Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates
Abstract
:1. Introduction
2. Phenolic and Polyphenolic Antioxidants in HRs
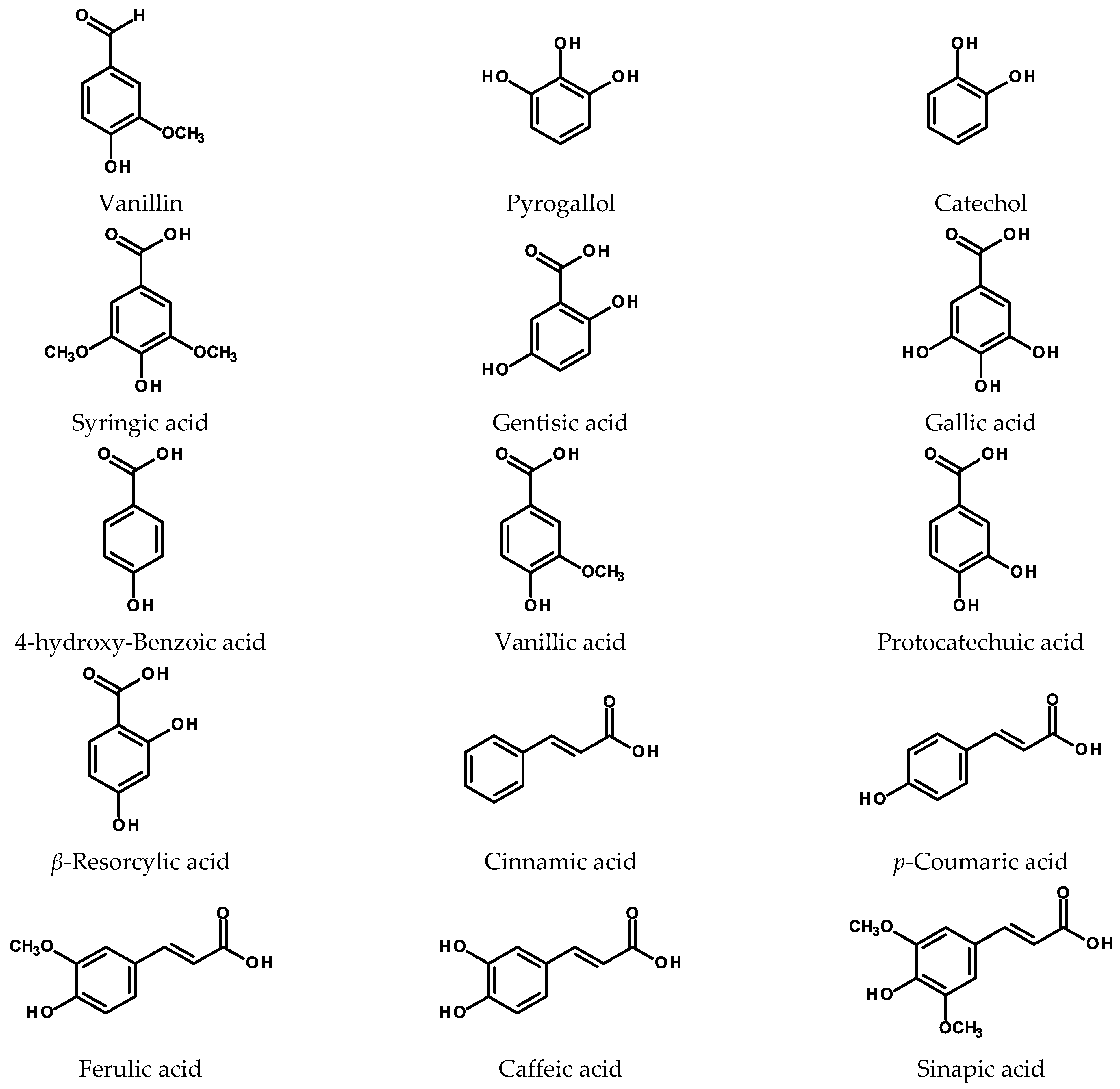
2.1. Simple Phenolics and Phenolic Acids
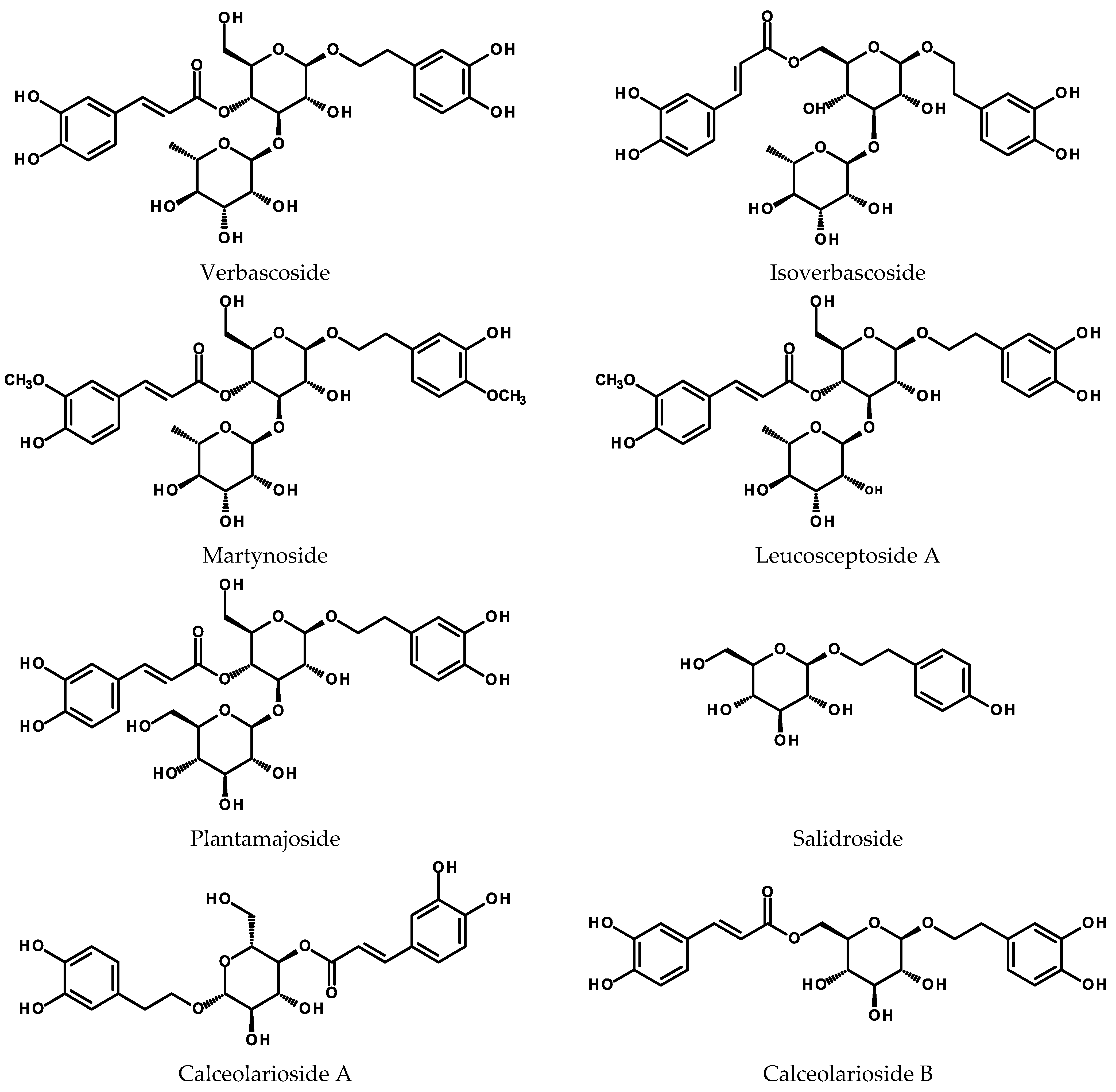
2.2. Phenylethanoids
2.3. Hydroxycinnamates
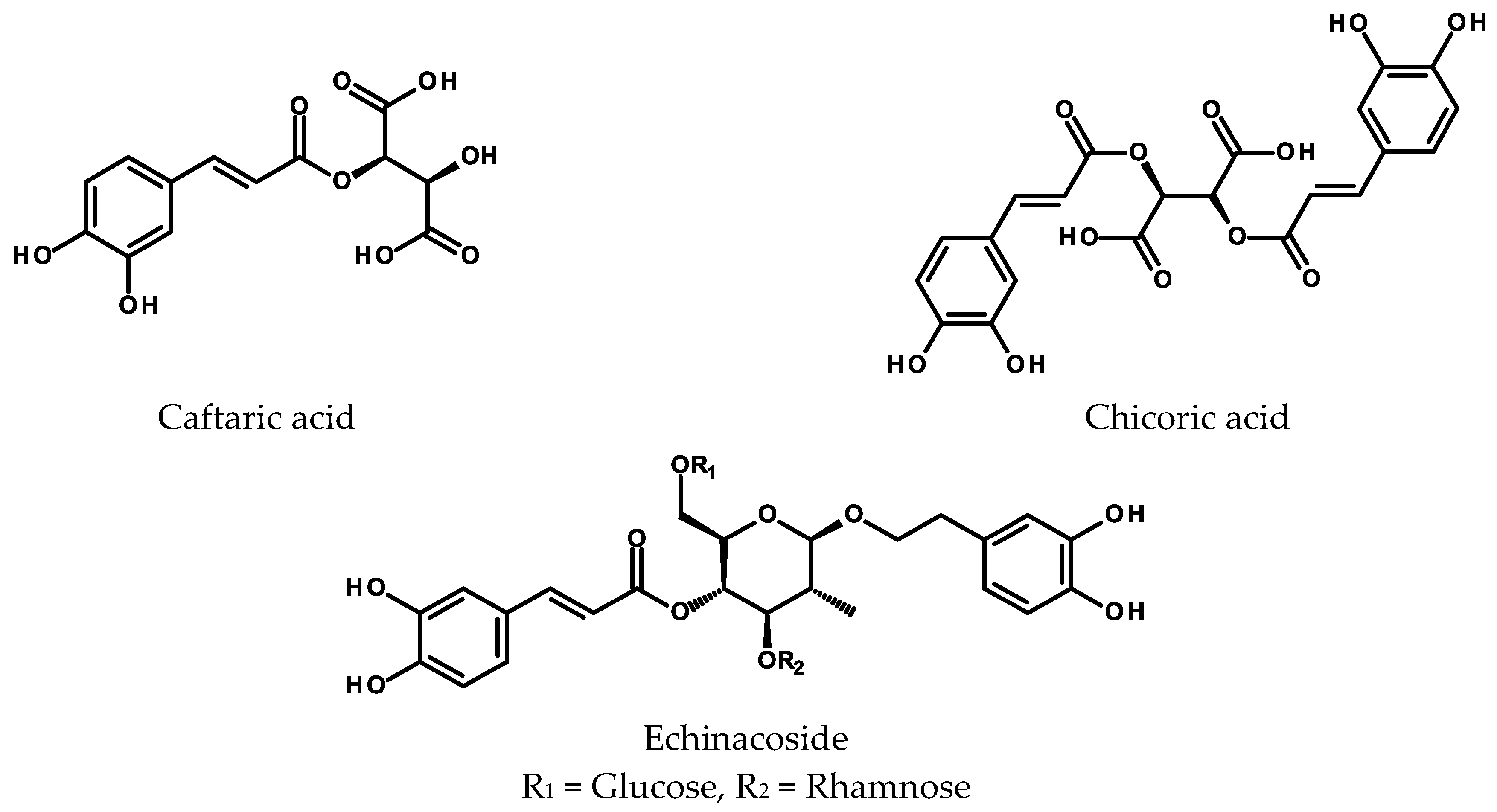
2.3.1. Conjugates with Tartaric Acid
2.3.2. Conjugates with Quinic Acid
2.3.3. Rosmarinic Acid, Salvianolic Acids, and Miscellaneous Compounds
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3:5-DCQA | 3,5-Dicaffeoylquinic acid |
| 4CL | 4-Coumarate:CoA ligase |
| 5-CQA | 5-Caffeoylquinic acid |
| AChE | Acetylcholinesterase |
| ALDH | Alcohol dehydrogenase |
| AOX | Alternative oxidase |
| BChE | Butyrylcholinesterase |
| C3H | Coumaroylquinate (coumaroylshikimate) 3’-monooxygenase |
| C4H | Cinnamate-4-hydroxylase |
| CGAs | Chlorogenic acids |
| CTA | Caftaric acid |
| CuAO | Copper-containing amine oxidase |
| DCTA | Chicoric acid |
| DW | Dry weight |
| DXR | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase |
| FW | Fresh weight |
| GB5 | Gamborg’s B5 |
| HBD | Hydroxybenzoate dehydrogenase |
| HBS | p-Hydroxybenzaldehyde synthase |
| HCHL | 4-Hydroxycinnamoyl-CoA hydratase/lyase |
| HCT | Shikimate O-hydroxycinnamoyltransferase |
| HPLC-PDA-ESI-MS | High performance liquid chromatography with photodiode detector electrospray ion source and mass spectrometry |
| HPPR | Hydroxyphenylpyruvate reductase |
| HR | Hairy root |
| MeJa | Methyl jasmonate |
| MS | Murashige and Skoog |
| PAL | Phenylalanine ammonia lyase |
| PAP1 | Production of anthocyanin pigment 1 |
| PG | Propyl gallate |
| p-HBA | p-Hydroxybenzoic acid (4-hydroxybenzoic acid) |
| PK | Pyruvate kinase |
| PPO | Polyphenol oxidase |
| RA | Rosmarinic acid |
| RAS | Rosmarinic acid synthase |
| SA | Salicylic acid |
| SHAM | Salicylhydroxamic acid |
| SKHD | Shikimate dehydrogenase |
| SOD | Superoxide dismutase |
| TAT | Tyrosine aminotransferase |
