Myeloid Trem2 Dynamically Regulates the Induction and Resolution of Hepatic Ischemia-Reperfusion Injury Inflammation
Abstract
1. Introduction
2. Results
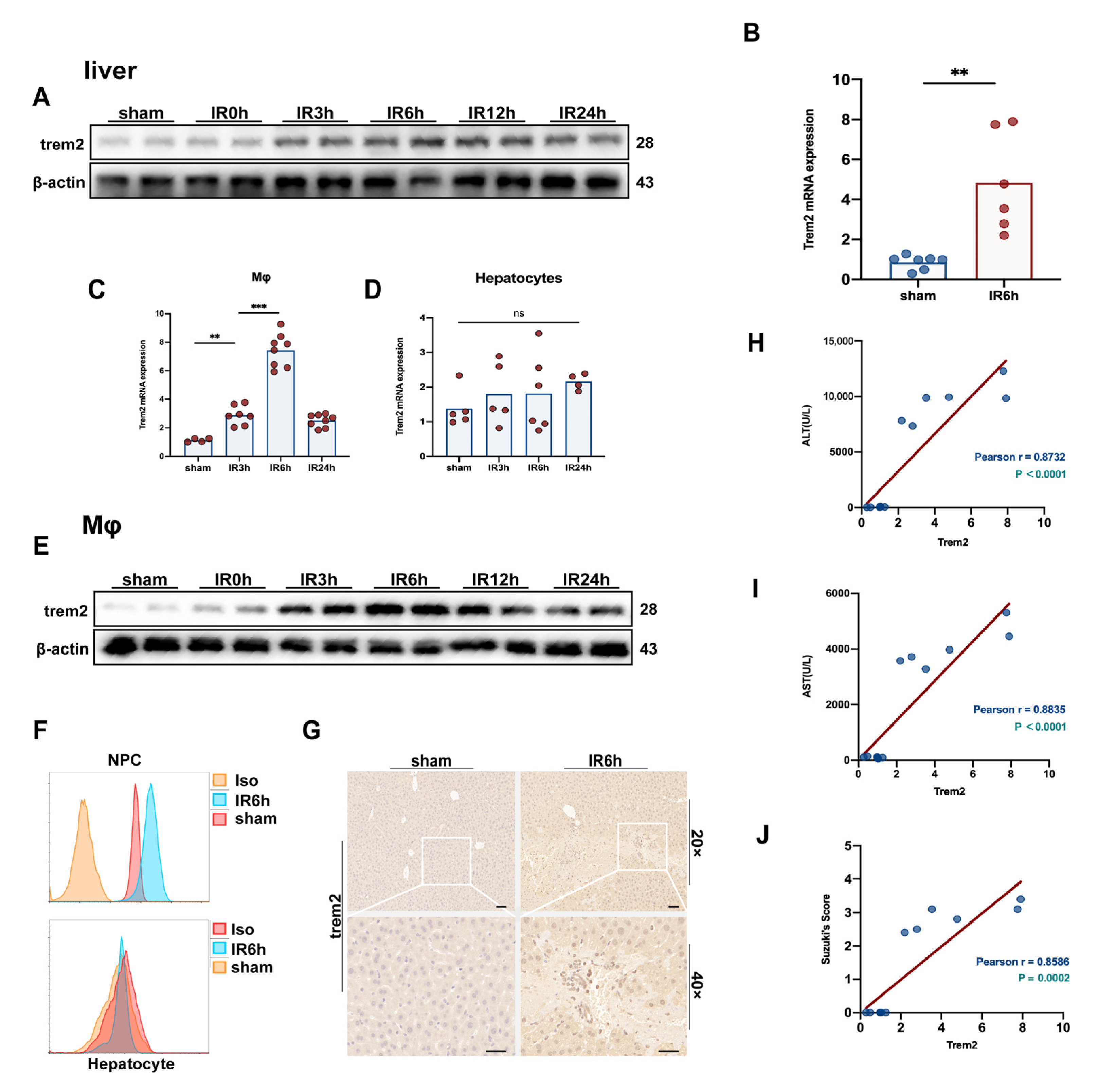
2.1. The Expression of Trem2 in Monocyte-Derived Macrophages Was in Response to Activated Reperfusion Injury after Hepatic Ischemia
2.2. Deficiency of Myeloid Trem2 Alleviates Liver Injury in the Early Stages of Reperfusion
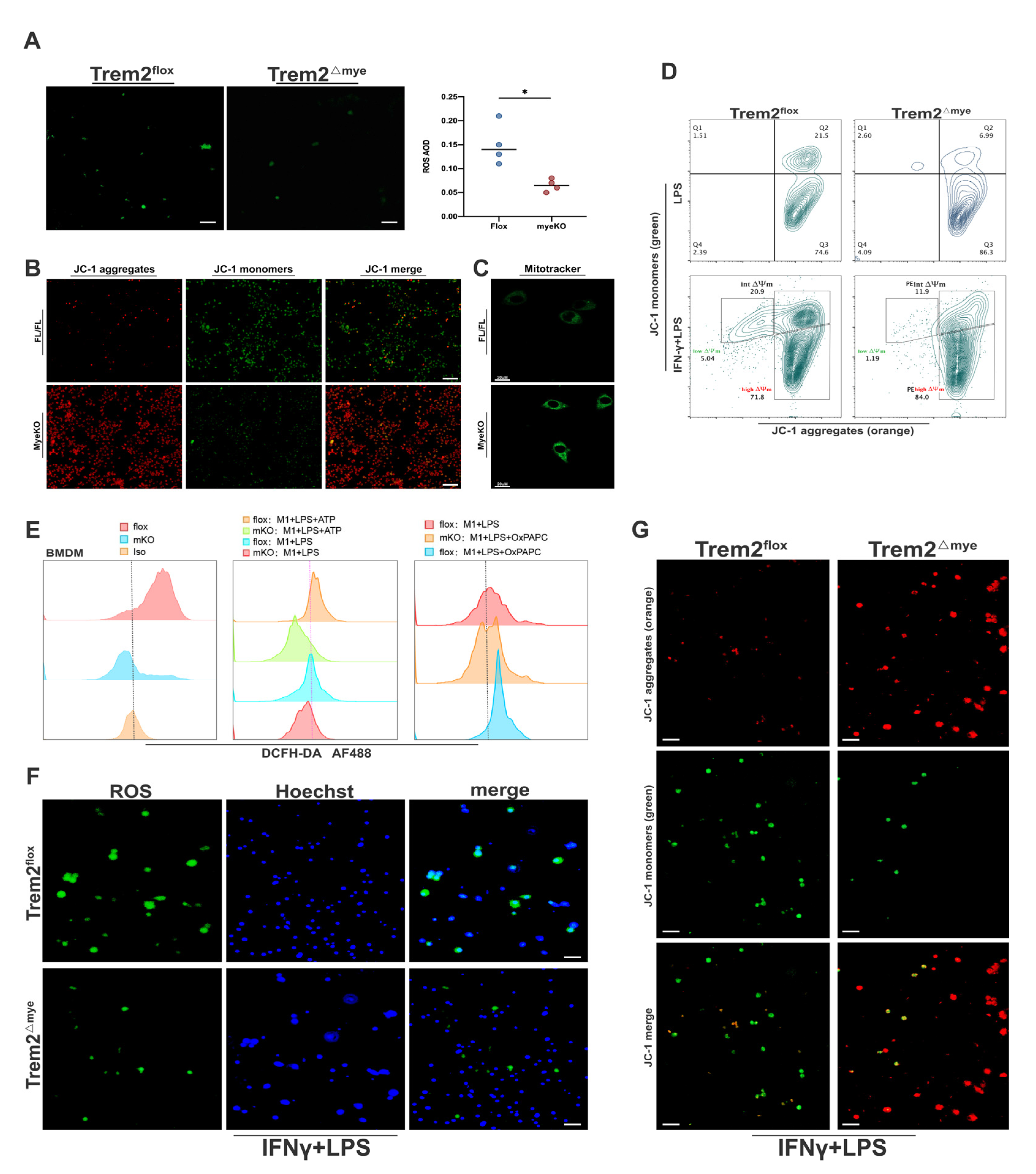
2.3. Mitochondrial Damage and ROS Accumulation Are Significantly Reduced in Trem2-Depleted Macrophages
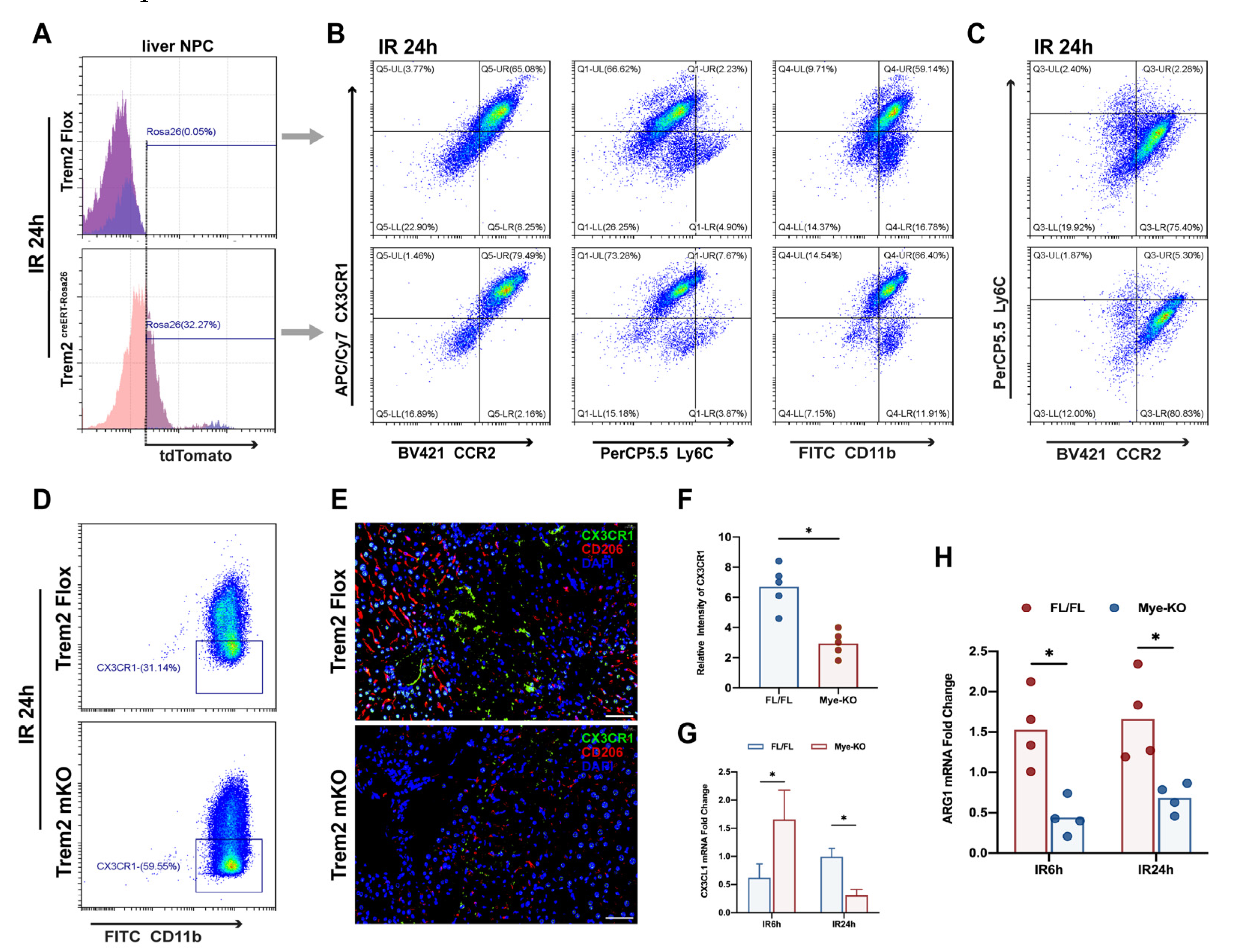
2.4. Myeloid Trem2 is Responsible for Driving the Phenotypic Transformation of Monocyte-Derived Macrophages
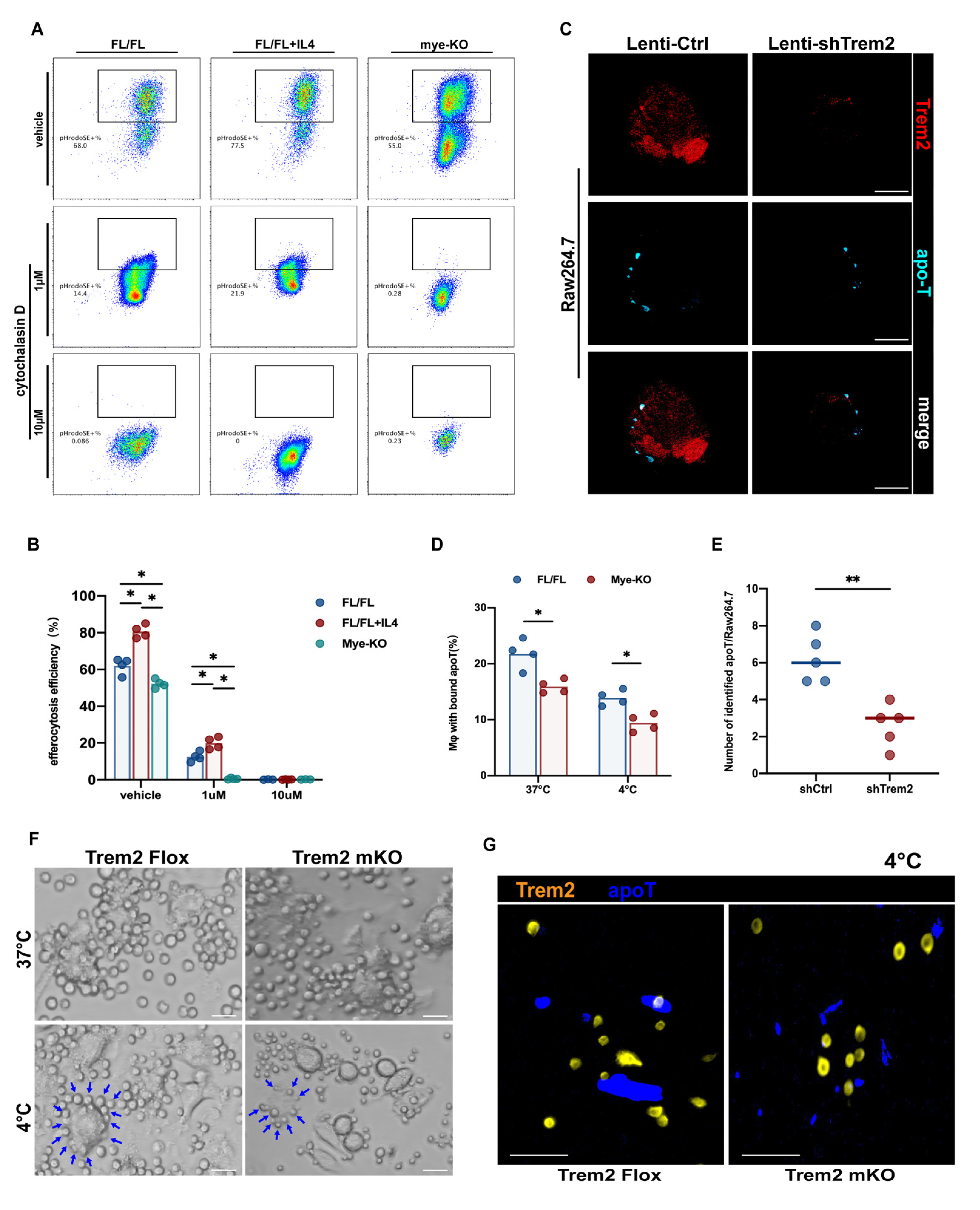
2.5. Myeloid Trem2 is Essential to Mφ Efferocytosis on the Stage of Liver IR Inflammation Resolution
2.6. Trem2 is Involved in the Recognition and Internalization of Apoptotic Cells
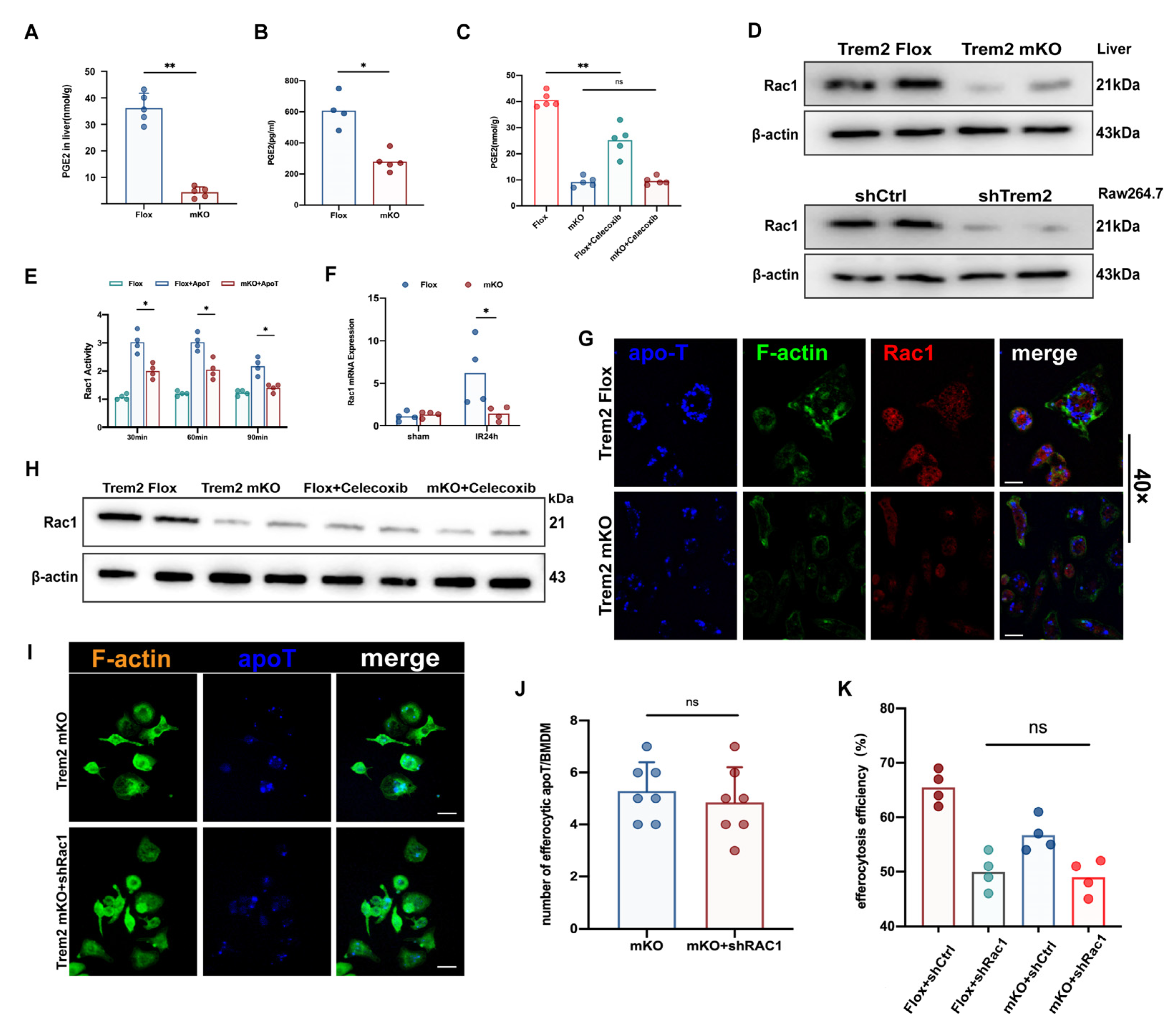
2.7. Myeloid Trem2 Regulates Cox2/PGE2-Mediated Rac1 Activation in Efferocytic Mφs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Mouse Liver Partial Warm Ischemia Model
4.3. Liver NPC Isolation
4.4. Measurement of mtDNA
4.5. Measurement of ROS and Mitochondrial Membrane Potential
4.6. Lentiviral Preparation of shTrem2 and shRac1
4.7. Mass Spectrometry Analysis
4.8. Extraction and Culture of Bone Marrow-Derived Macrophages (BMDMs)
4.9. Induction and Isolation of Peritoneal Macrophages
4.10. Isolation of Thymocytes
4.11. Preparation of Apoptotic Thymocytes
4.12. Efferocytosis Assay
4.13. Immunohistochemistry and Immunofluorescence
4.14. Flow Cytometry
