Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells
Abstract
:1. Introduction
2. Oogenesis, Oocyte Growth, and Oocyte Maturation
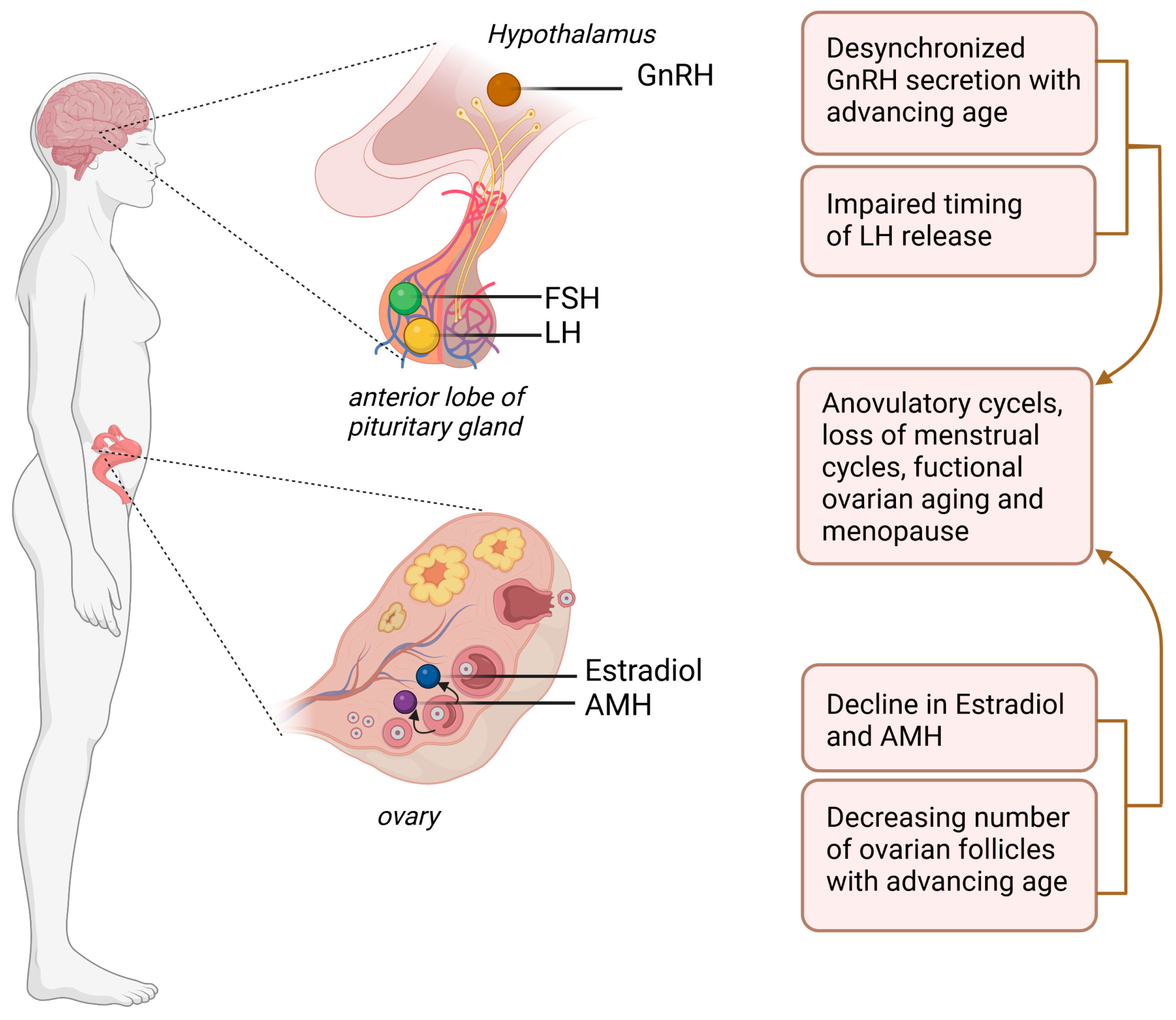
3. Oocyte Release, Relevant Hormones, Environment, and Maternal to Zygotic Transition
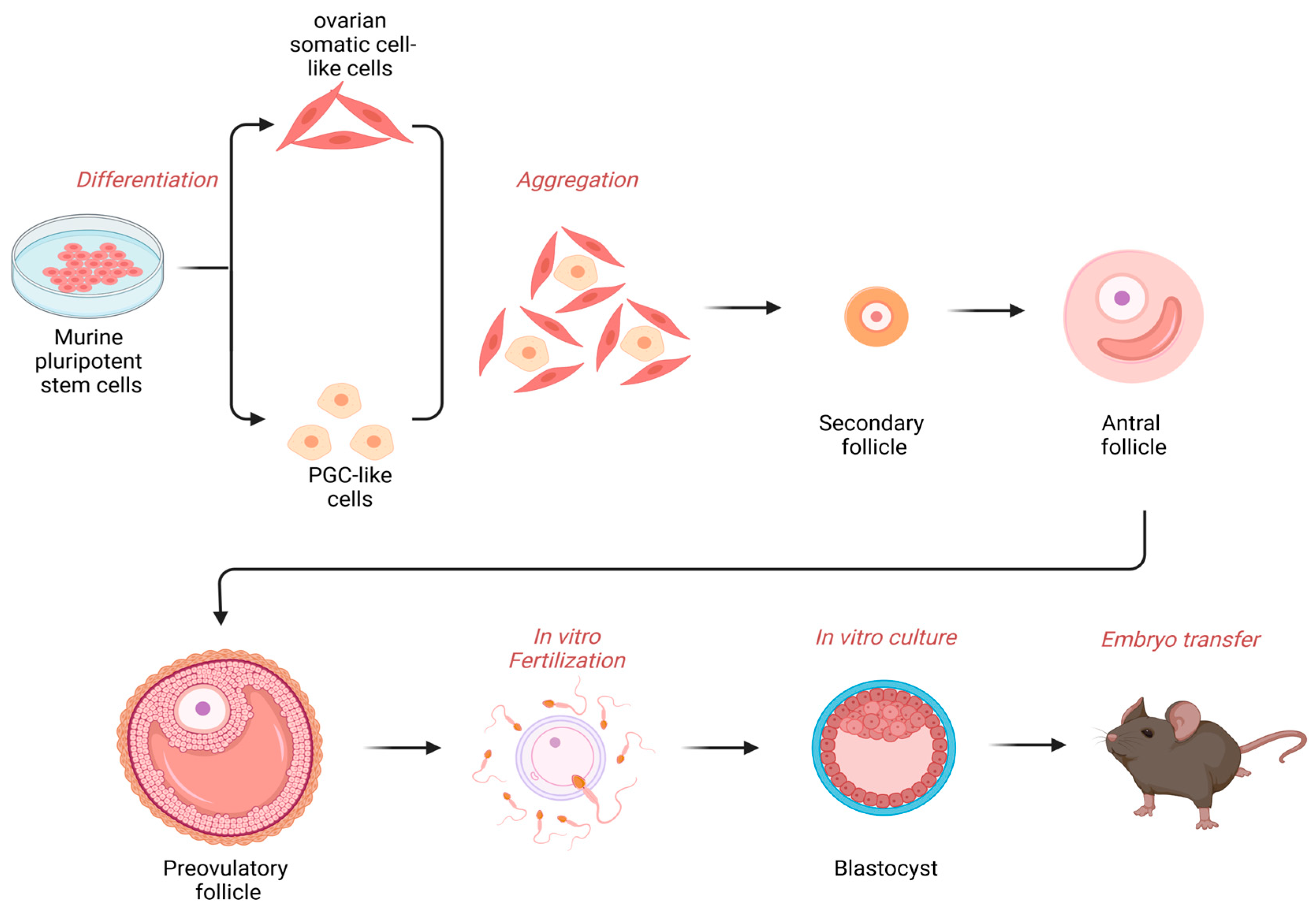
4. Implementations of Stem Cells for Reproductive and Regenerative Medicine
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verlhac, M.-H.; Terret, M.-E. Oocyte Maturation and Development. F1000Research 2016, 5, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A Comparative Analysis of Oocyte Development in Mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Khawar, M.B.; Li, W. Autophagy in Reproduction. Adv. Exp. Med. Biol. 2019, 1206, 453–468. [Google Scholar] [PubMed]
- Sanchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virant-Klun, I. Postnatal oogenesis in humans: A review of recent findings. Stem Cells Cloning 2015, 8, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Yatsenko, S.A.; Rajkovic, A. Genetics of human female infertility. Biol. Reprod. 2019, 101, 549–566. [Google Scholar] [CrossRef]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, M.; Crichton, J.H.; Playfoot, C.J.; Adams, I.R. Oocyte development, meiosis and aneuploidy. Semin. Cell Dev. Biol. 2015, 45, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Zickler, D.; Kleckner, N. Recombination, Pairing, and Synapsis of Homologs during Meiosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a016626. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.; Rajkovic, A. Genomic Markers of Ovarian Reserve. Semin. Reprod. Med. 2013, 31, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Handel, M.A.; Schimenti, J.C. Genetics of mammalian meiosis: Regulation, dynamics and impact on fertility. Nat. Rev. Genet 2010, 11, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Tora, L.; Vincent, S.D. What Defines the Maternal Transcriptome? Biochem. Soc. Trans. 2021, 49, 2051–2062. [Google Scholar] [CrossRef]
- Christou-Kent, M.C.; Dhellemmes, M.; Lambert, E.; Ray, P.F.; Arnoult, C. Diversity of RNA-Binding Proteins Modulating Post-Transcriptional Regulation of Protein Expression in the Maturing Mammalian Oocyte. Cells 2020, 9, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissen, G.A.; Garcia-Rudaz, C.; Ojeda, S.R. Role of Neurotrophic Factors in Early Ovarian Development. Semin. Reprod. Med. 2009, 27, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikanfar, S.; Oghbaei, H.; Rezaei, Y.R.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Nejabati, H.R.; Bahrami, Z.; Bleisinger, N.; Samadi, N.; Fattahi, A.; et al. Role of adipokines in the ovarian function: Oogenesis and steroidogenesis. J. Steroid. Biochem. Mol. Biol. 2021, 209, 105852. [Google Scholar] [CrossRef]
