Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells
Abstract
:1. Introduction
2. Fer and FerT: Newly Established Links between Sperm and Cancer Cells
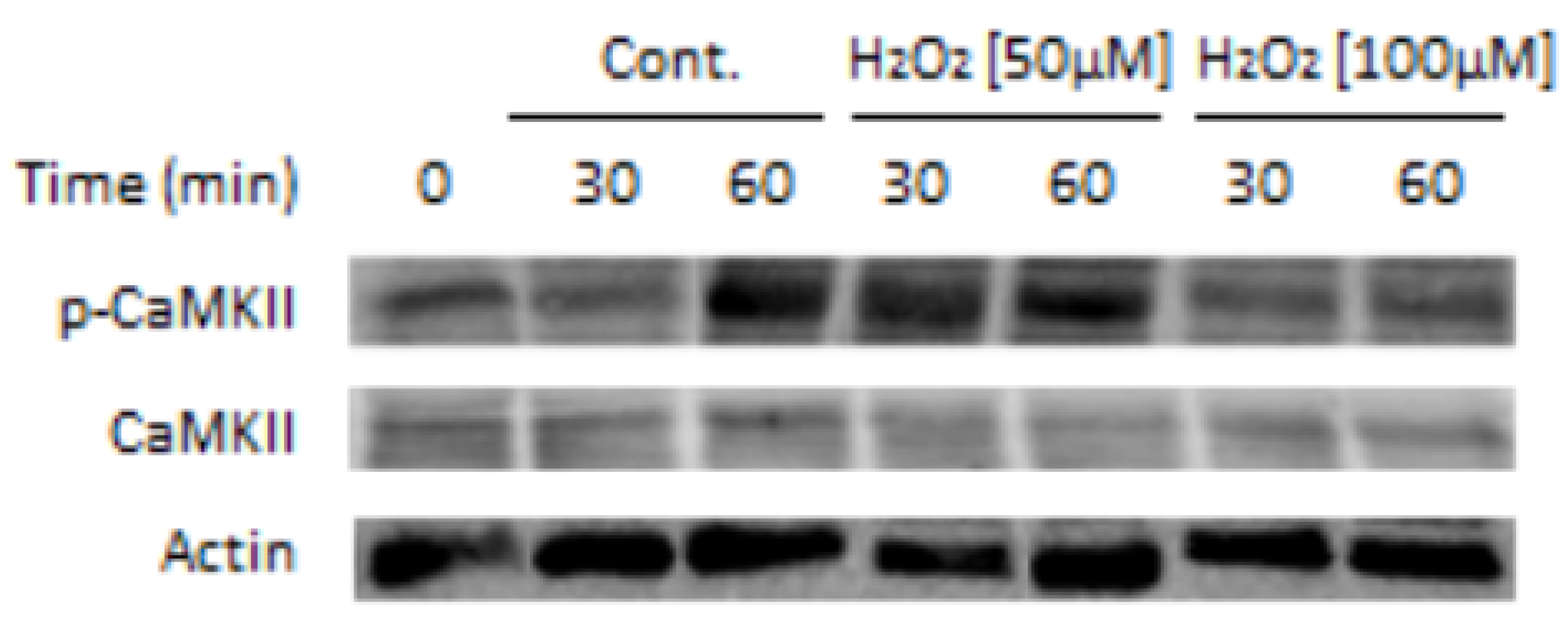
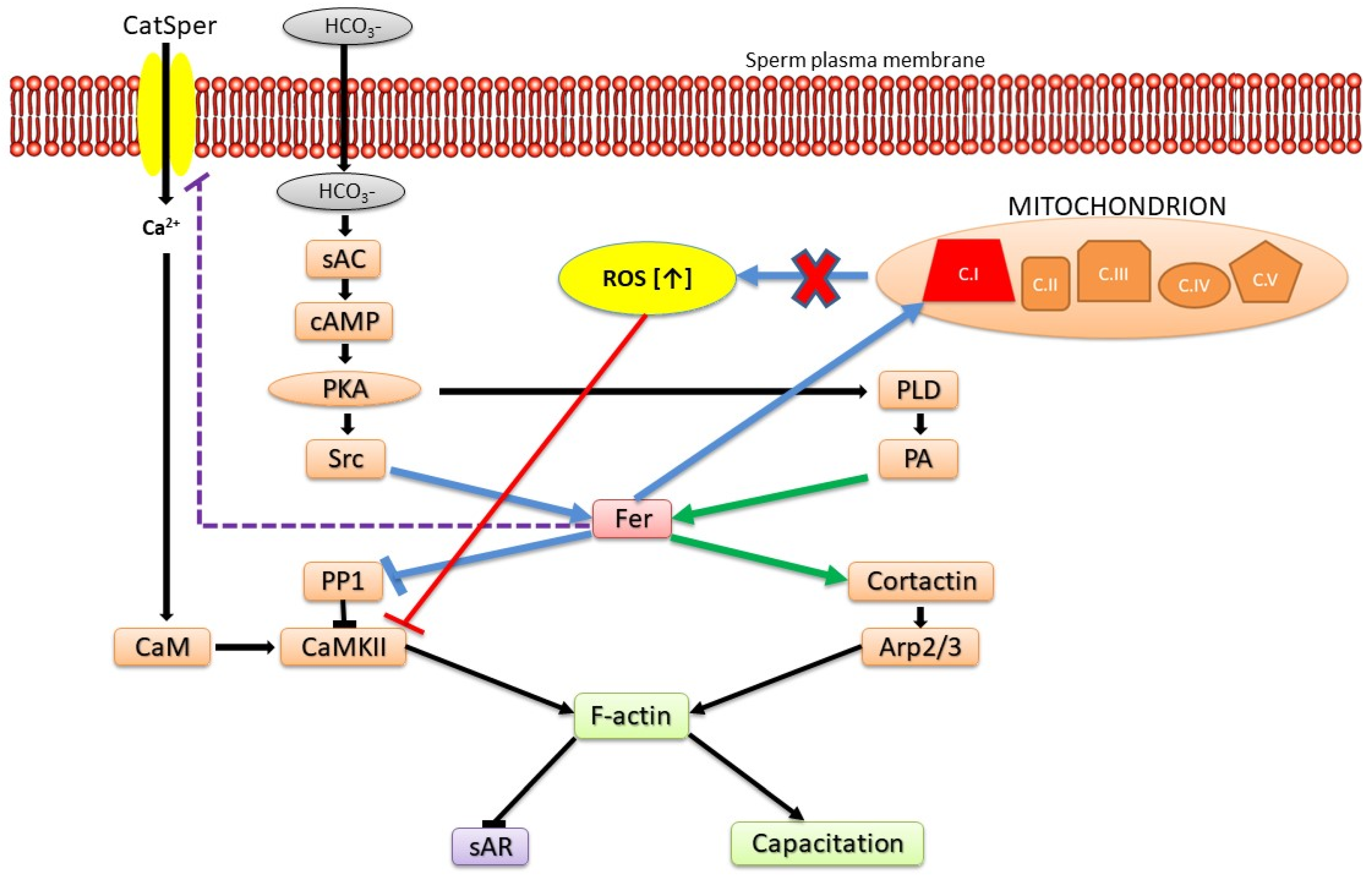
3. Roles of Fer in Modulating Sperm Functions
4. Oxidative Stress and Male Infertility: Current Knowledge of Pathophysiology and the Role of Antioxidant Therapy in Disease Management
Funding
Conflicts of Interest
References
- Greer, P. Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell Biol. 2002, 3, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Bern, O.; Tennenbaum, T.; Nir, U. Cell cycle-dependent nuclear accumulation of the p94fer tyrosine kinase is regulated by its NH2 terminus and is affected by kinase domain integrity and ATP binding. Cell Growth Differ. 1999, 10, 113–129. [Google Scholar] [PubMed]
- Heath, R.J.; Insall, R.H. F-BAR domains: Multifunctional regulators of membrane curvature. J. Cell. Sci. 2008, 121, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Stanicka, J.; Rieger, L.; O’Shea, S.; Cox, O.; Coleman, M.; O’Flanagan, C.; Addario, B.; McCabe, N.; Kennedy, R.; O’Connor, R. FES-related tyrosine kinase activates the insulin-like growth factor-1 receptor at sites of cell adhesion. Oncogene 2018, 37, 3131–3150. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, E.; Hikri, E.; Elkis, Y.; Cohen, O.; Segal, A.; Makovski, A.; Varvak, A.; Shpungin, S.; Nir, U. Oncogenic properties of a spermatogenic meiotic variant of fer kinase expressed in somatic cells. Cancer Res. 2014, 74, 6474–6485. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; Arulanantham, S.; Barr, K.; Cepeda, M.; Parkins, K.M.; Hamilton, A.M.; Johnston, D.; Penuela, S.; Hess, D.A.; Ronald, J.A.; et al. Targeting FER Kinase Inhibits Melanoma Growth and Metastasis. Cancers 2019, 11, 419. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, S.; Chen, Y.; Xiong, X.; Li, X.; Tonks, N.K.; Fan, G. Spatial regulation of signaling by the coordinated action of the protein tyrosine kinases MET and FER. Cell. Signal. 2018, 50, 100–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Zhu, Q.; Zhang, J.; Chen, S.; Wang, Y.; Cao, J.; Chen, L.; Hou, L.; Zhao, X.; et al. FER-mediated phosphorylation and PIK3R2 recruitment on IRS4 promotes AKT activation and tumorigenesis in ovarian cancer cells. eLife 2022, 11, e76183. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Vermeulen, J.F.; Ercan, C.; Houthuijzen, J.M.; Saig, F.A.; Vlug, E.J.; van der Wall, E.; van Diest, P.J.; Vooijs, M.; Derksen, P.W. FER kinase promotes breast cancer metastasis by regulating alpha6- and beta1-integrin-dependent cell adhesion and anoikis resistance. Oncogene 2013, 32, 5582–5592. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Z.; Liu, X.; Liu, Z.; Miao, X.; Li, D.; Zou, Q.; Yuan, Y. Clinicopathological significances of Feline sarcoma-related protein and beta2-adrenoceptor expression in pancreatic ductal adenocarcinomas. Int. J. Clin. Exp. Pathol. 2019, 12, 3390–3398. [Google Scholar]