4.15. Quantitative RT-PCR
4.16. ELISA Assay
4.17. Western Blot
4.18. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2012, 10, 79–89. [Google Scholar] [CrossRef]
- Davies, L.C.; Rosas, M.; Jenkins, S.J.; Liao, C.-T.; Scurr, M.J.; Brombacher, F.; Fraser, D.J.; Allen, J.E.; Jones, S.A.; Taylor, P.R. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. 2013, 4, 1886. [Google Scholar] [CrossRef]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P.; Leibovich, S.; et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 155, 56–65. [Google Scholar] [CrossRef]
- Mulder, K.; Patel, A.A.; Kong, W.T.; Piot, C.; Halitzki, E.; Dunsmore, G.; Khalilnezhad, S.; Irac, S.E.; Dubuisson, A.; Chevrier, M.; et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 2021, 54, 1883–1900.e5. [Google Scholar] [CrossRef]
- Zigmond, E.; Samia-Grinberg, S.; Pasmanik-Chor, M.; Brazowski, E.; Shibolet, O.; Halpern, Z.; Varol, C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 2014, 193, 344–353. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, J.; Sosa, R.; Zhang, H.; Wang, H.; Jin, D.; Crowley, K.; Naini, B.; Reed, F.E.; Busuttil, R.W.; et al. T-Cell Immunoglobulin and Mucin Domain-Containing Protein-4 Is Critical for Kupffer Cell Homeostatic Function in the Activation and Resolution of Liver Ischemia Reperfusion Injury. Hepatology 2021, 74, 2118–2132. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Koike, M.; Spusta, S.C.; Niemi, E.C.; Yenari, M.; Nakamura, M.C.; Seaman, W.E. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J. Neurochem. 2009, 109, 1144–1156. [Google Scholar] [CrossRef]
