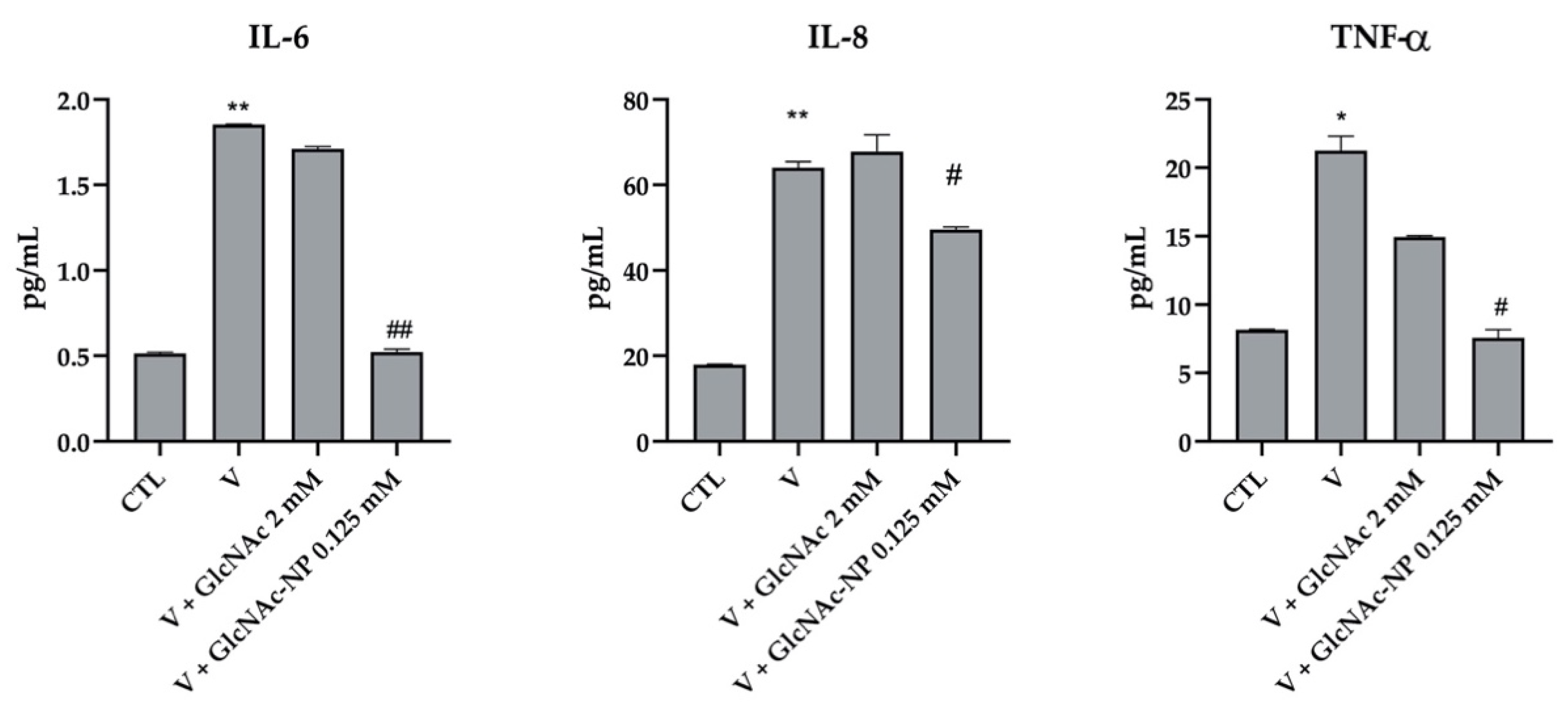
2.2.6. Cytokine Secretion Assay
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Viruses
4.3. Characterization of N-acetylglucosamine (GlcNAc)
4.3.1. Dynamic Light Scattering Characterization
4.3.2. Zeta Potential
4.3.3. Scanning Electron Microscopy/Energy Dispersive X-ray Spectroscopy (SEM/EDX) Characterization
4.4. Biological Assays
4.4.1. Cytotoxicity Assay
4.4.2. In Vitro Antiviral Activity of GlcNAc and GlcNAc-NPs towards IAV Infection
4.4.3. In Vitro Antiviral Activity of GlcNAc and GlcNAc-NPs towards Adv Infection
4.4.4. Cytokine Secretion Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waite, S.; Jeudy, J.; White, C.S. Acute Lung Infections in Normal and Immunocompromised Hosts. Radiol. Clin. North Am. 2006, 44, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Pattemore, P.K.; Jennings, L.C. Epidemiology of Respiratory Infections. In Pediatric Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2008; pp. 435–452. [Google Scholar]
- Asher, M.I.; Grant, C.C. Infections of the Upper Respiratory Tract. In Pediatric Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2008; pp. 453–480. [Google Scholar]
- Wat, D. The common cold: A review of the literature. Eur. J. Intern. Med. 2004, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.B.; Varkey, B. Viral infections in patients with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2008, 14, 89–94. [Google Scholar] [CrossRef]
- Kunz, A.N.; Ottolini, M. The Role of Adenovirus in Respiratory Tract Infections. Curr. Infect. Dis. Rep. 2010, 12, 81–87. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Lee, H.-J.; Piedra, P.A.; Choi, E.-H.; Park, K.-H.; Koh, Y.-Y.; Kim, W.-S. Lower Respiratory Tract Infections due to Adenovirus in Hospitalized Korean Children: Epidemiology, Clinical Features, and Prognosis. Clin. Infect. Dis. 2001, 32, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Kajon, A.E.; Lamson, D.M.; St George, K. Emergence and re-emergence of respiratory adenoviruses in the United States. Curr. Opin. Virol. 2019, 34, 63–69. [Google Scholar] [CrossRef]
- Kleinehr, J.; Wilden, J.J.; Boergeling, Y.; Ludwig, S.; Hrincius, E.R. Metabolic modifications by common respiratory viruses and their potential as new antiviral targets. Viruses 2021, 13, 2068. [Google Scholar] [CrossRef]
- Moon, S.M.; Choe, J.; Na, S.J.; Chung, C.R.; Suh, G.Y.; Jeon, K. Comparative Study on the Effect of Cidofovir Treatment for Severe Adenovirus Pneumonia. J. Intensive Care Med. 2021, 36, 1436–1442. [Google Scholar] [CrossRef]
- Legrand, F.; Berrebi, D.; Houhou, N.; Freymuth, F.; Faye, A.; Duval, M.; Mougenot, J.F.; Peuchmaur, M.; Vilmer, E. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 2001, 27, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza virus-related critical illness: Prevention, diagnosis, treatment. Crit. Care 2019, 23, 214. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef]
- Carraro, S.; Scheltema, N.; Bont, L.; Baraldi, E. Early-life origins of chronic respiratory diseases: Understanding and promoting healthy ageing. Eur. Respir. J. 2014, 44, 1682–1696. [Google Scholar] [CrossRef] [Green Version]
