Abstract
High blood pressure (HBP) is the leading risk factor for cardiovascular disease (CVD) and all-cause mortality worldwide. The progression of the disease leads to structural and/or functional alterations in various organs and increases cardiovascular risk. Currently, there are significant deficiencies in its diagnosis, treatment, and control. Vitamin D is characterized by its functional versatility and its involvement in countless physiological processes. This has led to the association of vitamin D with many chronic diseases, including HBP and CVD, due to its involvement in the regulation of the renin–angiotensin–aldosterone system. The aim of this study was to evaluate the effect of 13 single nucleotide polymorphisms (SNPs) related to the vitamin D metabolic pathway on the risk of developing HBP. An observational case-control study was performed, including 250 patients diagnosed with HBP and 500 controls from the south of Spain (Caucasians). Genetic polymorphisms in CYP27B1 (rs4646536, rs3782130, rs703842, and rs10877012), CYP2R1 rs10741657, GC rs7041, CYP24A1 (rs6068816, and rs4809957), and VDR (BsmI, Cdx2, FokI, ApaI, and TaqI) were analyzed by real-time PCR using TaqMan probes. Logistic regression analysis, adjusted for body mass index (BMI), dyslipidemia, and diabetes, showed that in the genotypic model, carriers of the GC rs7041 TT genotype were associated with a lower risk of developing HBP than the GG genotype (odds ratio (OR) = 0.44, 95% confidence interval (CI): 0.41–0.77, p = 0.005, TT vs. GG). In the dominant model, this association was maintained; carriers of the T allele showed a lower risk of developing HBP than carriers of the GG genotype (OR = 0.69, 95% CI: 0.47–1.03; TT + TG vs. GG, p = 0.010). Finally, in the additive model, consistent with previous models, the T allele was associated with a lower risk of developing HBP than the G allele (OR = 0.65, 95% CI: 0.40–0.87, p = 0.003, T vs. G). Haplotype analysis revealed that GACATG haplotypes for SNPs rs1544410, rs7975232, rs731236, rs4646536, rs703842, and rs10877012 were associated with a marginally significant lower risk of developing HBP (OR = 0.35, 95% CI: 0.12–1.02, p = 0.054). Several studies suggest that GC 7041 is associated with a lower active isoform of the vitamin D binding protein. In conclusion, the rs7041 polymorphism located in the GC gene was significantly associated with a lower risk of developing HBP. This polymorphism could therefore act as a substantial predictive biomarker of the disease.
Keywords:
high blood pressure; vitamin D; risk; genetic polymorphisms; CYP2R1; CYP27B1; CYP24A1; GC; VDR 1. Introduction
The European Society of Cardiology and the European Society of Hypertension define arterial hypertension or high blood pressure (HBP) in adults as systolic blood pressure (SBP) greater than or equal to 140 mmHg or diastolic blood pressure (DBP) greater than or equal to 90 mmHg, measured in the consulting room [,]. The American College of Cardiology/American Heart Association, for their part, locate the reference values as SBP/DBP greater than or equal to 130/80 mmHg []. The societies mentioned, together with the World Health Organization (WHO), agree that HBP is the main risk factor for developing cardiovascular disease (CVD) and for premature mortality [,,,,,]. According to the data reported by the non-communicable disease risk factor collaboration (NCD-RisC) and the WHO for the year 2019, the prevalence of HBP in adults was 32% in women and 34% in men, and it is estimated that there are 1278 million people with the disease [,].
There is a wide range of modifiable risk factors that contribute to the development of HBP, including age, sex, an unbalanced diet, overweight, obesity, alcohol abuse and smoking, vitamin D deficiency, lack of physical activity, psychological stress, socioeconomic factors, and inadequate access to healthcare [,,,,]. Given the diversity of symptoms and complex etiology of HBP, in most patients with this disease, no underlying cause is detected [,,,,].
The existence of a causal relationship between vitamin D and HBP is due to the participation of the active isoform of vitamin D (calcitriol, 1,25-dihydroxyvitamin D or 1,25-(OH)2D) in regulating the renin–angiotensin–aldosterone system (RAAS) by inhibiting expression of the gene that codes for renin, a key component in controlling blood pressure (BP) [,,,]. Moreover, given the ability of vitamin D to regulate the immune system and its anti-inflammatory activity, a deficiency of this vitamin is associated with the triggering of cytokine-mediated inflammatory processes, which lead to endothelial dysfunction and increased stiffness, contributing to raised BP [,,,,,,,].