- Kober, D.L.; Brett, T.J. TREM2-Ligand Interactions in Health and Disease. J. Mol. Biol. 2017, 429, 1607–1629. [Google Scholar] [CrossRef]
- Esparza-Baquer, A.; Labiano, I.; Sharif, O.; Agirre-Lizaso, A.; Oakley, F.; Rodrigues, P.M.; Zhuravleva, E.; O’Rourke, C.J.; Hijona, E.; Jimenez-Agüero, R.; et al. TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut 2020, 70, 1345–1361. [Google Scholar] [CrossRef]
- Gonçalves, L.A.; Rodrigues-Duarte, L.; Rodo, J.; de Moraes, L.V.; Marques, I.; Penha-Gonçalves, C. TREM2 governs Kupffer cell activation and explains belr1 genetic resistance to malaria liver stage infection. Proc. Natl. Acad. Sci. USA 2013, 110, 19531–19536. [Google Scholar] [CrossRef]
- Nakao, T.; Ono, Y.; Dai, H.; Nakano, R.; Perez-Gutierrez, A.; Camirand, G.; Huang, H.; Geller, D.A.; Thomson, A.W. DNAX Activating Protein of 12 kDa/Triggering Receptor Expressed on Myeloid Cells 2 Expression by Mouse and Human Liver Dendritic Cells: Functional Implications and Regulation of Liver Ischemia-Reperfusion Injury. Hepatology 2018, 70, 696–710. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Gilfillan, S.; Cella, M.; Aoshi, T.; Miller, M.; Piccio, L.; Hernandez, M.; Colonna, M. Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 2006, 177, 3520–3524. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Esparza-Baquer, A.; Oakley, F.; Labiano, I.; Korosec, A.; Jais, A.; Mann, J.; Tiniakos, D.; Santos-Laso, A.; Arbelaiz, A.; et al. Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage. Gut 2018, 68, 533–546. [Google Scholar] [CrossRef]