- Stocks, J.; Sonnappa, S. Early life influences on the development of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2013, 7, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Wertheim, J.O. A Glimpse Into the Origins of Genetic Diversity in the Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2020, 71, 721–722. [Google Scholar] [CrossRef] [Green Version]
- Hegele, R.G.; Hayashi, S.; Hogg, J.C.; Paré, P.D. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am. J. Respir. Crit. Care Med. 1995, 151, 1659–1664. [Google Scholar] [CrossRef]
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef]
- Sanders, C.J.; Doherty, P.C.; Thomas, P.G. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2011, 343, 13–21. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Viral Pneumonia in Older Adults. Clin. Infect. Dis. 2006, 42, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Rudan, I.; O’Brien, K.L.; Nair, H.; Liu, L.; Theodoratou, E.; Qazi, S.; Lukšić, I.; Walker, C.L.F.; Black, R.E.; Campbell, H. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health 2013, 3, 010401. [Google Scholar] [CrossRef] [PubMed]
- Kelleni, M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021, 133, 110982. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, G.; Scholtissek, C.; Rott, R. Inhibition of the Multiplication of Enveloped RNA-viruses by Glucosamine and 2-Deoxy-D-Glucose. J. Gen. Virol. 1972, 14, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C.; Rott, R.; Klenk, H.-D. Two different mechanisms of the inhibition of the multiplication of enveloped viruses by glucosamine. Virology 1975, 63, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, R.A.; Berghe, D.A. Vanden Inhibition of the Multiplication of Enveloped and Non-enveloped Viruses by Glucosamine. J. Pharm. Pharmacol. 2011, 40, 488–493. [Google Scholar] [CrossRef]
- Laine, R.A. The case for re-examining glycosylation inhibitors, mimetics, primers and glycosylation decoys as antivirals and anti-inflammatories in COVID19. Glycobiology 2020, 30, 763–767. [Google Scholar] [CrossRef]
- Hassan, A.E. An observational cohort study to assess N-acetylglucosamine for COVID-19 treatment in the inpatient setting. Ann. Med. Surg. 2021, 68, 102574. [Google Scholar] [CrossRef]
- Konopka, J.B. N-Acetylglucosamine Functions in Cell Signaling. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Osaki, T.; Wakuda, T.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Suppressive Effects of N-Acetyl-d-Glucosamine on Rheumatoid Arthritis Mouse Models. Inflammation 2012, 35, 1462–1465. [Google Scholar] [CrossRef]
- Richter, J.; Čapková, K.; Hříbalová, V.; Vannucci, L.; Danyi, I.; Malý, M.; Fišerová, A. Collagen-induced arthritis: Severity and immune response attenuation using multivalent N-acetyl glucosamine. Clin. Exp. Immunol. 2014, 177, 121–133. [Google Scholar] [CrossRef]
