Actin Depolymerization Factor ADF1 Regulated by MYB30 Plays an Important Role in Plant Thermal Adaptation
Abstract
:1. Introduction
2. Results
2.1. The Expression of AtADF1 Is Down-Regulated under High Temperature
2.2. AtADF1 Is a Negative Regulator of Plant Growth and Actin Filament Stability under High Temperature
2.3. The Expression of AtADF1 Is Positively Regulated by AtMYB30 under High Temperature and AtADF1 Is a Direct Target Gene of AtMYB30
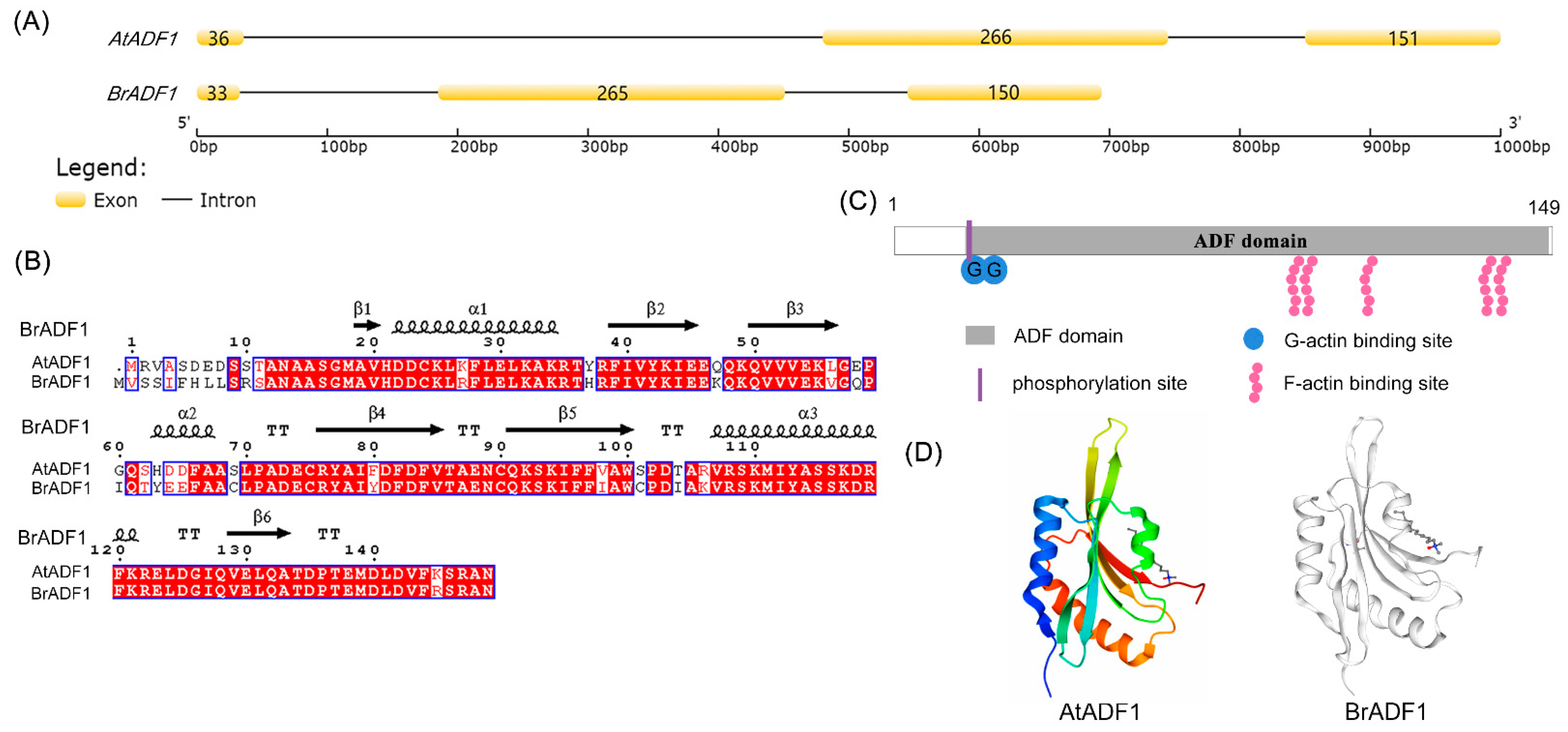
2.4. Sequence Analysis of Chinese Cabbage ADF1 (BrADF1)
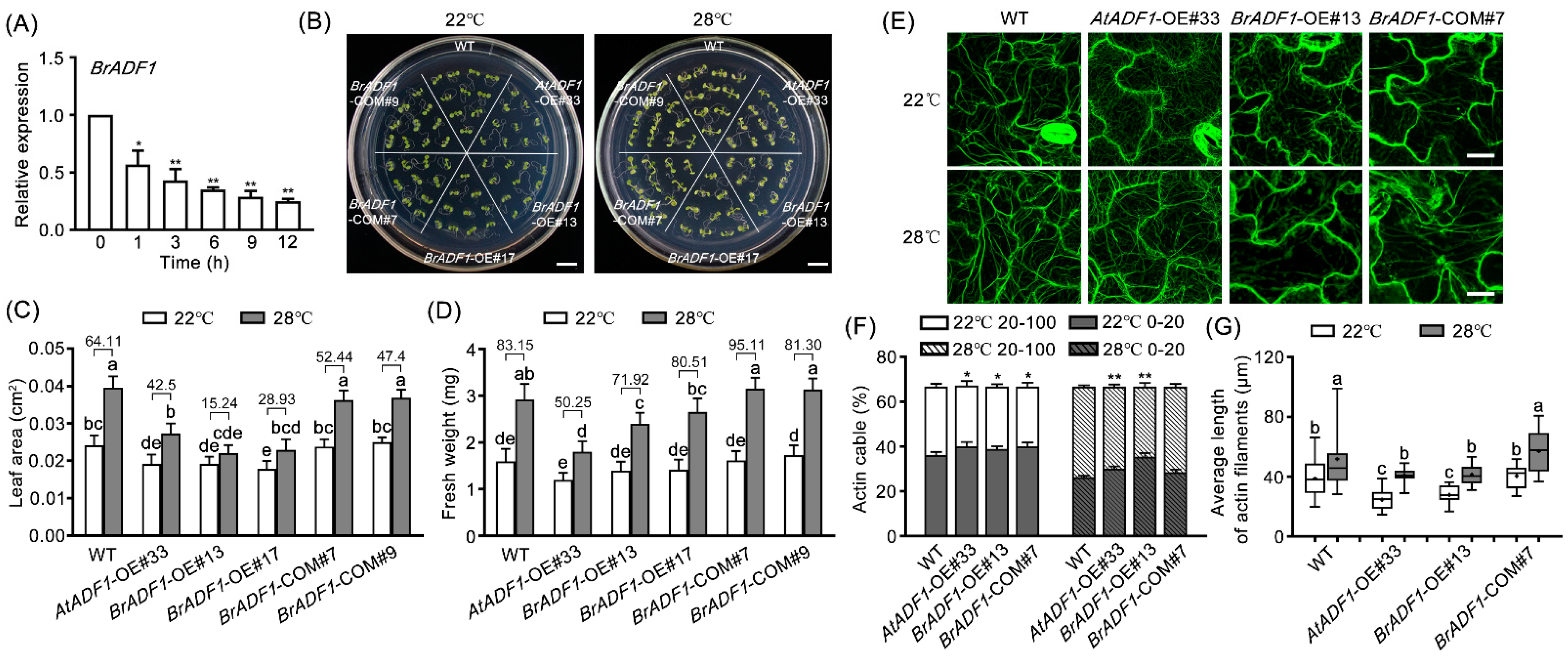
2.5. BrADF1 Inhibits Plant Growth and Actin Filaments Stability under High Temperature
3. Discussion
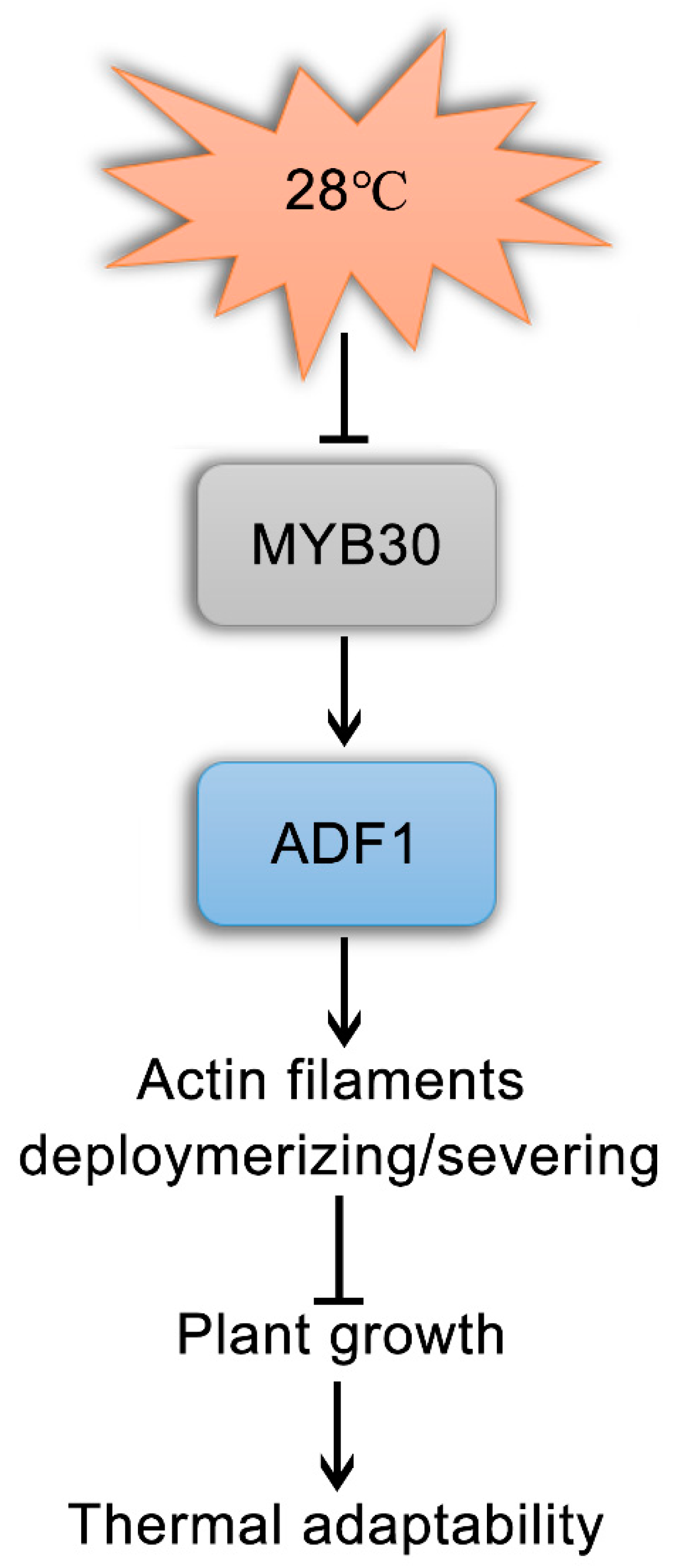
3.1. AtADF1, Mediated by AtMYB30, Plays a Negative Role in Plant Thermal Adaptation by Affecting Actin Filament Organization
3.2. BrADF1 Plays a Similar and Important Role to AtADF1 in Plant Thermal Adaptation
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Heat Treatments
4.2. Gene Expression and GUS Activity Analysis
4.3. Chromatin Immunoprecipitation-Quantitative PCR (ChIP-qPCR) Assay
4.4. Electrophoretic Mobility Shift Assays (EMSA)
4.5. Sequence Analysis of BrADF1
4.6. Plasmid Construction and Plant Transformation
4.7. Visualization and Analysis of Actin Filament Architecture and the Subcellular Localization of BrADF1
4.8. Western Blot Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruelland, E.; Zachowski, A. How Plants Sense Temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Song, C.; Lee, J.; Kim, T.; Hong, J.C.; Lim, C.O. VOZ1, a Transcriptional Repressor of DREB2C, Mediates Heat Stress Responses in Arabidopsis. Planta 2018, 247, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wang, T.-Y.; Weng, C.-P.; Duong, N.K.T.; Wu, S.-J. AtJ3, a Specific HSP40 Protein, Mediates Protein Farnesylation-Dependent Response to Heat Stress in Arabidopsis. Planta 2019, 250, 1449–1460. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Luo, A.; Davis, S.J.; Liu, J.-X. Timing to Grow: Roles of Clock in Thermomorphogenesis. Trends Plant Sci. 2021, 26, 1248–1257. [Google Scholar] [CrossRef]