The activity of vitamin D depends on its complex metabolism being carried out correctly. The term vitamin D comprises both the active and the inactive isoforms. The inactive ones are cholecalciferol and ergocalciferol. The first is synthesized in the dermis through the action of ultraviolet (UV) radiation, especially UVB (290–320 nm), on 7-dehydrocholesterol, while both the first and the second are incorporated through the diet [,,]. Both molecules are transported in the blood bound to vitamin D binding protein (VDBP) or GC, which carries them to the liver, where the CYP2R1 or 25-hydroxylase enzyme carries out a hydroxylation at position 25 of the molecule, producing calcidiol or 25(OH)D. This is the metabolite that remains in the blood for longer and is, therefore, the one that is measured when determining serum levels [,,]. It then travels to the kidney, where the CYP27B1 or α-1-hydroxylase enzyme includes a hydroxyl group at position 1, giving rise to calcitriol. To perform the vitamin’s activity, calcitriol binds to the vitamin D receptor (VDR), found in the cell membrane [,,,]. Once they are bound, the VDR translocates to the nucleus to form a complex with the retinoid X receptor (RXR), an orphan retinoid/steroid hormone receptor, which will act as a transcription factor in the expression of various genes involved in numerous physiological phenomena [,,,,,,,]. Finally, calcitriol will be degraded by successive hydroxylation reactions catalyzed by the CYP24A1 enzyme, located in the mitochondria, with the object of increasing the solubility of the molecule and eliminating it by renal excretion [,,,].
The genes which code for the transporter, enzymes, and receptor that participate in vitamin D metabolism are characterized as being highly polymorphic. Consequently, the presence of single nucleotide polymorphisms (SNPs) in the GC, CYP2R1, CYP27B1, CYP24A1, and VDR genes influences serum levels of vitamin D, and hence its activity [,,,,,,]. Therefore, the presence of SNPs in the genes mentioned may influence the development of HBP.
This study was designed with the aim of evaluating the SNP-type genetic polymorphisms in the genes involved in vitamin D metabolism and their relationship to the risk of suffering from HBP in Caucasian patients, specifically those residing in southern Spain.
2. Results
2.1. Study Subjects Characteristics
A total of 750 study participants were included: 250 cases diagnosed with HBP and 500 controls. Their sociodemographic and clinicopathological characteristics are described in Table 1.

Table 1.
Clinicopathological characteristics of HBP cases and controls.
The group of cases comprised 52.4% (131/250) women, and the median age was 66 (range: 60–73) years. With regard to smoking status, 60.7% (147/242) were non-smokers, 33.1% (80/242) were ex-smokers, and 25.2% (61/242) current smokers. In terms of drinking status, 71.2% (163/229) were classified as non-drinkers, 26.6% (61/229) as current drinkers, and 2.2% (5/229) as ex-drinkers. Most of the HBP patients were categorized as obese (44.5%, 94/211), 39.3% as overweight, and 16.1% as normal weight. A total of 50.8% (127/250) did not have dyslipidemia, and 58.4% (146/250) did not suffer from diabetes. As for the clinicopathological features of the disease, 58.4% (146/250) showed elevated SBP levels, and 58.4% (146/250) had normal DBP levels; total cholesterol and LDL cholesterol levels were normal in 55% (137/249) and 63.7% (142/223), respectively, and HDL cholesterol levels showed medium values in 53.3% (121/227); 69.2% (171/247) had normal triglyceride levels; mean glucose was 114 ± 47 mg/dL.
The control group was made up of 51.4% (257/500) women, and the median age was 65 (range: 60–73) years. There were 51.9% (246/474) non-smokers, 25.5% (121/474) ex-smokers, and 22.6% (107/474) current smokers. With regard to alcohol drinking status, 77.7% (351/452) were classified as non-drinkers, 18.4% (83/452) as current drinkers, and 4% (18/452) as ex-drinkers. The majority (39.8%) were overweight (135/339), 32.7% (111/339) were normal weight, and 27.4% (93/339) were obese. A total of 73.4% (367/500) had no dyslipidemia, and 89.1% (434/487) did not suffer from diabetes.
There were significant differences between the cases and the controls with respect to drinking status (odds ratio (OR) = 1.58, 95% confidence interval (CI): 1.08–2.31, p = 0.027, for drinker vs. non-drinker; and OR = 0.60, 95% CI: 0.19–1.53, p = 0.027, for ex-drinker vs. non-drinker), body mass index (BMI) (OR = 3.30, 95% CI: 2.06–5.38, p < 0.001, for obesity vs. normal weight and OR = 2.01, 95% CI: 1.26–3.24, p < 0.001, for overweight vs. normal weight), dyslipidemia (OR = 2.67, 95% CI: 1.95–3.68, p < 0.001), and diabetes (OR = 5.83; 95% CI: 4–8.58, p < 0.001), respectively. No statistically significant differences were observed for sex (p = 0.796) or age (p = 0.989).