- Coelho, I.; Duarte, N.; Barros, A.; Macedo, M.P.; Penha-Gonçalves, C. Trem-2 Promotes Emergence of Restorative Macrophages and Endothelial Cells During Recovery From Hepatic Tissue Damage. Front. Immunol. 2021, 11, 616044. [Google Scholar] [CrossRef]
- Li, Y.; Long, W.; Gao, M.; Jiao, F.; Chen, Z.; Liu, M.; Yu, L. TREM2 Regulates High Glucose-Induced Microglial Inflammation via the NLRP3 Signaling Pathway. Brain Sci. 2021, 11, 896. [Google Scholar] [CrossRef]
- Molgora, M.; Esaulova, E.; Vermi, W.; Hou, J.; Chen, Y.; Luo, J.; Brioschi, S.; Bugatti, M.; Omodei, A.S.; Ricci, B.; et al. TREM2 Modulation Remodels the Tumor Myeloid Landscape Enhancing Anti-PD-1 Immunotherapy. Cell 2020, 182, 886–900.e17. [Google Scholar] [CrossRef]
- Krenkel, O.; Hundertmark, J.; Abdallah, A.T.; Kohlhepp, M.; Puengel, T.; Roth, T.; Branco, D.P.P.; Mossanen, J.C.; Luedde, T.; Trautwein, C.; et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut 2019, 69, 551–563. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Deng, L.; Huang, X.; Yang, G.; Cheng, Q.; Guo, T.; Guo, L.; Niu, C.; Yang, X.; et al. Hepatic RACK1 deficiency protects against fulminant hepatitis through myeloid-derived suppressor cells. Theranostics 2022, 12, 2248–2265. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Cui, P.; Zhou, Y.; Liu, C.; Wu, X.; Ji, Y.; Wang, S.; Cheng, B.; Ye, H.; et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J. Clin. Investig. 2021, 131, e135197. [Google Scholar] [CrossRef]
- Nakano, R.; Tran, L.M.; Geller, D.A.; Macedo, C.; Metes, D.M.; Thomson, A.W. Dendritic Cell-Mediated Regulation of Liver Ischemia-Reperfusion Injury and Liver Transplant Rejection. Front. Immunol. 2021, 12, 705465. [Google Scholar] [CrossRef]