- Horvath, I.; Glatz, A.; Varvasovszki, V.; Torok, Z.; Pali, T.; Balogh, G.; Kovacs, E.; Nadasdi, L.; Benko, S.; Joo, F.; et al. Membrane Physical State Controls the Signaling Mechanism of the Heat Shock Response in Synechocystis PCC 6803: Identification of Hsp17 as a “Fluidity Gene”. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.-Y.; Kaplan, F.; Lee, K.-J.; Guy, C.L. Acquired Tolerance to Temperature Extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Mathur, J. Cell Shape Development in Plants. Trends Plant Sci. 2004, 9, 583–590. [Google Scholar] [CrossRef]
- Müller, J.; Menzel, D.; Šamaj, J. Cell-Type-Specific Disruption and Recovery of the Cytoskeleton in Arabidopsis Thaliana Epidermal Root Cells Upon Heat Shock Stress. Protoplasma 2007, 230, 231–242. [Google Scholar] [CrossRef]
- Dreiza, C.M.; Brophy, C.M.; Komalavilas, P.; Furnish, E.J.; Joshi, L.; Pallero, M.A.; Murphy-Ullrich, J.E.; Von Rechenberg, M.; Ho, Y.-S.J.; Richardson, B.; et al. Transducible Heat Shock Protein 20 (HSP20) Phosphopeptide Alters Cytoskeletal Dynamics. FASEB J. 2005, 19, 261–263. [Google Scholar] [CrossRef]
- Pivovarova, A.V.; Chebotareva, N.A.; Chernik, I.S.; Gusev, N.B.; Levitsky, D.I. Small Heat Shock Protein Hsp27 Prevents Heat-Induced Aggregation of F-Actin by Forming Soluble Complexes with Denatured Actin: Stable Complexes of Small HSP with Denatured Actin. FEBS J. 2007, 274, 5937–5948. [Google Scholar] [CrossRef] [PubMed]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of Actin Filament Turnover by Severing and Nucleation at Different Concentrations of ADF/Cofilin. Mol. Cell 2006, 24, 13–23. [Google Scholar] [CrossRef]
- Henty-Ridilla, J.L.; Li, J.; Blanchoin, L.; Staiger, C.J. Actin Dynamics in the Cortical Array of Plant Cells. Curr. Opin. Plant Biol. 2013, 16, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Hotulainen, P.; Paunola, E.; Vartiainen, M.K.; Lappalainen, P. Actin-Depolymerizing Factor and Cofilin-1 Play Overlapping Roles in Promoting Rapid F-Actin Depolymerization in Mammalian Nonmuscle Cells. Mol. Biol. Cell 2005, 16, 649. [Google Scholar] [CrossRef] [Green Version]