| TCM | Traditional Chinese medicine |
| TDC | Tyrosine decarboxylase |
| TyDC | Tyrosine/DOPA decarboxylase |
| UGT | UDP-Glucose glucosyltransferase |
| WP | Woody plant |
| YE | Yeast extract |
References
- Young, J.M.; Kuykendall, L.D.; Martínez-Romero, E.; Kerr, A.; Sawada, H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 2001, 51, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Hamill, J.D.; Lidgett, A.J. Hairy Root Cultures—Opportunities and Key Protocols for Studies in Metabolic Engineering. In Hairy Roots. Culture and Applications, 1st ed.; Doran, P., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 1–29. [Google Scholar]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.M.; Ritala, A.; Cardon, F. Hairy root cultures—A versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological exploration of transformed root culture for value-added products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root cultures, a boon for the production of valuable compounds: A comparative review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy root culture technology: Applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food ingredients and food made with plant cell and tissue cultures: State-of-the art and future trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Krishnajah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants against cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Shin, S.-A.; Moon, S.Y.; Kim, W.-Y.; Paek, S.-M.; Park, H.H.; Lee, C.S. Structure-based classification and anti-cancer effects of plant metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [Green Version]
- Olivero-David, R.; Ruiz-Roso, M.B.; Caporaso, N.; Perez-Olleros, L.; De las Heras, N.; Lahera, V.; Ruiz-Roso, B. In vivo bioavailability of polyphenols from grape by-product extracts, and effect on lipemia of normocholesterolemic Wistar rats. J. Sci. Food Agric. 2018, 98, 5581–5590. [Google Scholar] [CrossRef]