2.2. Genotype Distributions
The observed genotype frequencies did not significantly deviate from the expected frequency values according to the Hardy–Weinberg Equilibrium test. With the exceptions of CYP27B1 rs3782130 (p = 0.002769), CYP27B1 rs703842 (p = 0.03971), and CYP24A1 rs6068816 (p = 0.03246) for the control group (Table S1). For these three variants, we found no statistically significant differences from those described in the Iberian population: CYP27B1 rs3782130 C allele: 0.3050 vs. 0.2850, p = 0.9753; CYP27B1 rs703842 C allele: 0.2507 vs. 0.2940; p = 0.9452; and CYP24A1 rs6068816 T allele: 0.1109 vs. 0.1070; p = 0.9929, respectively []. The linkage disequilibrium (LD) r2 and D’ values are shown in Table S2. The SNP pairs rs4646536-rs10877012 (r2 = 0.731762, D’ = 0.905063), rs4646536-rs703842 (r2 = 0.747667, D’ = 0.89186), and rs10877012-rs703842 (r2 = 0.758471, D’ = 0.904393) were in strong LD based on D’ values (Figure 1). All the polymorphisms showed minor allele frequencies (MAFs) greater than 1%, and none of them were excluded from the analysis (Table S3). The haplotype frequency estimates are presented in Table S4.
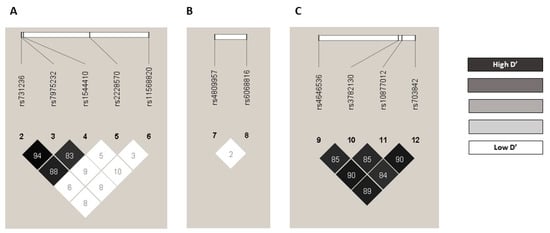
Figure 1.
Pairwise LD D’ plots for SNPs located in VDR gene, CYP24A1 gene, and CYP27B1 gene in the whole population. Numbers inside the squares are the D’ values expressed as a percent: (A) pairwise LD D’ plots for 5 SNPs located in VDR gene, (B) pairwise LD D’ plots for 2 SNPs located in CYP24A1 gene, and (C) pairwise LD D’ plots for 4 SNPs located in CYP27B1 gene. LD: linkage disequilibrium; SNP: single nucleotide polymorphism.
2.3. Influence of Genetic Polymorphisms on the Risk of HBP
The bivariate analysis between the 13 genetic polymorphisms included in the study and the risk of HBP was evaluated through various analytical models: genotypic, allelic, dominant, recessive, and additive (Table S5). We observed a statistically significant association for the GC rs7041 polymorphism in the additive (p = 0.028) and allelic (p = 0.031) models, and a tendency toward statistical significance in the dominant (p = 0.054) and genotypic (p = 0.088) models (Table S5). In the allelic model, the T allele was associated with a lower risk of HBP (OR = 0.79, 95% CI: 0.63–0.98, T vs. G), and the additive model was in line with this association (OR = 0.78, 95% CI: 0.62–0.97). In the genotypic model, carriers of the GC rs7041 TT genotype were associated with a lower risk of developing HBP (OR = 0.61, 95% CI: 0.39–0.95, TT vs. GG), as were carriers of the GC rs7041 TG genotype (OR = 0.76. 95% CI: 0.53–1.10, TG vs. GG). In addition, in the dominant model, it was observed that those individuals carrying the T allele showed a lower risk of suffering from HBP (OR = 0.72; 95% CI: 0.51–1.00; TT + TG vs. GG) (Table 2). The logistic regression analysis adjusted for BMI, dyslipidemia, and diabetes showed that in the genotypic model, carriers of the GC rs7041 TT and TG genotypes were associated with a lower risk of developing HBP (OR = 0.44, 95% CI: 0.41–0.77, p = 0.005, TT vs. GG; and OR = 0.64, 95% CI: 0.41–0.99, p = 0.045, TG vs. GG). This association was maintained in the dominant model, where carriers of the T allele showed a lower risk of developing HBP (OR = 0.69, 95% CI: 0.47–1.03, p = 0.01, TT + TG vs. GG). The recessive model showed that the GC rs7041 TT genotype presented a lower risk (OR = 0.58, 95% CI: 0.35–0.95, p = 0.035, TT vs. TG + GG). Finally, the additive model, in line with the previous models, revealed the GC rs7041 T allele was associated with a lower risk of suffering from HBP compared to the G allele (OR = 0.65, 95% CI: 0.40–0.87, p = 0.003, T vs. G) (Table 3). The remaining SNPs studied did not show a statistically significant association with the risk of developing HBP in any of the models analyzed (Table S5). The haplotype analysis was performed taking into account those polymorphisms that were in strong LD, and it was discovered that the GACATG haplotype—for the 6 SNPs located on Chromosome 12, i.e., rs1544410 (VDR), rs7975232 (VDR), rs731236 (VDR), rs4646536 (CYP27B1), rs703842 (CYP27B1), and rs10877012 (CYP27B1)—was associated with a lower risk of developing HBP (OR = 0.35; 95% CI: 0.12–1.02, p = 0.054) (Table 4).

Table 2.
Influence of GC rs7041 SNP on risk of HBP.

Table 3.
Influence of clinical characteristics and GC rs7041 SNP on risk of HBP.

Table 4.