- Clément, M.; Ketelaar, T.; Rodiuc, N.; Banora, M.Y.; Smertenko, A.; Engler, G.; Abad, P.; Hussey, P.J.; de Almeida Engler, J. Actin-Depolymerizing Factor2-Mediated Actin Dynamics Are Essential for Root-Knot Nematode Infection of Arabidopsis. Plant Cell 2009, 21, 2963–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-C.; Huang, W.-L.; Hong, C.-Y.; Lur, H.-S.; Chang, M.-C. Comprehensive Analysis of Differentially Expressed Rice Actin Depolymerizing Factor Gene Family and Heterologous Overexpression of OsADF3 Confers Arabidopsis Thaliana Drought Tolerance. Rice 2012, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, N. Plant Actin Depolymerizing Factor: Actin Microfilament Disassembly and More. J. Plant Res 2017, 130, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Qian, D.; Zhang, Z.; He, J.; Zhang, P.; Ou, X.; Li, T.; Niu, L.; Nan, Q.; Niu, Y.; He, W.; et al. Arabidopsis ADF5 Promotes Stomatal Closure by Regulating Actin Cytoskeleton Remodeling in Response to ABA and Drought Stress. J. Exp. Bot 2019, 70, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Xie, Y.; Jiang, Y.; Qu, X.; Huang, S. Arabidopsis ACTIN-DEPOLYMERIZING FACTOR7 Severs Actin Filaments and Regulates Actin Cable Turnover to Promote Normal Pollen Tube Growth. Plant Cell 2013, 25, 3405–3423. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.H.; Xia, G.X.; Hong, Y.; Ramachandran, S.; Kost, B.; Chua, N.H. ADF Proteins Are Involved in the Control of Flowering and Regulate F-Actin Organization, Cell Expansion, and Organ Growth in Arabidopsis. Plant Cell 2001, 6, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Baisakh, N.; Subudhi, P.K. Heat Stress Alters the Expression of Salt Stress Induced Genes in Smooth Cordgrass (Spartina Alterniflora L.). Plant Physiol. Bioch. 2009, 47, 232–235. [Google Scholar] [CrossRef]