- Cioffi, J.A.; VanBlerkom, J.; Antczak, M.; Shafer, A.; Wittmer, S.; Snodgrass, H.R. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol. Hum. Reprod. 1997, 3, 467–472. [Google Scholar] [CrossRef]
- Almog, B.; Gold, R.; Tajima, K.; Dantes, A.; Salim, K.; Rubinstein, M.; Barkan, D.; Homburg, R.; Lessing, J.B.; Nevo, N.; et al. Leptin Attenuates Follicular Apoptosis and Accelerates the Onset of Puberty in Immature Rats. Moll. Cell. Endocrinol. 2001, 183, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Hamm, M.L.; Bhat, G.K.; Thompson, W.E.; Mann, D.R. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol. Reprod. 2004, 71, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilbao, M.G.; Di Yorio, M.P.; Galarza, R.A.; Varone, C.L.; Faletti, A.G. Regulation of the Ovarian Oxidative Status by Leptin during the Ovulatory Process in Rats. Reproduction 2015, 149, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Tsai, E.-M.; Wu, L.-C.; Chen, S.-Y.; Chang, Y.-H.; Jong, S.-B.; Chan, T.-F. Higher Basal Adiponectin Levels Are Associated with Better Ovarian Response to Gonadotropin Stimulation during in Vitro Fertilization. Gynecol. Obstet. Investig. 2005, 60, 167–170. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tsai, E.-M.; Chen, H.-S.; Liu, Y.-H.; Lee, C.-H.; Chou, F.-H.; Chen, I.-J.; Chen, S.-Y.; Jong, S.-B.; Chan, T.-F. Serum Resistin Level Is a Predictor of Ovarian Response in in Vitro Fertilisation Cycle. Acta Obstet. Gynecol. Scand. Acta Obstet. Gynecol. Scand. 2007, 86, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. J. Reprod. Dev. 2020, 66, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, C.; Silva, J.V.; Silva, V.; Torgal, I.; Fardilha, M. Signalling Pathways Involved in Oocyte Growth, Acquisition of Competence and Activation. Hum. Fertil. 2015, 18, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The Pivotal Role of Glucose Metabolism in Determining Oocyte Developmental Competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, M.; Li, Y.; Zhang, Y. Profiling and functional characterization of maternal mRNA translation during mouse maternal-to-zygotic transition. Sci. Adv. 2022, 8, eabj3967. [Google Scholar] [CrossRef]
- Gahurova, L.; Tomizawa, S.I.; Smallwood, S.A.; Stewart-Morgan, K.R.; Saadeh, H.; Kim, J.; Andrews, S.R.; Chen, T.; Kelsey, G. Transcription and chromatin determinants of de novo DNA methylation timing in oocytes. Epigenet. Chromatin 2017, 10, 25. [Google Scholar] [CrossRef]
- Eckersley-Maslin, M.A.; Alda-Catalinas, C.; Reik, W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat. Rev. Mol. Cell Biol. 2018, 19, 436–450. [Google Scholar] [CrossRef]