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Sima, A.V.; Stancu, C.S. Phenolic Compounds Exerting Lipid-Regulatory, Anti-Inflammatory and Epigenetic Effects as Complementary Treatments in Cardiovascular Diseases. Biomolecules 2020, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic bioactives from plant-based foods for glycemic control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostič, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural polyphenols for the preservation of meat and dairy products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Overview of plant extracts as natural preservatives in meat. J. Food Process. Preserv. 2022, 46, e16796. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Munekata, P.E.S.; Nieto, G.; Bangar, S.P.; Dhama, K.; Lorenzo, J.M. Bioactive compounds from leaf vegetables as preservatives. Foods 2023, 12, 637. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Plants and natural products for the treatment of skin hyperpigmentation—A review. Planta Med. 2018, 84, 988–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Abouzeid, M.; Evidente, A. Polyphenols as fungal phytotoxins, seed germination stimulants and phytoalexins. Phytochem. Rev. 2013, 12, 653–672. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Yudina, Y.V.; Stojakowska, A. Hairy Root Cultures as a Source of Polyphenolic Antioxidants: Flavonoids, Stilbenoids and Hydrolyzable Tannins. Plants 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.R.; Verpoorte, R. Chorismate derived C6C1 compounds in plants. Planta 2005, 222, 1–5. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 137–184. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Sircar, D.; Mitra, A. Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota 2: Confirming biosynthetic steps through feeding of inhibitors and precursors. J. Plant Physiol. 2009, 166, 1370–1380. [Google Scholar] [CrossRef]