- González-Schain, N.; Dreni, L.; Lawas, L.M.F.; Galbiati, M.; Colombo, L.; Heuer, S.; Jagadish, K.S.V.; Kater, M.M. Genome-Wide Transcriptome Analysis During Anthesis Reveals New Insights into the Molecular Basis of Heat Stress Responses in Tolerant and Sensitive Rice Varieties. Plant Cell Physiol. 2016, 57, 57–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, Q.; Qian, D.; Niu, Y.; He, Y.; Tong, S.; Niu, Z.; Ma, J.; Yang, Y.; An, L.; Wan, D.; et al. Plant Actin-Depolymerizing Factors Possess Opposing Biochemical Properties Arising from Key Amino Acid Changes throughout Evolution. Plant Cell 2017, 29, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Ruzicka, D.R.; Kandasamy, M.K.; McKinney, E.C.; Burgos-Rivera, B.; Meagher, R.B. The Ancient Subclasses of Arabidopsis ACTIN DEPOLYMERIZING FACTOR Genes Exhibit Novel and Differential Expression: Arabidopsis ACTIN DEPOLYMERIZING FACTOR Genes Exhibit Novel and Differential Expression. Plant J. 2007, 52, 460–472. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Ren, J.; Yang, R.; Fan, J.; Li, Y.; Wang, X.; Joseph, S.; Deng, W.; Zhai, L. Genome-Wide Identification and Characterization of Actin-Depolymerizing Factor (ADF) Family Genes and Expression Analysis of Responses to Various Stresses in Zea Mays L. Int. J. Mol. Sci. 2020, 21, 1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB Gene Family in Arabidopsis Thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M.; Kim, R.J.-A.; Moore, C.M.; Chen, M. Daytime Temperature Is Sensed by Phytochrome B in Arabidopsis Through a Transcriptional Activator HEMERA. Nat. Commun. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Li, C.; Dong, X.; Li, H.; Zhang, D.; Zhou, Y.; Jiang, B.; Peng, J.; Qin, X.; Cheng, J.; et al. MYB30 Is a Key Negative Regulator of Arabidopsis Photomorphogenic Development That Promotes PIF4 and PIF5 Protein Accumulation in the Light. Plant Cell 2020, 32, 2196–2215. [Google Scholar] [CrossRef]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 Transcription Factor Regulates Oxidative and Heat Stress Responses Through ANNEXIN-mediated Cytosolic Calcium Signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Pang, W.; Piao, Z. Omics Meets Phytonutrients in Vegetable Brassicas: For Nutritional Quality Breeding. Hortic. Plant J. 2017, 3, 247–254. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Lu, Y.; de Ruiter, M.; Cariaso, M.; Prins, M.; van Tunen, A.; He, Y. Identification of Conserved and Novel MicroRNAs That Are Responsive to Heat Stress in Brassica Rapa. J. Exp. Bot 2012, 63, 1025–1038. [Google Scholar] [CrossRef]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of Three Brassica Species to High Temperature Stress During Reproductive Growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Li, R.; Xia, Y.; Bai, G.; Siddique, K.H.M.; Zhang, H.; Zheng, Y.; Yang, X.; Guo, P. Comparative Analysis of MiRNA Expression Profiles between Heat-Tolerant and Heat-Sensitive Genotypes of Flowering Chinese Cabbage Under Heat Stress Using High-Throughput Sequencing. Genes 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Song, H.; Han, C.-T.; Lim, Y.P.; Chung, S.-M.; Hur, Y. Expression Characteristics of Heat Shock Protein Genes in Two Comparable Inbred Lines of Chinese Cabbage, Chiifu and Kenshin. Genes Genom. 2010, 32, 247–257. [Google Scholar] [CrossRef]
- Wang, A.; Hu, J.; Huang, X.; Li, X.; Zhou, G.; Yan, Z. Comparative Transcriptome Analysis Reveals Heat-Responsive Genes in Chinese Cabbage (Brassica Rapa Ssp. Chinensis). Front. Plant Sci. 2016, 7, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarra, R.; Xue, Y. Ectopic Expression of Nucleolar DEAD-Box RNA Helicase OsTOGR1 Confers Improved Heat Stress Tolerance in Transgenic Chinese Cabbage. Plant Cell Rep. 2020, 39, 1803–1814. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 Is a Direct Target of BES1 and Cooperates with BES1 to Regulate Brassinosteroid-Induced Gene Expression. Plant J. 2009, 58, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qiu, T.; Yue, J.; Guo, N.; He, Y.; Han, X.; Wang, Q.; Jia, P.; Wang, H.; Li, M.; et al. Arabidopsis ADF1 Is Regulated by MYB73 and Is Involved in Response to Salt Stress Affecting Actin Filament Organization. Plant Cell Physiol. 2021, 62, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ren, H.; Li, J. An Auxin Transport Inhibitor Targets Villin-Mediated Actin Dynamics to Regulate Polar Auxin Transport. Plant Physiol. 2019, 181, 161–178. [Google Scholar] [CrossRef] [Green Version]
