Potential Anti-Candida albicans Mechanism of Trichoderma Acid from Trichoderma spirale
Abstract
:1. Introduction
2. Results
2.1. Anti-C. albicans Activity of TA
2.2. Cytotoxicity Assay of TA
2.3. Laser Confocal and Scanning Electron Microscopy and Observation of TA-Treated C. albicans
2.4. Detection of Mitochondrial Membrane Potential and ROS
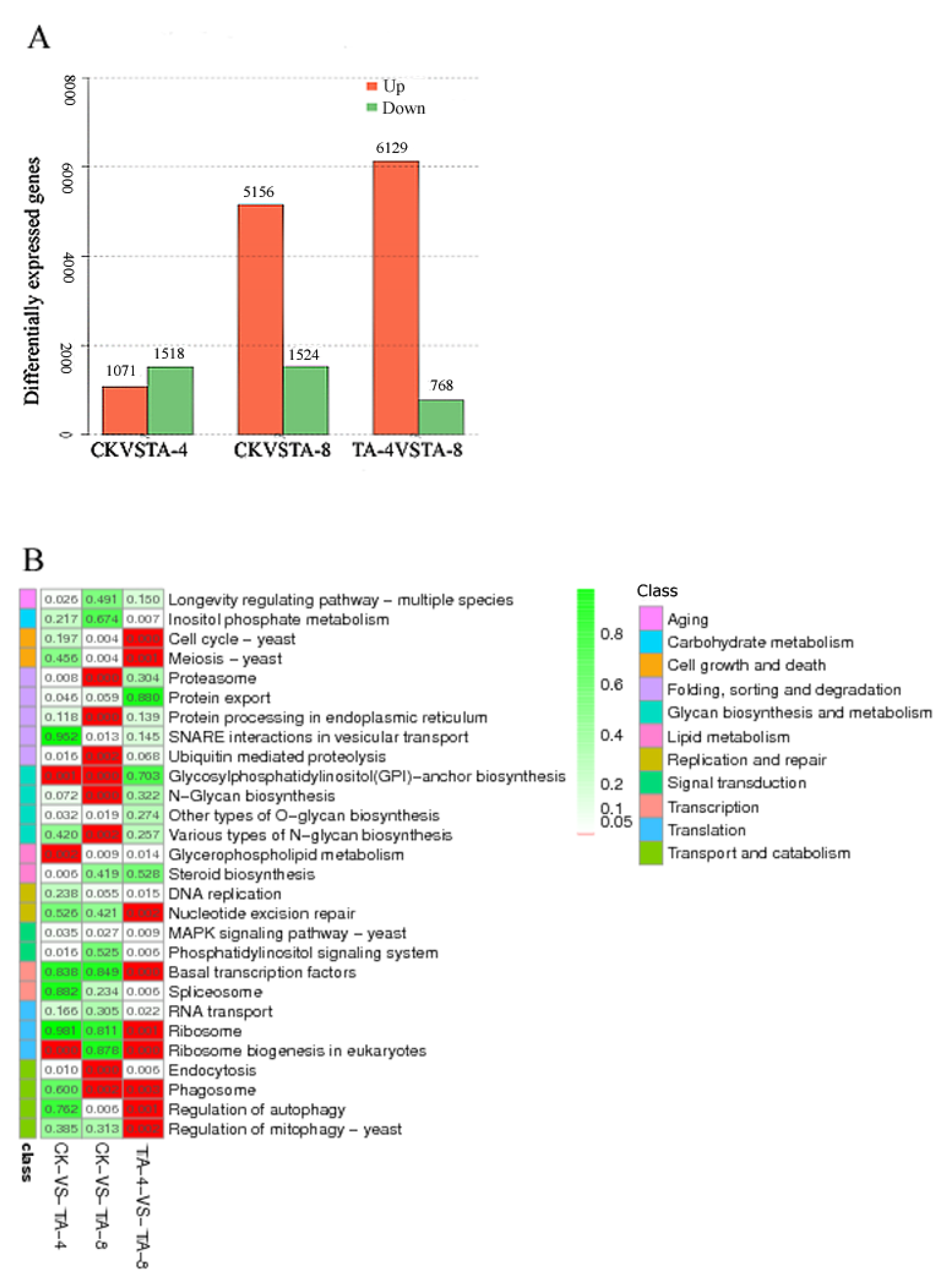
2.5. Transcriptome Sequencing of TA-Treated C. albicans
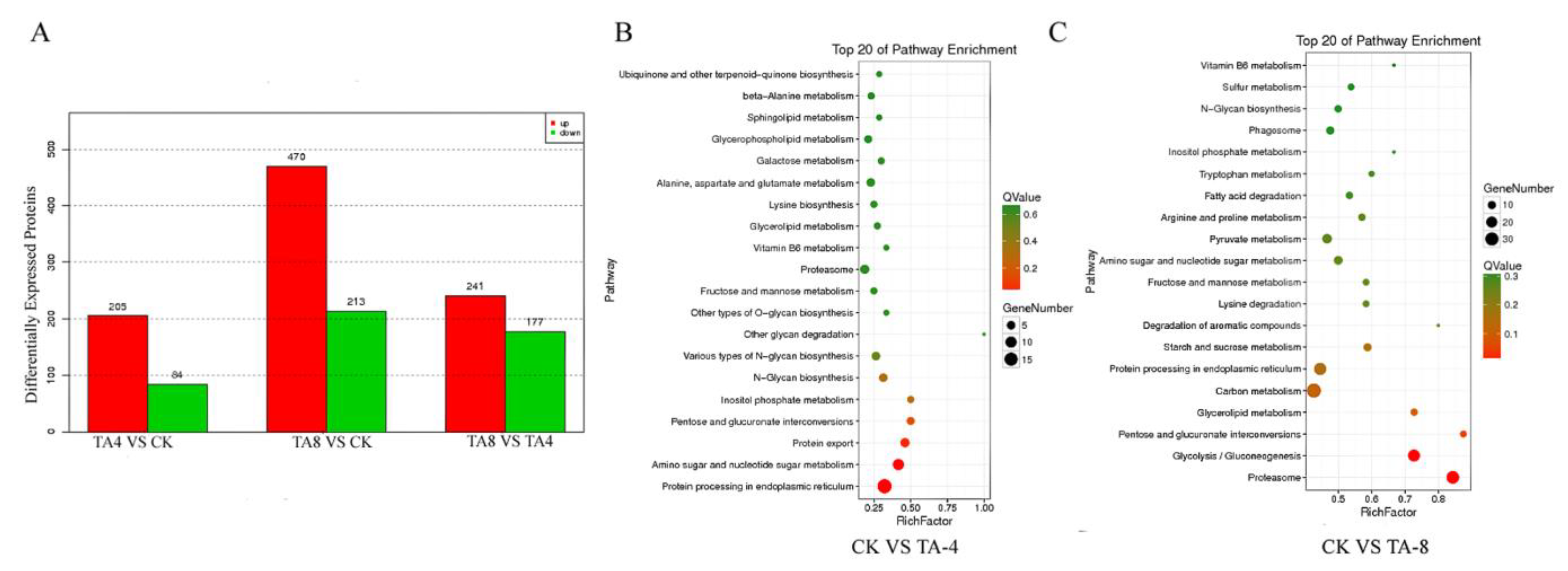
2.6. Proteomic Analysis of TA-Treated C. albicans
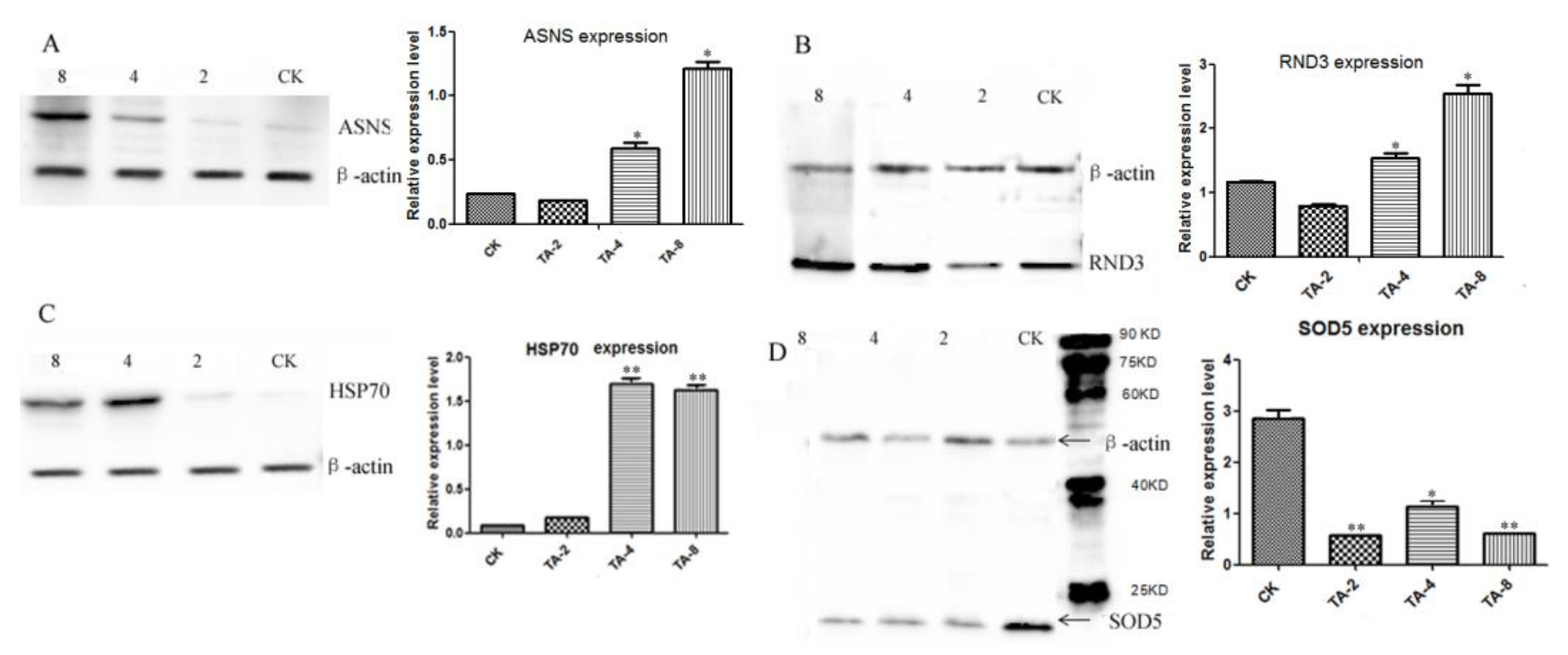
2.7. Validation of Significantly Differentially Expressed Proteins by Western Blot Analysis
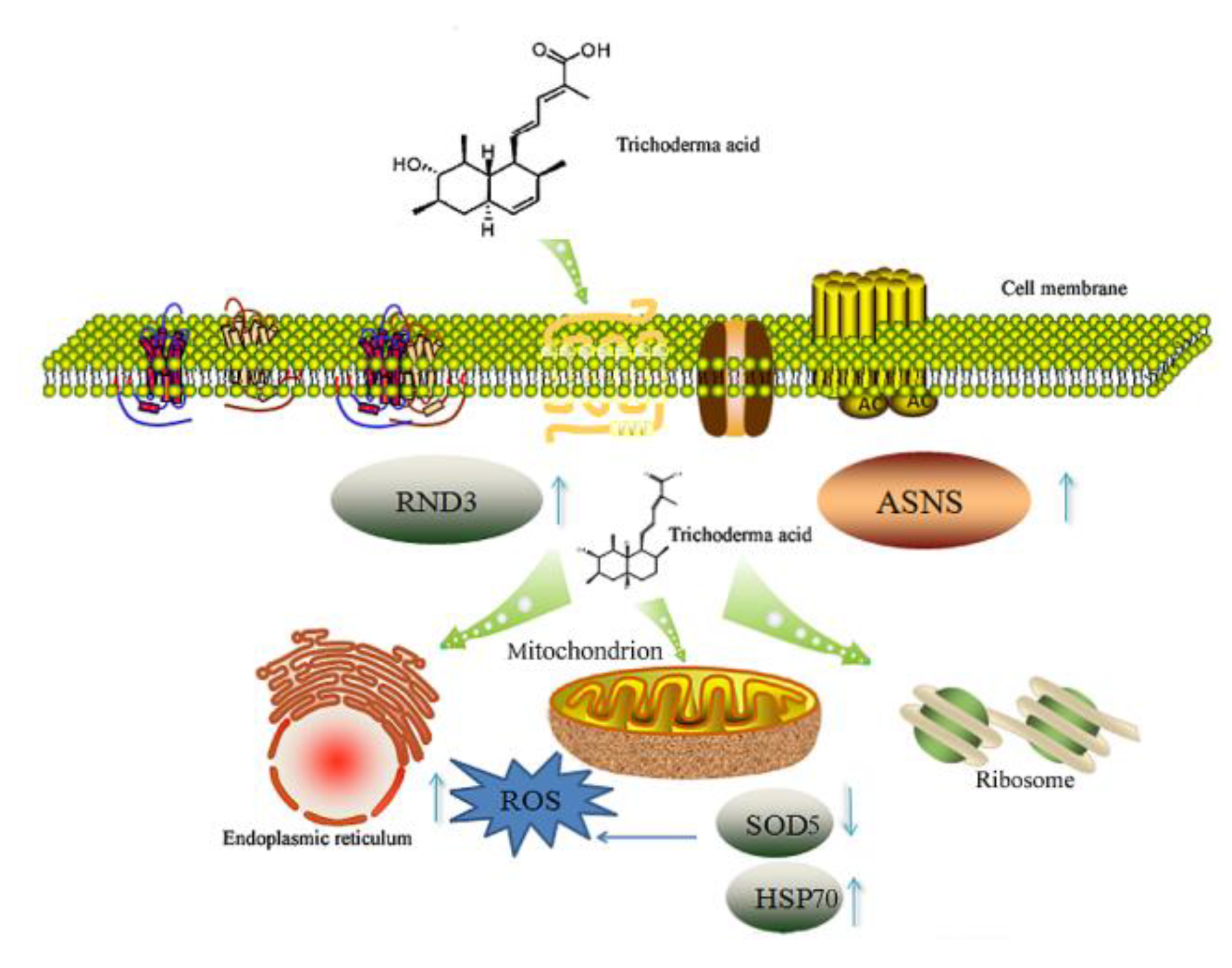
3. Discussion
4. Materials and Methods
4.1. The Strains, Cells and Compounds
4.2. Anti-C. albicans Activity Assay
4.3. Cytotoxicity Assay
4.4. The Flow Cytometry, Laser Confocal Microscopy Analysis and ROS Detection
4.5. The Transcriptomic Analysis of TA-Treated C. albicans
4.6. The Differentially Expressed Unigenes in TA-Treated C. albicans
4.7. The Proteomic Assay of TA-Treated C. albicans
4.8. Protein Identification and Data Analysis
4.9. The Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Roman, E.; Correia, I.; Salazin, A.; Fradin, C.; Jouault, T.; Poulain, D.; Liu, F.T.; Pla, J. The Cek1-mediated MAP kinase pathway regulates exposure of α-1,2 and β-1,2-mannosides in the cell wall of Candida albicans modulating immune recognition. Virulence 2016, 7, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.; Lee, H.; Jayaraja, S.; Suram, S.; Murphy, R.C.; Leslie, C.C. Prostaglandins from cytosolic phospholipase A2α/cyclooxygenase-1 pathway and mitogen-activated protein kinases regulate gene expression in Candida albicans-infected macrophages. J. Biol. Chem. 2016, 291, 7070–7086. [Google Scholar] [CrossRef] [Green Version]