- Chen, Z.H.; Yan, P.Y.; Tao, J.; Liu, S.; Tseng, G.; Nalesnik, M.; Hamilton, R.; Bhargava, R.; Nelson, J.B.; Pennathur, A.; et al. MAN2A1-FER Fusion Gene Is Expressed by Human Liver and Other Tumor Types and Has Oncogenic Activity in Mice. Gastroenterology 2017, 153, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Debackere, K.; van der Krogt, J.A.; Tousseyn, T.; Ferreiro, J.A.; Van Roosbroeck, K.; Marcelis, L.; Graux, C.; Dierickx, D.; Ameye, G.; Vandenberghe, P.; et al. FER and FES tyrosine kinase fusions in follicular T-cell lymphoma. Blood 2020, 135, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Drieux, F.; Ruminy, P.; Sater, V.; Marchand, V.; Fataccioli, V.; Lanic, M.D.; Viennot, M.; Viailly, P.J.; Sako, N.; Robe, C.; et al. Detection of Gene Fusion Transcripts in Peripheral T-Cell Lymphoma Using a Multiplexed Targeted Sequencing Assay. J. Mol. Diagn. 2021, 23, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, Z.; Xu, J.; Mei, X.; Bi, M.; Jiang, F.; Yu, D.; Zhong, C. Role of feline sarcoma-related protein in the viability and apoptosis of bladder cancer cells. Mol. Med. Rep. 2019, 19, 5219–5226. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, S.; Gao, Y.; Greer, P.A.; Tonks, N.K. HGF-independent regulation of MET and GAB1 by nonreceptor tyrosine kinase FER potentiates metastasis in ovarian cancer. Genes Dev. 2016, 30, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Fan, G. FER mediated HGF-independent regulation of HGFR/MET activates RAC1-PAK1 pathway to potentiate metastasis in ovarian cancer. Small GTPases 2020, 11, 155–159. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.J.; Ge, W.L.; Chen, L.; Yuan, H.; Meng, L.D.; Huang, X.M.; Shen, P.; Miao, Y.; Jiang, K.R. YY1 inhibits the migration and invasion of pancreatic ductal adenocarcinoma by downregulating the FER/STAT3/MMP2 signaling pathway. Cancer Lett. 2019, 463, 37–49. [Google Scholar] [CrossRef]
- Sluimer, L.M.; Bullock, E.; Ratze, M.A.K.; Enserink, L.; Overbeeke, C.; Hornsveld, M.; Brunton, V.G.; Derksen, P.W.B.; Tavares, S. SKOR1 mediates FER kinase-dependent invasive growth of breast cancer cells. J. Cell Sci. 2023, 136, jcs260243. [Google Scholar] [CrossRef]