- Henty, J.L.; Bledsoe, S.W.; Khurana, P.; Meagher, R.B.; Day, B.; Blanchoin, L.; Staiger, C.J. Arabidopsis Actin Depolymerizing Factor4 Modulates the Stochastic Dynamic Behavior of Actin Filaments in the Cortical Array of Epidermal Cells. Plant Cell 2011, 23, 3711–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Bai, J.; Li, S.; Li, X.; Yang, L.; He, Y. HTT2 Promotes Plant Thermotolerance in Brassica Rapa. BMC Plant Biol. 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusamy, M.; Kim, J.H.; Kim, S.H.; Park, S.Y.; Lee, S.I. BrPP5.2 Overexpression Confers Heat Shock Tolerance in Transgenic Brassica Rapa through Inherent Chaperone Activity, Induced Glucosinolate Biosynthesis, and Differential Regulation of Abiotic Stress Response Genes. Int. J. Mol. Sci. 2021, 22, 6437. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Bharti, K.; Scharf, K.-D. Arabidopsis and The Heat Stress Transcription Factor World: How Many Heat Stress Transcription Factors Do We Need? Cell Stress 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Carlier, M.-F.; Laurent, V.; Santolini, J.; Melki, R.; Didry, D.; Xia, G.-X.; Hong, Y.; Chua, N.-H.; Pantaloni, D. Actin Depolymerizing Factor (ADF/Cofilin) Enhances the Rate of Filament Turnover: Implication in Actin-Based Motility. J. Cell Biol. 1997, 136, 1307–1322. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Yuan, M. Salt Tolerance Requires Cortical Microtubule Reorganization in Arabidopsis. Plant Cell Physiol. 2007, 48, 1534–1547. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-H.; Hong, Y. Arabidopsis CDPK6 Phosphorylates ADF1 at N-Terminal Serine 6 Predominantly. Plant Cell Rep. 2013, 32, 1715–1728. [Google Scholar] [CrossRef]