- Radfan, A.; Santosh, K.; Santhakumari, B.; Rajendra, P.; Mahesh, K.; Gajanan, Z. Serum responsive proteome reveals correlation between oxidative phosphorylation and morphogenesis in Candida albicans ATCC10231. J. Proteom. 2018, 185, 25–38. [Google Scholar]
- Moudgal, V.; Sobel, J. Antifungals to treat Candida albicans. Expert Opin. Pharmacother. 2018, 11, 2037–2048. [Google Scholar] [CrossRef]
- Pierce, C.G.; Lopez-Ribot, J.L. Candidasis drug discovery and development: New approaches targeting virulence for discovery and identifying new drugs. Expert Opin. Drug Dis. 2013, 8, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Ellepola, A.N.; Samaranayake, L.P.; Khan, Z.U. Extracellular phospholipase production of oral Candida albicans isolates from smokers, diabetics, asthmatics, denture wearers and healthy individuals following brief exposure to polyene, echinocandin and azole antimycotics. Braz. J. Microbiol. 2016, 47, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Francois, I.E.J.A.; Cammue, B.P.A.; Borgers, M.; Ausma, J.; Dispersyn, G.D.; Thevissen, K. Azoles: Mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect. Agents Med. Chem. 2006, 5, 3–13. [Google Scholar]
- Mahmoud, G.; D’Angelo, M. Anidulafungin: A potent antifungal that targets Candida and Aspergillus. Infect. Dis. Clin. Pract. 2005, 13, 158–164. [Google Scholar]
- Serrano-Wu, M.; Du, X.; Balasubramanian, N.; Laurent-St, D.R. Novel Thio-Derivatives of Sordarin as Antifungal Agents. PCT International Application, WO2002022567. 21 March 2002. [Google Scholar]