- Hu, M.; Lin, Y.; Men, X.; Wang, S.; Sun, X.; Zhu, Q.; Lu, D.; Liu, S.; Zhang, B.; Cai, W.; et al. High-salt diet downregulates TREM2 expression and blunts efferocytosis of macrophages after acute ischemic stroke. J. Neuroinflam. 2021, 18, 90. [Google Scholar] [CrossRef]
- Wang, J.; Kubes, P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell 2016, 165, 668–678. [Google Scholar] [CrossRef]
- Lantz, C.; Radmanesh, B.; Liu, E.; Thorp, E.B.; Lin, J. Single-cell RNA sequencing uncovers heterogenous transcriptional signatures in macrophages during efferocytosis. Sci. Rep. 2020, 10, 14333. [Google Scholar] [CrossRef]
- Morioka, S.; Perry, J.S.A.; Raymond, M.H.; Medina, C.B.; Zhu, Y.; Zhao, L.; Serbulea, V.; Onengut-Gumuscu, S.; Leitinger, N.; Kucenas, S.; et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 2018, 563, 714–718. [Google Scholar] [CrossRef]
- Yurdagul, A., Jr.; Subramanian, M.; Wang, X.; Crown, S.B.; Ilkayeva, O.R.; Darville, L.; Kolluru, G.K.; Rymond, C.C.; Gerlach, B.D.; Zheng, Z.; et al. Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell Metab. 2020, 31, 518–533.e10. [Google Scholar] [CrossRef]
- Ampomah, P.B.; Cai, B.; Sukka, S.R.; Gerlach, B.D.; Yurdagul, A.; Wang, X.; Kuriakose, G.; Darville, L.N.F.; Sun, Y.; Sidoli, S.; et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 2022, 4, 444–457. [Google Scholar] [CrossRef]
- Meriwether, D.; Jones, A.E.; Ashby, J.W.; Solorzano-Vargas, R.S.; Dorreh, N.; Noori, S.; Grijalva, V.; Ball, A.B.; Semis, M.; Divakaruni, A.S.; et al. Macrophage COX2 Mediates Efferocytosis, Resolution Reprogramming, and Intestinal Epithelial Repair. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1095–1120. [Google Scholar] [CrossRef]