Influence of haplotypes formed by 6 SNPs located on Chromosome 12 on risk of HBP.
3. Discussion
Arterial hypertension, which is also commonly referred to as HBP, is the main avoidable risk factor for CVD and all-cause mortality. Recent studies have related vitamin D deficiency with HBP and CVD and suggested that vitamin D serum status is a predictive factor of cardiovascular morbidity and mortality [,,,,,]. This study was conducted with the objectives of evaluating the impacts of various SNPs involved in the vitamin D metabolic pathway individually on HBP risk and assessing the haplotypic associations of six SNPs located on Chromosome 12 with risk of HBP.
The results obtained in this study showed that the T allele of the GC rs7041 SNP was associated with a lower risk of HBP in all the models studied. Several reviews indicate that the T allele of the GC rs7041 is associated with the slower transport phenotype of VDBP [,]. Up to now, the relationship between GC rs7041 and the risk of suffering from HBP has been studied by only very few studies. However, there are studies in which the presence of this polymorphism is related to the development of CVD. The study carried out by Kiani et al. (2019), with 249 cases and 182 controls in a Caucasian population (Iran) diagnosed with heart disease by angiography, indicated that individuals carrying the TG + TT genotypes in GC rs7041 SNP in the dominant model had higher SBP/DBP levels than those with the TT genotype (p < 0.01) []. These results are in line with our study, in which the T allele showed a protective effect against the risk of HBP. On the other hand, the study conducted by Daffara et al. (2017) in a cohort composed of 1080 Caucasian patients (Italy) found no relationship between the prevalence of heart disease and the GC rs7041 SNP (OR = 1.26, 95% CI: 0.73–2.19, p = 0.41, GT vs. TT; and OR = 1.25, 95% CI: 0.82–1.91, p = 0.30, GG vs. TT) []. Similarly, Michos et al. (2015), in their study comprising a cohort of 11945 Caucasian and African American patients (United States) participating in the Atherosclerosis Risk in Communities (ARIC) Study, in which the GC rs7041 SNP and vitamin D levels were assessed in relation to coronary heart disease (CHD), found no statistically significant interaction between 25(OH)D and the GC rs7041 SNP in relation to risk of CHD (p-interaction = 0.87) [].
In our study, no statistically significant associations were observed for the five VDR gene SNPs examined, i.e., rs731236, rs7975232, rs1544410, rs2228570, and rs11568820, in any of the models analyzed (Table S5). In line with our results is the meta-analysis by Zhu et al. (2019) in populations of diverse ancestries (China, Italy, United States, and India) []. For VDR rs2228570 (FokI) SNP, the meta-analysis included 4011 cases and 4847 controls from populations of diverse ancestries (China, Italy, United States, and India) showed no association with greater susceptibility to HBP is found in any of the models ((OR = 1.02, 95% CI: 0.75–1.38, p = 0.90, and I2 = 86%, p(I2) < 0.0001, TC + CC vs. TT), (OR = 0.98, 95% CI: 0.88–1.09, p = 0.68, I2 = 32%, p(I2) = 0.18, CC vs. TT + TC), (OR = 0.97; 95% CI: 0.77–1.23, p = 0.82, I2 = 78%; p(I2) = 0.0001, TC vs. TT + CC), (OR = 1.02, 95% CI: 0.84–1.23, p = 0.86, I2 = 82%, p(I2) < 0.0001, T vs. C)). Nor has any association been demonstrated between the VDR rs7975232 (ApaI) polymorphism in two Asian populations (both from China), with 517 cases and 355 controls, and the development of HBP in any of the models ((OR = 1.05, 95% CI: 0.84–1.32, p = 0.66, I2 = 36%, p(I2) = 0.21, GT vs. TT vs. GG), (OR = 1.21, 95% CI: 0.54–2.71, p = 0.64, I2 = 70%; p(I2) = 0.07, TT vs. GG + GT), (OR = 0.95; 95% CI: 0.54–1.67, p = 0.87, I2 = 81%; (I2) = 0.02, GT vs. GG + TT), (OR = 1.04, 95% CI: 0.87–1.23, p = 0.68, I2 = 0%, p(I2) = 0.83, G vs. T)). However, in three populations (from United States, China, and Italy), with 826 cases and 627 controls, the presence of the genotypes GA + AA of the VDR rs1544410 (BsmI) SNP as a risk factor for HBP was evident in the dominant model, whereas the presence of the heterozygous genotype GA of the VDR rs1544410 (BsmI) SNP was a risk factor for HBP in the heterozygous model compared to the homozygous genotypes ((OR = 1.32, 95% CI: 1.05–1.68, p = 0.02, I2 = 39%, p(I2) = 0.19, GA + AA vs. GG), (OR = 1.27, 95% CI: 1.01–1.60, p = 0.04, I2 = 41%, p(I2) = 0.18, GA vs. GG + AA, respectively)) []. By contrast, Muray et al. (2003) conducted a cross-sectional study of 590 apparently healthy subjects of Caucasian origin (Spain), which showed the presence of the GG genotype of the VDR rs1544410 (BsmI) SNP as a risk factor for HBP in men (p < 0.006) [].