- Vastenhouw, N.L.; Cao, W.X.; Lipshitz, H.D. The maternal-to-zygotic transition revisited. Development 2019, 146, dev161471. [Google Scholar] [CrossRef] [Green Version]
- Nitta, M.; Yogo, K.; Ohashi, M.; Akiyama, M.; Kunitomo, Y.; Ogawa, T.; Ishida-Kitagawa, N.; Miyoshi, J.; Sato, E.; Takeya, T. Identification and Expression Analysis of Connexin-45 and Connexin-60 as Major Connexins in Porcine Oocytes. J. Anim. Sci. 2010, 88, 3269–3279. [Google Scholar] [CrossRef] [Green Version]
- Schilffarth, S.; Antoni, B.; SChams, D.; Meyer, H.H.; Berisha, B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int. J. Biol. Sci. 2009, 5, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, S.; Mauro, A.; Cellini, V.; Patacchiola, F. The role of Akt signalling in the mammalian ovary. Int. J. Dev. Biol. 2012, 56, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susor, A.; Kubelka, M. Translational Regulation in the Mammalian Oocyte. In Oocytes: Maternal Information and Functions, Results and Problems in Cell Differentiation; Kloc, M., Ed.; Springer International Publishing AG: Cham, Switzerland, 2007; Volume 63, pp. 260–262. ISBN 978-3-319-60855-6. [Google Scholar]
- Sankar, A.; Lerdrup, M.; Manaf, A.; Johansen, J.V.; Martin-Gonzalez, J.; Borup, R.; Blanshard, R.; Klungland, A.; Hansen, K.; Andersen, C.Y.; et al. KDM4A regulates the maternal-to-zygotic transition by protecting broad H3K4me3 domains from H3K9me3 invasion in oocytes. Nat. Cell Biol. 2020, 22, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Fauque, P.; De Mouzon, J.; Devaux, A.; Epelboin, S.; Gervoise-Boyer, M.J.; Levy, R.; Valentin, M.; Viot, G.; Bergere, A.; De Vienne, C.; et al. Reproductive technologies, female infertility, and the risk of imprinting-related disorders. Clin. Epigenetics 2020, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Luong, X.G.; Maria Daldello, E.; Rajkovic, G.; Yang, C.-R. Genome-wide analysis reveals a switch in the translational program upon oocyte meiotic resumption. Nucleic Acids Res. 2020, 48, 3257–3276. [Google Scholar] [CrossRef] [Green Version]
- Ungricht, R.; Kutay, U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 229–245. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Sen, A.; Caiazza, F. Oocyte maturation: A story of arrest and release. FBS 2013, 5, 451–477. [Google Scholar] [CrossRef] [Green Version]
- Richani, D.; Gilchrist, R.B. The Epidermal Growth Factor Network: Role in Oocyte Growth, Maturation and Developmental Competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Ma, Y.; Shang, Y.; Huo, R.; Li, W. Post-translational regulation of the maternal-to-zygotic transition. Cell. Mol. Life Sci. 2018, 75, 1707–1722. [Google Scholar] [CrossRef]