- Fischman, K.; Edman, J.C.; Shackleford, G.M.; Turner, J.A.; Rutter, W.J.; Nir, U. A murine fer testis-specific transcript (ferT) encodes a truncated Fer protein. Mol. Cell Biol. 1990, 10, 146–153. [Google Scholar]
- Hazan, B.; Bern, O.; Carmel, M.; Lejbkowicz, F.; Goldstein, R.S.; Nir, U. ferT encodes a meiosis-specific nuclear tyrosine kinase. Cell Growth Differ. 1993, 4, 443–449. [Google Scholar]
- Makovski, A.; Yaffe, E.; Shpungin, S.; Nir, U. Intronic promoter drives the BORIS-regulated expression of FerT in colon carcinoma cells. J. Biol. Chem. 2012, 287, 6100–6112. [Google Scholar] [CrossRef] [PubMed]
- Kierszenbaum, A.L.; Rivkin, E.; Tres, L.L. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis 2011, 1, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Makovski, A.; Yaffe, E.; Shpungin, S.; Nir, U. Down-regulation of Fer induces ROS levels accompanied by ATM and p53 activation in colon carcinoma cells. Cell Signal 2012, 24, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef]
- Elkis, Y.; Cohen, M.; Yaffe, E.; Satmary-Tusk, S.; Feldman, T.; Hikri, E.; Nyska, A.; Feiglin, A.; Ofran, Y.; Shpungin, S.; et al. A novel Fer/FerT targeting compound selectively evokes metabolic stress and necrotic death in malignant cells. Nat. Commun. 2017, 8, 940. [Google Scholar] [CrossRef] [PubMed]
- Grinshtain, E.; Shpungin, S.; Baum, M.; Nir, U.; Breitbart, H. The Fer tyrosine kinase protects sperm from spontaneous acrosome reaction. Dev. Biol. 2022, 487, 24–33. [Google Scholar] [CrossRef]
- Breitbart, H.; Rubinstein, S.; Nass-Arden, L. The role of calcium and Ca2+-ATPase in maintaining motility in ram spermatozoa. J. Biol. Chem. 1985, 260, 11548–11553. [Google Scholar] [CrossRef]
- Mason, J.A.; Hagel, K.R.; Hawk, M.A.; Schafer, Z.T. Metabolism during ECM Detachment: Achilles Heel of Cancer Cells? Trends Cancer 2017, 3, 475–481. [Google Scholar] [CrossRef]
- Jin, L.; Alesi, G.N.; Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 2016, 35, 3619–3625. [Google Scholar] [CrossRef]
- Celia-Terrassa, T.; Kang, Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016, 30, 892–908. [Google Scholar] [CrossRef]
- Mehazri, L.; Shpungin, S.; Bel, S.; Nir, U. Loss of Fer Jeopardizes Metabolic Plasticity and Mitochondrial Homeostasis in Lung and Breast Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 3387. [Google Scholar] [CrossRef]
- Pan, D.A.; Hardie, D.G. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem. J. 2002, 367, 179–186. [Google Scholar] [CrossRef]
- Yi, X.; Long, X.; Liu, C. Activating autophagy and ferroptosis of 3-Chloropropane-1,2-diol induces injury of human umbilical vein endothelial cells via AMPK/mTOR/ULK1. Mol. Med. Rep. 2023, 27, 76. [Google Scholar] [CrossRef]