With respect to the SNPs present in the CYP27B1 gene, the study conducted by Wang et al. (2014), with the use of genome-wide genotype data in a Caucasian population (23,294 cases, Europe) to evaluate the impact of the polymorphisms involved in the vitamin D signaling and metabolic pathways on BP, concluded that the CYP27B1 rs4646536 and CYP27B1 rs703842 polymorphisms are not related to HBP (p > 0.05) in the Women’s Genome Health Study (WGHS) or International Consortium of Blood Pressure (ICBP), respectively []. These results are in line with those shown in our study, where no statistical relationship was found between the presence of those SNPs and the risk of suffering from HBP.
As for the SNPs in the CYP2R1 gene, no significant relationship between CYP2R1 rs10741657 and the risk of HBP was found in our study. In contrast to our findings, Ye et al. (2019) described the presence of this SNP as a protective factor against the risk of HBP in an Asian population (n = 324 cases/525 controls, China). This study showed how the presence of the CYP2R1 rs10741657 TT genotype acted as a protective factor, regardless of vitamin D levels, for the additive model (OR = 0.81, 95% CI: 0.66–0.98, p < 0.05) and for the dominant model (OR = 0.73, 95% CI: 0.56–0.97, p < 0.05) [].
Finally, in our study, the SNPs located in the CYP24A1 gene (rs6068816 and rs4809957) were found not to be associated with the risk of suffering from HBP. The results shown by the previous study conducted by Ye et al. (2019) in an Asian population are in line with our results, since none of the polymorphisms of the CYP24A1 gene was associated with the risk of developing HBP [].
This study has several limitations, and a major limitation is the sample size since a larger cohort might make it possible to detect associations between the genetic variants being studied and the risk of HBP with greater statistical power. Moreover, a larger number of cases would enable us to strengthen the statistically significant causal relationship that was obtained. In addition, other limitations should be mentioned, such as the inherent ones from the retrospective studies, the ethnic differences and geographical variability in the studies with which the results are being compared, together with the lack of studies confirming an association between the GC rs7041 SNP and the risk of HBP.
The results shown in this study suggested that the presence of the T allele in the GC rs7041 SNP could be a protective factor against HBP. However, these results must be interpreted with caution, given the need for more scientific evidence to enable us to determine which of these SNPs could be used as biomarkers for the risk of HBP. Continued research with a larger cohort is still needed. The strengths of our study were the homogeneity of the sample, especially in terms of the cases, which consisted of only University Hospital Virgen de las Nieves patients diagnosed by the same team and also from the same geographical area, thus increasing uniformity.
4. Materials and Methods
We conducted an observational retrospective case-control study.
4.1. Study Subjects
This study included 250 individuals diagnosed with HBP and 500 controls with no HBP diagnosis, such that both cases and controls were randomly enrolled, of Caucasian origin, from southern Spain. Subjects were followed up retrospectively from the enrolment. The inclusion criteria for the cases were age 18 years or over, diagnosis of HBP at the Hospital Universitario Virgen de las Nieves (between 1998 and 2018), and available clinical data. The controls were individuals aged over 18 years with no HBP diagnosis who had lived in the same geographical area and were recruited at the same hospital.
This case-control study was carried out in accordance with the Declaration of Helsinki, with the approval of the Ethics and Research Committee of the Andalusian Public Health Service’s Biobank (Code: 0957-N-21). The subjects signed a written informed consent form for the collection of blood or saliva samples and their donation to the Biobank. The samples were coded and treated confidentially.
4.2. Sociodemographic and Clinical Variables
The sociodemographic data compiled included sex, age at diagnosis, weight, height, and drinking status. From the weight and height values, the BMI was calculated and classified into three categories, according to the WHO scale (normal weight for BMI < 25 kg/m2, overweight for BMI ≥ 25 kg/m2 and <30 kg/m2, and obesity for BMI ≥ 30 kg/m2, respectively). Individuals were classified by standard drink units (SDUs) as non-drinkers if they were teetotalers or did not consume alcohol regularly, as current drinkers if their alcohol consumption was greater than 4 SDUs per day in men and greater than 2.5 SDUs per day in women, and as ex-drinkers, if their alcohol consumption had been greater than 4 SDUs per day in men and greater than 2.5 SDUs in women, but they did not currently drink []. The HBP diagnosis in the patients was performed in accordance with the criteria of the European Society of Cardiology and the European Society of Hypertension [,]. Cardiovascular morbidity was assessed through the presence of other pathologies, such as diabetes diagnosis (yes/no) and dyslipidemia diagnosis (yes/no), according to the criteria of the Clinical Practice Guidelines on the management of diabetes and dyslipidemia [,].