- Nakaya, M.; Kitano, M.; Matsuda, M.; Nagata, S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9198–9203. [Google Scholar] [CrossRef]
- Richens, T.R.; Linderman, D.J.; Horstmann, S.A.; Lambert, C.; Xiao, Y.-Q.; Keith, R.L.; Boé, D.M.; Morimoto, K.; Bowler, R.P.; Day, B.J.; et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 2009, 179, 1011–1021. [Google Scholar] [CrossRef]
- Frasch, S.C.; Fernandez-Boyanapalli, R.F.; Berry, K.Z.; Leslie, C.C.; Bonventre, J.V.; Murphy, R.C.; Henson, P.M.; Bratton, D.L. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J. Biol. Chem. 2011, 286, 12108–12122. [Google Scholar] [CrossRef]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef]
- Kinoshita, M.; Uchida, T.; Sato, A.; Nakashima, M.; Nakashima, H.; Shono, S.; Habu, Y.; Miyazaki, H.; Hiroi, S.; Seki, S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 2010, 53, 903–910. [Google Scholar] [CrossRef]
- Klein, I.; Cornejo, J.C.; Polakos, N.K.; John, B.; Wuensch, S.A.; Topham, D.J.; Pierce, R.H.; Crispe, I.N. Kupffer cell heterogeneity: Functional properties of bone marrow derived and sessile hepatic macrophages. Blood 2007, 110, 4077–4085. [Google Scholar] [CrossRef]
- Dal-Secco, D.; Wang, J.; Zeng, Z.; Kolaczkowska, E.; Wong, C.; Petri, B.; Ransohoff, R.M.; Charo, I.F.; Jenne, C.N.; Kubes, P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 2015, 212, 447–456. [Google Scholar] [CrossRef]