- Warn, P.A.; Sharp, A.; Morrissey, G.; Denning, D.W. Activity of aminocandin (IP960) compared with amphotericin B and fluconazole in a neutropenic murine model of disseminated infection caused by afluconazole-resistant strain of Candida tropicalis. J. Antimicrob. Chem. 2005, 56, 590–593. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.Q.; Wu, X.Z.; Cheng, A.X.; Zhang, L.; Ji, M.; Lou, H.X. Retigeric acid B exerts antifungal effect through enhanced reactive oxygen species and decreased cAMP. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.J.; Xue, T.J.; Wu, L.; Zhu, C.L.; Zhou, Z.T. In vitro antifungal activity of dictamnine against Candida albicans. J. Clin. Stomatol. 2011, 27, 654–657. [Google Scholar]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Lu, Z.Q.; Wang, Y.Z.; Zhang, M.; Wang, X.Y.; Tang, X.D.; Peng, X.; Zeng, H. Nerol triggers mitochondrial dysfunction and disruption via elevation of Ca2+ and ROS in Candida albicans. Int. J. Biochem. Cell Biol. 2017, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef]
- Li, D.L.; Chen, Y.C.; Li, Y.Y.; Tao, M.H.; Zhang, W.M. Isolation and identification of anti-tumor constituents from an endophytic fungus of Baimuxiang (Aquilaria sinensis). J. Microbiol. 2010, 30, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Yamada, N. Octahydronaphthalene Derivative and Medicine. PCT International Application, WO200507-856 A1. 24 December 2004. [Google Scholar]
- Fierro, I.M.; Barja-Fidalgo, C.; Cunha, F.Q.; Ferreira, S.H. The involvement of nitric oxide in the anti-Candida albicans activity of rat neutrophils. Immunology 1996, 89, 295–300. [Google Scholar] [CrossRef]