The clinical variables were collected at the time of diagnosis of the disease: SBP (normal < 120 mmHg; elevated 120–130 mmHg; high > 130 mmHg), DBP (normal < 80 mmHg; elevated 80–90 mmHg; high > 90 mmHg), and total cholesterol (normal < 200 mg/dL; elevated 200–240 mg/dL; high > 240 mg/dL), HDL cholesterol (at risk < 40 mg/dL; medium 40–60 mg/dL; optimum > 60 mg/dL), LDL cholesterol (normal < 130 mg/dL; elevated 130–160 mg/dL; high > 160 mg/dL), triglycerides (normal < 150 mg/dL; elevated 150–200 mg/dL; high > 200 mg/dL), and fasting blood sugar (mg/dL).
4.3. Genetic Variables
4.3.1. DNA Isolation
Blood samples (3 mL) were collected in BD Vacutainer K3E Plus blood collection tubes, and saliva samples in BD Falcon 50 mL conical tubes (BD, Plymouth, UK). DNA was extracted using the QIAamp DNA Mini extraction Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s instructions for purification of DNA from blood or saliva, and stored at −40 °C. The concentration and purity of the DNA were measured using the NanoDrop 2000 UV spectrophotometer with 280/260 and 280/230 absorbance ratios. The DNA samples, isolated from blood or saliva, were preserved in the Biobank of the Hospital Universitario Virgen de las Nieves, part of the Andalusian Public Health Service’s Biobank.
4.3.2. Detection of Gene Polymorphisms and Quality Control
The gene polymorphisms were determined by real-time polymerase chain reaction (PCR) allelic discrimination assay using TaqMan probes (ABI Applied Biosystems, QuantStudio 3 Real-Time PCR System, 96 wells), following the manufacturer’s instructions. Ten percent of the results were confirmed by Sanger sequencing (Table 5). Real-time PCR and Sanger sequencing were performed in the Pharmacogenetics Unit of the Hospital Universitario Virgen de las Nieves. The criteria for SNPs quality control were: (1) missing genotype rate per SNP < 0.05; (2) minor allele frequency > 0.01; (3) p-value > 0.05 in Hardy–Weinberg Equilibrium test; (4) missing genotype rate in the case group is less than 0.05, and in the control group is less than 0.05, respectively.

Table 5.
Gene SNPs and their TaqMan Assay IDs.
4.4. Statistical Analysis
Cases and controls were matched by age and sex using the 1:2 propensity score matching method []. Quantitative data were expressed as the result (± standard deviation) for variables with normal distribution and as medians or percentiles (25 and 75) for variables with non-normal distribution. The Shapiro–Wilks test was used to verify normality.
We determined the Hardy–Weinberg equilibrium and the haplotype frequency through the D’ and r2 coefficients. The bivariate association between risk of HBP and polymorphisms was evaluated for multiple models (genotypic, additive, allelic, dominant, and recessive), using the Pearson χ2 and Fisher’s exact tests, and assessed with the ORs and their corresponding 95% CIs. We defined the models as follows: genotypic (DD vs. Dd vs. dd), allelic (D vs. d), dominant (DD + Dd vs. dd), recessive (DD vs. Dd + dd), and additive (dd = 0, Dd = 1, DD = 2), where D is the minor allele and d the major allele. We considered unconditional multiple logistic regression models (genotypic, dominant, and recessive) to determine the influence of possible confounding variables on the risk of suffering from HBP.
All the tests were 2-sided, with a significance level of p < 0.05, and were estimated using PLINK and R 4.0.2 software [,]. We performed LD with Haploview 4.2 [] and haplotype analysis with SNPStats [].
5. Conclusions
This study shows that the GC rs7041 SNP is associated with hypertension development and could play a notable role as a biomarker for the risk of the disease. By contrast, no relationship was found between the rs1544410 (VDR BsmI), rs11568820 (VDR Cdx2), rs2228570 (VDR FokI), rs7975232 (VDR ApaI), rs731236 (VDR TaqI), rs10741657 (CYP2R1), rs4646536 (CYP27B1), rs3782130 (CYP27B1), rs10877012 (CYP27B1), rs703842 (CYP27B1), rs6068816 (CYP24A1), and rs4809957 (CYP24A1) SNPs and the risk of suffering from HBP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24065974/s1.
Author Contributions
Conceptualization, C.P.-R., L.E.P.-L., S.R.-T. and J.M.G.-N.; methodology, N.M.-P., A.F.-A., C.M.-J. and J.M.G.-N.; software, S.R.-T. and L.E.P.-L.; validation, C.P.-R., M.C.R.-T. and A.J.-M.; formal analysis, S.R.-T. and L.E.P.-L.; investigation, S.R.-T., J.M.G.-N., N.M.-P., A.F.-A., C.M.-J., and L.E.P.-L.; resources, C.P.-R. and A.J.-M.; data curation, C.P.-R.; writing—original draft preparation, J.M.G.-N., S.R.-T. and L.E.P.-L.; writing—review and editing, C.P.-R.; visualization, N.M.-P. and C.P.-R.; supervision, C.P.-R.; project administration, M.C.R.-T.; funding acquisition, A.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
ERDF funds (EU) from the Instituto de Salud Carlos III (PT13/0010/0039) supported by co-funding grants from the Biobank of the Hospital Universitario Virgen de las Nieves.