- Schuller, M.; Ahel, I. Beyond protein modification: The rise of non-canonical ADP-ribosylation. Biochem. J. 2022, 479, 463–477. [Google Scholar] [CrossRef]
- Bai, P.; Canto, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012, 16, 290–295. [Google Scholar] [CrossRef]
- Szekvolgyi, L.; Ohta, K.; Nicolas, A. Initiation of meiotic homologous recombination: Flexibility, impact of histone modifications, and chromatin remodeling. Cold Spring Harb. Perspect. Biol. 2015, 7, a016527. [Google Scholar] [CrossRef]
- Celik-Ozenci, C.; Tasatargil, A. Role of poly(ADP-ribose) polymerases in male reproduction. Spermatogenesis 2013, 3, e24194. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltran, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Megnagi, B.; Ickowicz, D.; Breitbart, H. Regulation of sperm motility by PIP2(4,5) and actin polymerization. Dev. Biol. 2013, 381, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, O.; Breitbart, H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016, 415, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef]
- Wiser, A.; Sachar, S.; Ghetler, Y.; Shulman, A.; Breitbart, H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: Preliminary results. Andrologia 2014, 46, 313–315. [Google Scholar] [CrossRef]
- Ackermann, F.; Zitranski, N.; Borth, H.; Buech, T.; Gudermann, T.; Boekhoff, I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J. Cell Sci. 2009, 122, 4547–4557. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Role and regulation of sperm gelsolin prior to fertilization. J. Biol. Chem. 2010, 285, 39702–39709. [Google Scholar] [CrossRef]
- Dahan, T.; Breitbart, H. Involvement of metabolic pathway in the sperm spontaneous acrosome reaction. Theriogenology 2022, 192, 38–44. [Google Scholar] [CrossRef]
- Endo, D.; Kon, S.; Sato, T.; Toyama, F.; Katsura, Y.; Nakauchi, Y.; Takayama-Watanabe, E.; Watanabe, A. NMDA-type glutamate receptors mediate the acrosome reaction and motility initiation in newt sperm. Mol. Reprod. Dev. 2019, 86, 1106–1115. [Google Scholar] [CrossRef]
- Xiao, Y.; Wen, Z.Z.; Wu, B.; Zhu, H.X.; Zhang, A.Z.; Li, J.Y.; Gao, J.G. Deletion of Aldh4a1 Leads to Impaired Sperm Maturation in Mice. Mol. Biol. 2022, 56, 585–594. [Google Scholar] [CrossRef]
- Visconti, P.E.; Galantino-Homer, H.; Ning, X.; Moore, G.D.; Valenzuela, J.P.; Jorgez, C.J.; Alvarez, J.G.; Kopf, G.S. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 1999, 274, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Rotfeld, H.; Hillman, P.; Ickowicz, D.; Breitbart, H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction 2014, 147, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Tsirulnikov, E.; Huta, Y.; Breitbart, H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology 2019, 128, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshana, C.; Setiawan, R.; Tajima, A.; Asano, A. Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus). PLoS ONE 2020, 15, e0241181. [Google Scholar] [CrossRef]