- Vazquez-Torres, A.; Jones-Carson, J.; Balish, E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect. Immun. 1996, 64, 3127–3133. [Google Scholar] [CrossRef] [Green Version]
- Madariaga-Venegas, F.; Fernández-Soto, R.; Duarte, L.F.; Suarez, N.; Delgadillo, D.; Jara, J.A.; Fernández-Ramires, R.; Urzua, B.; Molina-Berrios, B. A characterization of a novel antibiofilm effect of nitric oxide-releasing aspirin (NCX-4040) on Candida albicans isolates from denture stomatitis patients. PLoS ONE 2018, 12, e0176755. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.H.; Zhao, S.S.; Liu, D.Y.; Wang, Z.; Niu, L.L.; Hou, L.H.; Wang, C.L. ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chem.-Biol. Interact. 2011, 190, 16–27. [Google Scholar] [CrossRef]
- Bi, Y.L.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of stat3 signalling pathway. Phytomedicine 2018, 15, 10–16. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Li, S.J.; Austriaco, N. Aging and cell death in the other yeasts, Schizosaccharomyces pombe and Candida albicans. FEMS Yeast Res. 2014, 14, 119–135. [Google Scholar]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, J.E.; Park, W.H. Cytoprotective effect of rhamnetin on miconazole-induced H9c2 cell damage. Nutr. Res. Pract. 2015, 9, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.; Koo, C.Y.; Infante, E.; Giacomini, C.; Morris, J.D.H. Rnd3 interacts with TAO kinases and contributes to mitotic cell rounding and spindle positioning. J. Cell Sci. 2020, 133, 235895. [Google Scholar] [CrossRef]