- Mitra, A.; Mayer, M.J.; Mellon, F.A.; Michael, A.J.; Narbad, A.; Parr, A.J.; Waldron, K.W.; Walton, N.J. 4-Hydroxycinnamoyl-CoA hydratase/lyase, an enzyme of phenylpropanoid cleavage from Pseudomonas, causes formation of C6-C1 acid and alcohol glucose conjugates when expressed in hairy roots of Datura stramonium L. Planta 2002, 215, 79–89. [Google Scholar] [CrossRef]
- Ur Rahman, L.; Kouno, H.; Hashiguchi, Y.; Yamamoto, H.; Narbad, A.; Parr, A.; Walton, N.; Ikenaga, T.; Kitamura, Y. HCHL expression in hairy roots of Beta vulgaris yields a high accumulation of p-hydroxybenzoic acid (pHBA) glucose ester, and linkage of pHBA into cell walls. Bioresour. Technol. 2009, 100, 4836–4842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, M.; Shimomura, K.; Sasaki, K.; Yoshihira, K.; Ishimaru, K. Glucosylation of phenolics by hairy root cultures of Lobelia sessilifolia. Phytochemistry 1995, 40, 1149–1150. [Google Scholar] [CrossRef]
- Yan, C.-Y.; Yu, R.-M.; Zhang, Z.; Kong, L.-Y. Biotransformation of 4-hydroxybenzen derivatives by hairy root cultures of Polygonum multiflorum Thunb. J. Integr. Plant Biol. 2007, 49, 207–212. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Liu, J.-H.; Yu, B.-Y. Biotransformation of p-, m-, and o-hydroxybenzoic acids by Panax ginseng hairy root cultures. J. Mol. Catal. B Enzym. 2008, 54, 72–75. [Google Scholar] [CrossRef]
- Sircar, D.; Roychowdhury, A.; Mitra, A. Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota. J. Plant Physiol. 2007, 164, 1358–1366. [Google Scholar] [CrossRef]
- Sircar, D.; Mitra, A. Evidence for p-hydroxybenzoate formation involving enzymatic phenylpropanoid side-chain cleavage in hairy roots of Daucus carota. J. Plant Physiol. 2008, 165, 407–414. [Google Scholar] [CrossRef]
- Sircar, D.; Cardosoa, H.G.; Mukherjee, C.; Mitra, A.; Arnholdt-Schmitt, B. Alternative oxidase (AOX) and phenolic metabolism in methyl jasmonate-treated hairy root cultures of Daucus carota L. J. Plant Physiol. 2012, 169, 657–663. [Google Scholar] [CrossRef]