Institutional Review Board Statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics and Research Committee of the Sistema Andaluz de Salud (code: 0957-N-21).
Informed Consent Statement
All subjects involved in the study signed the written informed consent form.
Data Availability Statement
Data unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
BP: blood pressure; BMI: body mass index; CI: confidence interval; CYP24A1: cytochrome P450 family 24 subfamily A member 1; CYP27B1: cytochrome P450 family 27 subfamily B member 1; CYP2R1: cytochrome P450 family 2 subfamily R member 1; CVD: cardiovascular disease; DBP: diastolic blood pressure; GC: GC Vitamin D Binding Protein (gene); HBP: high blood pressure; HDL: high-density lipoprotein; LD: linkage disequilibrium; LDL: low-density lipoprotein; MAF: minor allele frequency; NCD-RisC: non-communicable disease risk factor collaboration; OR: odds ratio; PCR: polymerase chain reaction; RAAS: renin–angiotensin–aldosterone system; RXR: retinoid X receptor; SBP: systolic blood pressure; SDU: standard drink unit; SNP: single nucleotide polymorphism; UVB: ultraviolet B; VDBP: vitamin D binding protein; VDR: vitamin D receptor (gene); VDR: vitamin D receptor (protein); WHO: World Health Organization.
References
- Sociedad Europea de Cardiología/Sociedad Europea de Hipertensión. Guía ESC/ESH sobre el diagnóstico y tratamiento de la hipertensión. Rev. Esp. Cardiol. 2019, 72, 104–810. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jameson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, 23976. [Google Scholar] [CrossRef]
- Hypertension. World Health Organization (WHO) [Internet]. Available online: https://www.who.int/es/news-room/fact-sheets/detail/hypertension (accessed on 27 September 2022).
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Li, Z.Q.; Ding, N.; Yi, H.L. The association between vitamin D receptor gene polymorphism and susceptibility to hypertension: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9066–9074. [Google Scholar] [CrossRef]
- Nunes, I.F.O.C.; Cavalcante, A.A.C.M.; Alencar, M.V.O.B.; Carvalho, M.D.F.; Sermento, J.L.R.; Teixeira, N.S.C.C.A.; Paiva, A.A.; Carvalho, L.R.; Nascimento, L.C.F.; Cruz, M.S.P.; et al. Meta-Analysis of the Association Between the rs228570 Vitamin D Receptor Gene Polymorphism and Arterial Hypertension Risk. Adv. Nutr. 2020, 11, 1211–1220. [Google Scholar] [CrossRef]
- González-Rojo, P.; Pérez-Ramírez, C.; Gálvez-Navas, J.M.; Pineda-Lancheros, L.E.; Rojo-Tolosa, S.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Vitamin D-related single nucleotide polymorphisms as risk biomarker of cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 8686. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Park, K.M.; Lee, M.J.; Yang, D.H.; Kim, S.H.; Lee, S.Y. Vitamin D and Hypertension. Electrolyte Blood Press. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Kumutsor, S.K.; Burgess, S.; Munroe, P.B.; Khan, H. Vitamin D and high blood pressure: Causal association or epiphenomenon? Eur. J. Epidemiol. 2014, 29, 1–14. [Google Scholar] [CrossRef]
- Kumutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Couto Alves, A.; Heerspink, H.J.L.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian andomization study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Afzal, S.; Nordestgaard, B.G. Vitamin D, Hypertension, and Ischemic Stroke in 116 655 Individuals from the General Population: A Genetic Study. Hypertension 2017, 70, 499–507. [Google Scholar] [CrossRef]
- Márquez-Pete, N.; Pérez-Ramírez, C.; Maldonado Montoro, M.M.; Martínez-Martínez, F.; Farnández-Llimos, F.; Sánchez-Pozo, A.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Association of vitamin D receptor genes polymorphisms with rheumatoid arthritis. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Rojo-Tolosa, S.; Pineda-Lancheros, L.E.; Gálvez-Navas, J.M.; Sánchez-Martínez, J.A.; González-Gutiérrez, M.V.; Fernández-Alonso, A.; Morales García, C.; Jiménez-Morales, A.; Pérez-Ramírez, C. Association between Polymorphisms of the Vitamin D Metabolic Pathway and the Risk of Developing Asthma. Nutrients 2023, 15, 823. [Google Scholar] [CrossRef] [PubMed]
- Sirajudeen Shaf, I.; Al Menhali, A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef]
- Basit, S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Shen, H.; Bielak, B.F.; Ferguson, J.F.; Streeten, E.A.; Yerges-Amstrong, L.M.; Liu, J. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2648–2654. [Google Scholar] [CrossRef]