- Lee, R.E.B.; Liu, T.T.; Barker, K.S.; Lee, R.E.; David, R.P. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J. Antimicrob. Chemother. 2015, 55, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Liu, D.C.; Sun, T.T.; Zhang, A.M. Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J. Plant Physiol. 2005, 162, 81–89. [Google Scholar] [CrossRef]
- Ansari, M.A.; Fatima, Z.; Ahmad, K.; Hameed, S. Monoterpenoid perillyl a lcohol impairs metabolic flexibility of Candida albicans by inhibiting glyoxylate cycle. Biochem. Biophys. Res. Commun. 2018, 495, 560–566. [Google Scholar] [CrossRef]
- Canturk, Z. Evaluation of synergistic anticandidal and apoptotic effects of ferulic acid and caspofungin against Candida albicans. J. Food Drug Anal. 2018, 26, 439–443. [Google Scholar] [CrossRef]
- Fringuelli, R.; Pietrella, D.; Schiaffella, F.; Guarraci, A.; Vecchiarelli, A. Anti-Candida albicans properties of novel benzoxazine analogues. Bioorgan. Med. Chem. 2002, 10, 1681–1686. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Chagas, L.R.; Costa, M.S. Photodynamic antimicrobial chemotherapy (pact) inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. Lasers Med. Sci. 2014, 29, 1059. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity In Vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Pistoia, E.S.; Cosio, T.; Campione, E.; Pica, F.; Volpe, A.; Marino, D.; Di Francesco, P.; Monari, C.; Fontana, C.; Favaro, M.; et al. All-Trans Retinoic Acid Effect on Candida albicans Growth and Biofilm Formation. J. Fungi 2022, 8, 1049. [Google Scholar] [CrossRef]
- Kaneko, T.; Dougherty, T.J.; Magee, T.V. Macrolide antibiotics-Science Direct. Compr. Med. Chem. II 2007, 7, 519–566. [Google Scholar]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Wang, Q.L.; Wang, S.F.; Wu, J.Q.; Gao, Q.; Liu, W. Thiopeptide antibiotics exhibit a dual mode of action against intracellular pathogens by affecting both host and microbe. Chem. Biol. 2015, 22, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]