- Sha, Q.-Q.; Zheng, W.; Wu, Y.-W.; Li, S.; Guo, L.; Zhang, S.; Lin, G.; Ou, X.-H.; Fan, H.-Y. Dynamics and Clinical Relevance of Maternal MRNA Clearance during the Oocyte-to-Embryo Transition in Humans. Nat. Commun. 2020, 11, 4917. [Google Scholar] [CrossRef]
- Sha, Q.Q.; Zhu, Y.Z.; Li, S.; Jiang, Y.; Chen, L.; Sun, X.H.; Shen, L.; Ou, X.H.; Fan, H.Y. Characterization of zygotic genome activation-dependent maternal mRNA clearance in mouse. Nucleic Acids Res. 2020, 48, 879–894. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic genome activation during the maternal-to-zygotic transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Zhou, X.; Shen, K.; Liu, Z.; Cheng, T.; Liu, D.; Cheng, Y.; Peng, X.; Sun, M.-X. Two-step maternal-to-zygotic transition with two-phase parental genome contributions. Dev. Cell 2019, 49, 882–893. [Google Scholar] [CrossRef]
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2018, 20, 221–234. [Google Scholar] [CrossRef]
- Schultz, R.M. Regulation of zygotic gene activation in the mouse. Bioessays 1993, 15, 531–538. [Google Scholar] [CrossRef]
- Asami, M.; Lam, B.Y.H.; Ma, M.K.; Rainbow, K.; Braun, S.; VerMilyea, M.D.; Yeo, G.S.H.; Perry, A.C.F. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell 2022, 29, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.F.; Ellison, P.T. Normative Study of Age Variation in Salivary Progesterone Profiles. J. BioSoc. Sci. 1992, 24, 233–244. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Cittelly, D.M. Sex steroid hormone function in the brain niche: Implications for brain metastatic colonization and progression. Cancer Rep. 2020, 5, e1241. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, J.T.; Strauss, J.F., III. The Role of Lipoproteins in Steroidogenesis and Cholesterol Metabolism in Steroidogenic Glands. Endocr. Rev. 1982, 3, 299–329. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Sex Hormones, Growth Hormone, and the Cornea. Cells 2022, 11, 224. [Google Scholar] [CrossRef]
- Vannuccini, S.; Bocchi, C.; Severi, F.M.; Challis, J.R.; Petraglia, F. Endocrinology of human parturition. Ann. Endocrinol. 2016, 77, 105–113. [Google Scholar] [CrossRef]
- Chighizola, C.; Meroni, P.L. The role of environmental estrogens and autoimmunity. Autoimmun. Rev. 2012, 11, A493–A501. [Google Scholar] [CrossRef]
- Saadia, Z. Follicle stimulating hormone (LH: FSH) ratio in polycystic ovary syndrome (PCOS)—Obese vs. non- obese women. Med. Arch. 2020, 74, 289–293. [Google Scholar] [CrossRef]
- Mahesh, V.B.; Murphy, L.L.; O’Conner, J.L. Selective Modulation of FSH and LH Secretion by Steroids. Adv. Exp. Med. Biol. 1987, 219, 131–152. [Google Scholar]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef] [Green Version]