- Kon, S.; Takaku, A.; Toyama, F.; Takayama-Watanabe, E.; Watanabe, A. Acrosome reaction-inducing substance triggers two different pathways of sperm intracellular signaling in newt fertilization. Int. J. Dev. Biol. 2019, 63, 589–595. [Google Scholar] [CrossRef]
- Choi, Y.J.; Uhm, S.J.; Song, S.J.; Song, H.; Park, J.K.; Kim, T.; Park, C.; Kim, J.H. Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: Linking proteome analysis. J. Reprod. Dev. 2008, 54, 68–83. [Google Scholar] [CrossRef]
- Itach, S.B.; Finklestein, M.; Etkovitz, N.; Breitbart, H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev. Biol. 2012, 362, 154–161. [Google Scholar] [CrossRef]
- Pasder, O.; Shpungin, S.; Salem, Y.; Makovsky, A.; Vilchick, S.; Michaeli, S.; Malovani, H.; Nir, U. Downregulation of Fer induces PP1 activation and cell-cycle arrest in malignant cells. Oncogene 2006, 25, 4194–4206. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006, 40, 1045–1055. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: Superoxide dismutase as a major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Wiser, A.; Ickowicz, D.; Lubart, R.; Shulman, A.; Breitbart, H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum. Reprod. 2011, 26, 2274–2282. [Google Scholar] [CrossRef]
- Orta, G.; de la Vega-Beltran, J.L.; Martin-Hidalgo, D.; Santi, C.M.; Visconti, P.E.; Darszon, A. CatSper channels are regulated by protein kinase A. J. Biol. Chem. 2018, 293, 16830–16841. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Young, S.; Krenz, H.; Tuttelmann, F.; Ropke, A.; Krallmann, C.; Kliesch, S.; Zeng, X.H.; Brenker, C.; Strunker, T. The Ca2+ channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J. Biol. Chem. 2020, 295, 13181–13193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nir, U.; Grinshtain, E.; Breitbart, H. Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5256. https://doi.org/10.3390/ijms24065256
Nir U, Grinshtain E, Breitbart H. Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells. International Journal of Molecular Sciences. 2023; 24(6):5256. https://doi.org/10.3390/ijms24065256
Chicago/Turabian StyleNir, Uri, Elina Grinshtain, and Haim Breitbart. 2023. "Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells" International Journal of Molecular Sciences 24, no. 6: 5256. https://doi.org/10.3390/ijms24065256