- Doddapattar, P.; Dev, R.; Ghatge, M.; Patel, R.B.; Jain, M.; Dhanesha, N.; Lentz, S.R.; Chauhan, A.K. Myeloid Cell PKM2 Deletion Enhances Efferocytosis and Reduces Atherosclerosis. Circ. Res. 2022, 130, 1289–1305. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, J.; Chen, R.; Lin, Y.; Chen, H.; Ling, Q. The role of macrophage TAM receptor family in the acute-to-chronic progression of liver disease: From friend to foe? Liver Int. 2022, 42, 2620–2631. [Google Scholar] [CrossRef]
- Yue, S.; Zhou, H.; Wang, X.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Zhai, Y. Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J. Immunol. 2017, 198, 3588–3595. [Google Scholar] [CrossRef]
- Schlegel, M.; Köhler, D.; Körner, A.; Granja, T.; Straub, A.; Giera, M.; Mirakaj, V. The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology 2015, 63, 1689–1705. [Google Scholar] [CrossRef]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Subramanian, M.; Sansbury, B.E.; Lin, C.-S.; Spite, M.; Fredman, G.; Tabas, I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, 6526–6531. [Google Scholar] [CrossRef]
- Deberge, M.; Yeap, X.Y.; Dehn, S.; Zhang, S.; Grigoryeva, L.; Misener, S.; Procissi, D.; Zhou, X.; Lee, D.; Muller, W.A.; et al. MerTK Cleavage on Resident Cardiac Macrophages Compromises Repair After Myocardial Ischemia Reperfusion Injury. Circ. Res. 2017, 121, 930–940. [Google Scholar] [CrossRef]
- Glinton, K.E.; Ma, W.; Lantz, C.W.; Grigoryeva, L.S.; DeBerge, M.; Liu, X.; Febbraio, M.; Kahn, M.; Oliver, G.; Thorp, E.B. Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J. Clin. Investig. 2022, 132, e140685. [Google Scholar] [CrossRef]