- Talent, J.M.; Gracy, R.W. Pilot study of oral polymeric N-acetyl-d-glucosamine as a potential treatment for patients with osteoarthritis. Clin. Ther. 1996, 18, 1184–1190. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- Tamai, Y.; Miyatake, K.; Okamoto, Y.; Takamori, Y.; Sakamoto, K.; Minami, S. Enhanced healing of cartilaginous injuries by N-acetyl-d-glucosamine and glucuronic acid. Carbohydr. Polym. 2003, 54, 251–262. [Google Scholar] [CrossRef]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-Acetylglucosamine: Production and Applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, S.; Heuschkel, R.; Tomlin, S.; Davies, S.E.; Edwards, S.; Walker-Smith, J.A.; French, I.; Murch, S.H. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 1567–1579. [Google Scholar] [CrossRef]
- Grigorian, A.; Araujo, L.; Naidu, N.N.; Place, D.J.; Choudhury, B.; Demetriou, M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 2011, 286, 40133–40141. [Google Scholar] [CrossRef] [Green Version]
- Sy, M.; Brandt, A.U.; Lee, S.-U.; Newton, B.L.; Pawling, J.; Golzar, A.; Rahman, A.M.A.; Yu, Z.; Cooper, G.; Scheel, M.; et al. N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J. Biol. Chem. 2020, 295, 17413–17424. [Google Scholar] [CrossRef]
- Lee, S.-U.; Li, C.F.; Mortales, C.-L.; Pawling, J.; Dennis, J.W.; Grigorian, A.; Demetriou, M. Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity. PLoS ONE 2019, 14, e0214253. [Google Scholar] [CrossRef] [Green Version]
- Persiani, S.; Roda, E.; Rovati, L.C.; Locatelli, M.; Giacovelli, G.; Roda, A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthr. Cartil. 2005, 13, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Persiani, S.; Rotini, R.; Trisolino, G.; Rovati, L.C.; Locatelli, M.; Paganini, D.; Antonioli, D.; Roda, A. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthr. Cartil. 2007, 15, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotto d’Abusco, A.; Cicione, C.; Calamia, V.; Negri, R.; Giordano, C.; Grigolo, B.; Politi, L.; Scandurra, R. Glucosamine and its N-acetyl-phenylalanine derivative prevent TNF-α-induced transcriptional activation in human chondrocytes. Clin. Exp. Rheumatol. 2007, 25, 847–852. [Google Scholar] [PubMed]
- Scotto d’Abusco, A.; Politi, L.; Giordano, C.; Scandurra, R. A peptidyl-glucosamine derivative affects IKKα kinase activity in human chondrocytes. Arthritis Res. Ther. 2010, 12, R18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EC. Commission recommendation 2022/3689 of 10 June 2022 on the definition of nanomaterial. Off. J. Eur. Union 2022, C229, 1–5. [Google Scholar]
- Galeas-Pena, M.; McLaughlin, N.; Pociask, D. The role of the innate immune system on pulmonary infections. Biol. Chem. 2019, 400, 443–456. [Google Scholar] [CrossRef]
- Shi, T.; McLean, K.; Campbell, H.; Nair, H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta–analysis. J. Glob. Health 2015, 5, 010408. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, B.; Xiao, N.; Zhang, F.; Zhao, X.; Liu, Q.; Xie, Z.; Gao, H.; Duan, Z.; Zhong, L. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J. Med. Virol. 2019, 91, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Arnott, A.; Semogas, I.; Falsey, A.R.; Openshaw, P.; Wedzicha, J.A.; Campbell, H.; Nair, H.; Nair, H.; Campbell, H.; et al. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2020, 222, S563–S569. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.-Y.; Kim, M.-R.; Park, J.-Y.; Choi, E.-H.; Lee, H.-J.; Yun, C.-K. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr. Infect. Dis. J. 1995, 14, 1054–1059. [Google Scholar] [CrossRef]