- Pineda-Lancheros, L.E.; Rojo-Tolosa, S.; Gálvez-Navas, J.M.; Martínez-Martínez, F.; Sánchez-Martín, A.; Jiménez-Morales, A.; Pérez-Ramírez, C. Effect of Single Nucleotide Polymorphisms in the Vitamin D Metabolic Pathway on Susceptibility to Non-Small Cell Lung Cancer. Nutrients 2022, 14, 4668. [Google Scholar] [CrossRef]
- Nan, G.; Chu, X.-P.; Xuan, Y.-P.; Ren, D.-Q.; Wang, Y.; Ma, K.; Gao, H.-J.; Jiao, W.-J. Associations between abnormal vitamin D metabolism pathway function and non-small cell lung cancer. Oncol. Lett. 2017, 14, 7538–7544. [Google Scholar] [CrossRef]
- Pineda-Lancheros, L.E.; Pérez-Ramírez, C.; Sánchez-Martín, A.; Gálvez-Navas, J.M.; Martínez-Martínez, F.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Impact of Genetic Polymorphisms on the Metabolic Pathway of Vitamin D and Survival in Non-Small Cell Lung Cancer. Nutrients 2021, 13, 3783. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl. Neurodegener. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Man, Q.; Li, L.; Song, P.; Jia, S.; Song, S. Vitamin D receptor gene polymorphisms modify the association of serum 25-hydroxyvitamin D levels with handgrip strength in the elderly in Nothern China. Nutrition 2019, 57, 202–207. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef] [PubMed]
- Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2021, 59, 1–30. [Google Scholar] [CrossRef]
- Bowman, K.; Jones, L.; Pilling, L.C.; Delgado, J.; Kuchel, G.A.; Ferrucci, L.; Fortinsky, R.H.; Melzer, D. Vitamin D levels and risk of delirium: A mendelian randomization study in the UK Biobank. Neurology 2019, 92, e1387–e1394. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Artherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- De la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galdeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef]
- Legarth, C.; Grimm, D.; Wheland, M.; Bauer, J.; Krüger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef]
- Rendina, D.; De Filippo, G.; Muscariello, R.; De Palma, D.; Fiengo, A.; De Pascale, F.; Strazzullo, P. Vitamin D and cardiometabolic disorders. High Blood Press. Cardiovasc. Prev. 2014, 21, 251–256. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Kiani, A.; Mohamadi-Nori, E.; Vaisi-Raygani, A.; Tanhapour, M.; Elahi-Rad, S.; Bahrehmad, F.; Rahimi, Z.; Pourmotabbed, T. Vitamin D-binding protein and vitamin D receptor genotypes and 25-hydroxyvitamin D levels are associated with development of aortic and mitral valve calcification and coronary artery diseases. Mol. Biol. Rep. 2019, 5, 5225–5236. [Google Scholar] [CrossRef]
- Daffara, V.; Verdoia, M.; Rolla, R.; Nardin, M.; Marino, P.; Bellomo, G.; Carriero, A.; De Luca, G. Impact of polymorphism rs7041 and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 775–783. [Google Scholar] [CrossRef]
- Michos, E.D.; Misialek, J.R.; Selvin, E.; Folsom, A.R.; Pankow, J.S.; Post, W.S.; Lutsey, P.L. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incidental coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis 2015, 241, 12–17. [Google Scholar] [CrossRef]
- Muray, S.; Parisi, E.; Cardús, A.; Craver, L.; Fernández, E. Influence of vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D on blood pressure in apparently healthy subjects. J. Hypertens. 2003, 21, 2069–2075. [Google Scholar] [CrossRef]
- Wang, L.; Chu, A.; Buring, J.E.; Ridker, P.M.; Chasman, D.I.; Sesso, H.D. Common Genetic Variations in the Vitamin D Pathway in Relation to Blood Pressure. Am. J. Hypertens. 2014, 27, 1387–1395. [Google Scholar] [CrossRef]
- Ye, X.; Jia, J.; Zhang, N.; Ding, H.; Zhan, Y. Associations of genetic polymorphisms of the vitamin D pathway with blood pressure in a Han Chinese population. Clin. Exp. Hypertens. 2019, 41, 460–465. [Google Scholar] [CrossRef]
- Ministerio de Sanidad Servicios Sociales e Igualdad. Guía de Práctica Clínica Sobre Diabetes Tipo 2; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2008. [Google Scholar]
- Ministerio de Sanidad Servicios Sociales e Igualdad. Guía de Práctica Clínica Sobre El Manejo de Los Lípidos Como Factor de Riesgo Cardiovascular; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2017. [Google Scholar]
- Randolph, J.J.; Falbe, K.; Kureethara, A.M.; Balloun, J.L. A step-by-step guide to propensity score matching in R. Pract. Assess. Res. Eval. 2014, 19, 1–6. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 236–265. [Google Scholar] [CrossRef]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).