- Skenhan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancerdrug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Fang, Z.S.; Jiang, C.R.; Feng, Y.; Chen, R.X.; Lin, X.Y.; Zhang, Z.Q.; Han, L.H.; Chen, X.D.; Li, H.Y.; Guo, Y.B.; et al. Effects of G6PD activity inhibition on the viability, ROS generation and mechanical properties of cervical cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2245–2254. [Google Scholar] [CrossRef]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, J.; Anjo, S.I.; Fonseca, L.; Egas, C.; Manadas, B.; Abrantes, I. Bursaphelenchus xylophilus and B. mucronatus secretomes: A comparative proteomic analysis. Sci. Rep. 2016, 6, 39007. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Chen, Y.; Zhang, W.; Liu, T.; Liu, Y.; Li, M.; Li, S.; Xu, L.; Liu, H. Potential Anti-Candida albicans Mechanism of Trichoderma Acid from Trichoderma spirale. Int. J. Mol. Sci. 2023, 24, 5445. https://doi.org/10.3390/ijms24065445
Ye W, Chen Y, Zhang W, Liu T, Liu Y, Li M, Li S, Xu L, Liu H. Potential Anti-Candida albicans Mechanism of Trichoderma Acid from Trichoderma spirale. International Journal of Molecular Sciences. 2023; 24(6):5445. https://doi.org/10.3390/ijms24065445
Chicago/Turabian StyleYe, Wei, Yuchan Chen, Weimin Zhang, Taomei Liu, Yuping Liu, Mengran Li, Saini Li, Liqiong Xu, and Hongxin Liu. 2023. "Potential Anti-Candida albicans Mechanism of Trichoderma Acid from Trichoderma spirale" International Journal of Molecular Sciences 24, no. 6: 5445. https://doi.org/10.3390/ijms24065445