- Kim, J.H.; Nguyen, N.H.; Jeong, C.Y.; Nguyen, N.T.; Hong, S.-W.; Lee, H. Loss of the R2R3 MYB, AtMyb73, Causes Hyper-Induction of the SOS1 and SOS3 Genes in Response to High Salinity in Arabidopsis. J. Plant Physiol. 2013, 170, 1461–1465. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.W.; Chua, N.H. Agrobacterium-Mediated Transformation of Arabidopsis Thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yuan, M.; Ehrhardt, D.W.; Wang, Z.; Mao, T. Arabidopsis MICROTUBULE DESTABILIZING PROTEIN40 Is Involved in Brassinosteroid Regulation of Hypocotyl Elongation. Plant Cell 2012, 24, 4012–4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Qin, T.; Ma, Q.; Sun, J.; Liu, Z.; Yuan, M.; Mao, T. Light-Regulated Hypocotyl Elongation Involves Proteasome-Dependent Degradation of the Microtubule Regulatory Protein WDL3 in Arabidopsis. Plant Cell 2013, 25, 1740–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Bi, S.; Wang, L.; Li, H.; Gao, B.; Huang, S.; Qu, X.; Cheng, J.; Wang, S.; Liu, C.; et al. GLABRA2 Regulates Actin Bundling Protein VILLIN1 in Root Hair Growth in Response to Osmotic Stress. Plant Physiol. 2020, 184, 176–193. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cheng, J.; Bi, S.; Wang, J.; Cheng, X.; Liu, S.; Gao, Y.; Lan, Q.; Shi, X.; Wang, Y.; et al. Actin Depolymerization Factor ADF1 Regulated by MYB30 Plays an Important Role in Plant Thermal Adaptation. Int. J. Mol. Sci. 2023, 24, 5675. https://doi.org/10.3390/ijms24065675
Wang L, Cheng J, Bi S, Wang J, Cheng X, Liu S, Gao Y, Lan Q, Shi X, Wang Y, et al. Actin Depolymerization Factor ADF1 Regulated by MYB30 Plays an Important Role in Plant Thermal Adaptation. International Journal of Molecular Sciences. 2023; 24(6):5675. https://doi.org/10.3390/ijms24065675
Chicago/Turabian StyleWang, Lu, Jianing Cheng, Shuangtian Bi, Jinshu Wang, Xin Cheng, Shihang Liu, Yue Gao, Qingkuo Lan, Xiaowei Shi, Yong Wang, and et al. 2023. "Actin Depolymerization Factor ADF1 Regulated by MYB30 Plays an Important Role in Plant Thermal Adaptation" International Journal of Molecular Sciences 24, no. 6: 5675. https://doi.org/10.3390/ijms24065675