- Mohr, M.A.; Esparza, L.A.; Steffen, P.; Micevych, P.E.; Kauffman, A.S. Progesterone Receptors in AVPV Kisspeptin Neurons Are Sufficient for Positive Feedback Induction of the LH Surge. Endocrinology 2021, 162, bqab161. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R.; De Santis, L.; Cecconi, S. Female fertility and environmental pollution. Int. J. Environ. Res. Public Health 2020, 17, 8802. [Google Scholar] [CrossRef] [PubMed]
- Lager, C.; Ellison, P.T. Effect of Moderate Weight Loss on Ovarian Function Assessed by Salivary Progesterone Measurements. Am. J. Hum. Biol. 1990, 2, 303–312. [Google Scholar] [CrossRef]
- Constantini, N.W.; Dubnov, G.; Lebrun, C.M. The menstrual cycle and sport performance. Clin. Sports Med. 2005, 24, 51–82. [Google Scholar] [CrossRef] [PubMed]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H., Jr.; Paul, S.M. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557. [Google Scholar] [CrossRef] [Green Version]
- Breen, K.M.; Karsch, F.J. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front. Neuroendocrinol. 2006, 27, 233–245. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.-H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E. Dietary Patterns and Outcomes of Assisted Reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Reichman, M.E.; Judd, J.T.; Brown, C.; Longcope, C.; Schatzkin, A.; Forman, M.; Campbell, W.S.; Franz, C.; Kahle, L.; et al. Relation of Energy, Fat, and Fiber Intakes to Plasma Concentrations of Estrogens and Androgens in Premenopausal Women. Am. J. Clin. Nutr. 1996, 64, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Morimoto, Y.; Takata, Y.; Murphy, S.P.; Stanczyk, F.Z. Alcohol and dietary fibre intakes affect circulating sex hormones among premenopausal women. Public Health Nutr. 2006, 9, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Andronico, F.; Battaglia, R.; Ragusa, M.; Barbagallo, D.; Purrello, M.; Di Pietro, C. Extracellular vesicles in human oogenesis and implantation. Int. J. Mol. Sci. 2019, 20, 2162. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.F.; Zhou, Z.Y.; He, X.Y.; Tao, L.; Jiang, Y.T.; Lan, R.; Hong, Q.H.; Chu, M.X. Integrated analyses of miRNA-mRNA expression profiles of ovaries reveal the crucial interaction networks that regulate the prolificacy of goats in the follicular phase. BMC Genom. 2021, 22, 812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, Y.; Liu, Y.; Yang, X. MicroRNAs in ovarian function and disorders. J. Ovarian Res. 2015, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordowitzki, P.; Sokołowska, G.; Wasielak-Politowska, M.; Skowronska, A.; Skowronski, M.T. Pannexins and Connexins: Their Relevance for Oocyte Developmental Competence. Int. J. Mol. Sci. 2021, 22, 5918. [Google Scholar] [CrossRef] [PubMed]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Jacobson, E.C.; Collier, A.J.; Plath, K. The Transcription Factor Code in IPSC Reprogramming. Curr. Opin. Genet. Dev. 2021, 70, 89–96. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Dong, M.H.; Kim, Y.Y.; Ku, S.-Y. Identification of Stem Cell-like Cells in the Ovary. Tissue Eng. Regen. Med. 2022, 19, 675–685. [Google Scholar] [CrossRef]
- Bui, H.T.; Thuan, N.V.; Kwon, D.N.; Choi, Y.J.; Kang, M.H.; Han, J.W.; Kim, T.; Kim, J.H. Identification and characterization of putative stem cells in the adult pig ovary. Development 2014, 141, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Silvestris, E.; D’oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In vitro generation of oocytes from ovarian stem cells (OSCs): In search of major evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Bragança, J.; Lopes, J.A.; Mendes-Silva, L.; Almeida Santos, J.M. Induced Pluripotent Stem Cells, a Giant Leap for Mankind Therapeutic Applications. World J. Stem Cells 2019, 11, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Pappas, J.J.; Yang, P.C. Human ESC vs. iPSC-pros and cons. J. Cardiovasc. Transl. Res. 2008, 1, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.H.; Muller, Y.D.; Balaphas, A.; Morel, P.; Pascual, M.; Seebach, J.D.; Buhler, L.H. Xenotransplantation: Back to the future? Transpl. Int. 2018, 31, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Phimister, E.G. Genetic Modification in Pig-to-Human Transplantation. N. Engl. J. Med. 2022, 387, 79–82. [Google Scholar] [CrossRef]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef]
- Janotka, M.; Ostadal, P. Biochemical Markers for Clinical Monitoring of Tissue Perfusion. Mol. Cell. Biochem. 2021, 476, 1313–1326. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.; Atoui, R. Therapeutic Use of Stem Cells for Myocardial Infarction. Bioengineering 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajnik, K.; Mietkiewska, K.; Skowronska, A.; Kordowitzki, P.; Skowronski, M.T. Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells. Int. J. Mol. Sci. 2023, 24, 6837. https://doi.org/10.3390/ijms24076837
Krajnik K, Mietkiewska K, Skowronska A, Kordowitzki P, Skowronski MT. Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells. International Journal of Molecular Sciences. 2023; 24(7):6837. https://doi.org/10.3390/ijms24076837
Chicago/Turabian StyleKrajnik, Kornelia, Klaudia Mietkiewska, Agnieszka Skowronska, Pawel Kordowitzki, and Mariusz T. Skowronski. 2023. "Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells" International Journal of Molecular Sciences 24, no. 7: 6837. https://doi.org/10.3390/ijms24076837