- Tavares, L.P.; Teixeira, M.M.; Garcia, C.C. The inflammatory response triggered by Influenza virus: A two edged sword. Inflamm. Res. 2017, 66, 283–302. [Google Scholar] [CrossRef]
- Michelini, F.M.; Bueno, C.A.; Areco, Y.B.; Alché, L.E. A synthetic stigmastane displays antiadenoviral activity and reduces the inflammatory response to viral infection. Antiviral Res. 2020, 183, 104879. [Google Scholar] [CrossRef]
- Simmons, C.; Farrar, J. Insights into Inflammation and Influenza. N. Engl. J. Med. 2008, 359, 1621–1623. [Google Scholar] [CrossRef] [Green Version]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J. Med. Virol. 2001, 64, 262–268. [Google Scholar] [CrossRef]
- Yamaya, M.; Nadine, L.K.; Ota, C.; Kubo, H.; Makiguchi, T.; Nagatomi, R.; Nishimura, H. Magnitude of influenza virus replication and cell damage is associated with interleukin-6 production in primary cultures of human tracheal epithelium. Respir. Physiol. Neurobiol. 2014, 202, 16–23. [Google Scholar] [CrossRef]
- Hayden, F.G.; Fritz, R.; Lobo, M.C.; Alvord, W.; Strober, W.; Straus, S.E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Investig. 1998, 101, 643–649. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Wu, T.-Z.; Liu, D.-P.; Shao, P.-L.; Chang, L.-Y.; Lu, C.-Y.; Lee, C.-Y.; Huang, F.-Y.; Huang, L.-M. Influenza Pandemics: Past, Present and Future. J. Formos. Med. Assoc. 2006, 105, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhou, W.; Huang, H.; Zhu, H.; Zhou, P.; Zhu, H.; Ju, D. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit. Care 2013, 17, R301. [Google Scholar] [CrossRef] [Green Version]
- Alcorn, M.J.; Booth, J.L.; Coggeshall, K.M.; Metcalf, J.P. Adenovirus Type 7 Induces Interleukin-8 Production via Activation of Extracellular Regulated Kinase 1/2. J. Virol. 2001, 75, 6450–6459. [Google Scholar] [CrossRef] [Green Version]
- Hierholzer, J.C. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 1992, 5, 262–274. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Y.; Wang, H.; Deng, J. Adenovirus 7 Induces Interlukin-6 Expression in Human Airway Epithelial Cells via p38/NF-κB Signaling Pathway. Front. Immunol. 2020, 11, 551413. [Google Scholar] [CrossRef] [PubMed]
- Abbondanzo, S.L.; English, C.K.; Kagan, E.; McPherson, R.A. Fatal adenovirus pneumonia in a newborn identified by electron microscopy and in situ hybridization. Arch. Pathol. Lab. Med. 1989, 113, 1349–1353. [Google Scholar] [PubMed]
- Moro, M.R.; Bonville, C.A.; Suryadevara, M.; Cummings, E.; Faddoul, D.; Kobayaa, H.; Branigan, P.J.; Domachowske, J.B. Clinical Features, Adenovirus Types, and Local Production of Inflammatory Mediators in Adenovirus Infections. Pediatr. Infect. Dis. J. 2009, 28, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Philpott, N.J.; Nociari, M.; Elkon, K.B.; Falck-Pedersen, E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-α induction pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 6200–6205. [Google Scholar] [CrossRef] [Green Version]
- Benihoud, K.; Salone, B.; Esselin, S.; Opolon, P.; Poli, V.; Di Giovine, M.; Perricaudet, M.; Saggio, I. The role of IL-6 in the inflammatory and humoral response to adenoviral vectors. J. Gene Med. 2000, 2, 194–203. [Google Scholar] [CrossRef]