- Huang, W.; Wang, B.O.; Hou, Y.F.; Fu, Y.; Cui, S.-J.; Zhu, J.-H.; Zhan, X.-Y.; Li, R.-K.; Tang, W.; Wu, J.-C.; et al. JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism. J. Clin. Investig. 2022, 7, e158571. [Google Scholar] [CrossRef]
- Gerlach, B.D.; Ampomah, P.B.; Yurdagul, A., Jr.; Liu, C.; Lauring, M.C.; Wang, X.; Kasikara, C.; Kong, N.; Shi, J.; Tao, W.; et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 2021, 33, 2445–2463.e8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Li, X.; Xia, N.; Zhang, Y.; Yu, W.; Li, J.; Jiao, C.; Wang, Z.; Pu, L. Myeloid Trem2 Dynamically Regulates the Induction and Resolution of Hepatic Ischemia-Reperfusion Injury Inflammation. Int. J. Mol. Sci. 2023, 24, 6348. https://doi.org/10.3390/ijms24076348
Han S, Li X, Xia N, Zhang Y, Yu W, Li J, Jiao C, Wang Z, Pu L. Myeloid Trem2 Dynamically Regulates the Induction and Resolution of Hepatic Ischemia-Reperfusion Injury Inflammation. International Journal of Molecular Sciences. 2023; 24(7):6348. https://doi.org/10.3390/ijms24076348
Chicago/Turabian StyleHan, Sheng, Xiangdong Li, Nan Xia, Yu Zhang, Wenjie Yu, Jie Li, Chenyu Jiao, Ziyi Wang, and Liyong Pu. 2023. "Myeloid Trem2 Dynamically Regulates the Induction and Resolution of Hepatic Ischemia-Reperfusion Injury Inflammation" International Journal of Molecular Sciences 24, no. 7: 6348. https://doi.org/10.3390/ijms24076348
APA StyleHan, S., Li, X., Xia, N., Zhang, Y., Yu, W., Li, J., Jiao, C., Wang, Z., & Pu, L. (2023). Myeloid Trem2 Dynamically Regulates the Induction and Resolution of Hepatic Ischemia-Reperfusion Injury Inflammation. International Journal of Molecular Sciences, 24(7), 6348. https://doi.org/10.3390/ijms24076348