- McElvaney, N.G.; Crystal, R.G. IL-6 release and airway administration of human CFTR cDNA adenovirus vector. Nat. Med. 1995, 1, 182–184. [Google Scholar] [CrossRef]
- Crystal, R.G.; McElvaney, N.G.; Rosenfeld, M.A.; Chu, C.-S.; Mastrangeli, A.; Hay, J.G.; Brody, S.L.; Jaffe, H.A.; Eissa, N.T.; Danel, C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 1994, 8, 42–51. [Google Scholar] [CrossRef]
- Baggiolini, M.; Dewald, B.; Moser, B. lnterleukin-8 and Related Chemotactic Cytokines—CXC and CC Chemokines. Adv. Immunol. 1993, 55, 97–179. [Google Scholar] [CrossRef]
- Nocker, R.E.T.; Schoonbrood, D.F.M.; van de Graaf, E.A.; Hack, E.; Lutter, R.; Jansen, H.M.; Out, T.A. lnterleukin-8 in Airway Inflammation in Patients with Asthma and Chronic Obstructive Pulmonary Disease. Int. Arch. Allergy Immunol. 1996, 109, 183–191. [Google Scholar] [CrossRef]
- Leland Booth, J.; Metcalf, J.P. Type-Specific Induction of Interleukin-8 by Adenovirus. Am. J. Respir. Cell Mol. Biol. 1999, 21, 521–527. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Shokouhi Targhi, H.; Mehrbod, P.; Fotouhi, F.; Amininasab, M. In vitro anti-influenza assessment of anionic compounds ascorbate, acetate and citrate. Virol. J. 2022, 19, 88. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sarkar, M.C.; Chatterjee, T.; Sharma Dey, R.; Bag, P.; Chakraborti, S.; Khan, M.T.H. Recent advancements for the evaluation of anti-viral activities of natural products. N. Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef]
- Mehrbod, P.; Abdalla, M.A.; Njoya, E.M.; Ahmed, A.S.; Fotouhi, F.; Farahmand, B.; Gado, D.A.; Tabatabaian, M.; Fasanmi, O.G.; Eloff, J.N.; et al. South African medicinal plant extracts active against influenza A virus. BMC Complement. Altern. Med. 2018, 18, 112. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO 2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Superti, F.; Marchetti, M.; Rapetti Mogol, G. Inactivation of influenza virus by a blend of essential plant oil vapour and aerosol. J. Biol. Regul. Homeost. Agents 2021, 35, 1667–1675. [Google Scholar] [CrossRef]








| GlcNAc Form | CC50 (mM) | EC50 (mM) | SI |
|---|---|---|---|
| Bulk | >16 | 4 | >4 |
| NPs | >16 | 0.125 | >128 |
| GlcNAc Form | CC50 (mM) | EC50 (mM) | SI |
|---|---|---|---|
| Bulk | 8 | ND | ND |
| NPs | >16 | 0.125 | >128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; De Berardis, B.; Bigioni, I.; Mariano, A.; Superti, F.; Scotto d’Abusco, A. In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections. Int. J. Mol. Sci. 2023, 24, 5129. https://doi.org/10.3390/ijms24065129
Marchetti M, De Berardis B, Bigioni I, Mariano A, Superti F, Scotto d’Abusco A. In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections. International Journal of Molecular Sciences. 2023; 24(6):5129. https://doi.org/10.3390/ijms24065129
Chicago/Turabian StyleMarchetti, Magda, Barbara De Berardis, Irene Bigioni, Alessia Mariano, Fabiana Superti, and Anna Scotto d’Abusco. 2023. "In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections" International Journal of Molecular Sciences 24, no. 6: 5129. https://doi.org/10.3390/ijms24065129