- Mukherjee, C.; Sircar, D.; Chatterjee, M.; Das, S.; Mitra, A. Combating photooxidative stress in green hairy roots of Daucus carota cultivated under light irradiation. J. Plant Physiol. 2014, 171, 179–187. [Google Scholar] [CrossRef]
- Mukherjee, C.; Samanta, T.; Mitra, A. Redirection of metabolite biosynthesis from hydroxybenzoates to volatile terpenoids in green hairy roots of Daucus carota. Planta 2016, 243, 305–320. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of glucosinolates, phenolic compounds and associated gene expression profiles of hairy root cultures in turnip (Brassica rapa ssp. rapa). 3 Biotech 2016, 6, 175. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa. Bioprocess Biosyst. Eng. 2018, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of bioactive compounds and gene expression alterations in hairy root cultures of chinese cabbage elicited by copper oxide nanoparticles. Plant Cell Tissue Org. Cult. 2018, 134, 95–106. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; John, K.M.M.; Yang, Y.-S.; Kim, S.-H.; Chung, I.-M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Org. Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Chung, I.-M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hortic. 2016, 198, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Chung, I.-M.; Thiruvengadam, M. Evaluation of phenolic compounds, antioxidant and antimicrobial activities from transgenic hairy root cultures of gherkin (Cucumis anguria L.). S. Afr. J. Bot. 2015, 100, 80–86. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, E.-H.; Kim, S.-H.; Chung, I.-M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-T.; Lee, J.-D.; Ahn, M.-S.; Kim, S.-W.; Park, S.-Y. Enhanced production of phenolic compounds in hairy root cultures of Polygonum multiflorum and its metabolite discrimination using HPLC and FT-IR methods. Appl. Microbiol. Biotechnol. 2018, 102, 9563–9575. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, A.; Zhou, M.; Betekhtin, A. Buckwheat in Tissue Culture Research: Current Status and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 2298. [Google Scholar] [CrossRef]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M. Agrobacterium rhizogenes mediated transformation of Ficus carica L. for the efficient production of secondary metabolites. J. Sci. Food Agric. 2020, 100, 2185–2197. [Google Scholar] [CrossRef]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M.; Kumar, V. Piriformospora indica based elicitation for overproduction of phenolic compounds by hairy root cultures of Ficus carica. J. Biotechnol. 2021, 327, 43–53. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Thiruvengadam, M.; Chung, I.-M. Identification of elicitors enhances the polyphenolic compounds and pharmacological potential in hairy root cultures of Aster scaber. S. Afr. J. Bot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Ansari, M.A.; Chung, I.-M.; Rajakumar, G.; Alzohairy, M.A.; Almatroudi, A.; Khanna, V.G.; Thiruvengadam, M. Evaluation of polyphenolic compounds and pharmacological activities in hairy root cultures of Ligularia fischeri Turcz. f. spiciformis (Nakai). Molecules 2019, 24, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, S.; Salma, U.; Ali, M.N.; Hazra, A.K.; Mandal, N. Development of transgenic hairy roots and augmentation of secondary metabolites by precursor feeding in Sphagneticola calendulacea (L.) Pruski. Ind. Crops Prod. 2018, 121, 206–215. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Rijo, P.; Białas, A.J.; Wielanek, M.; Wysokińska, H.; Garcia, C.; Toma, M.; Śliwiński, T.; Skała, E. Over-expression of AtPAP1 transcriptional factor enhances phenolic acid production in transgenic roots of Leonurus sibiricus L. and their biological activities. Mol. Biotechnol. 2018, 60, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tissue Org. Cult. 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Isca, V.M.S.; Picot, L.; Wielanek, M.; Śliwiński, T.; Sitarek, P. Preliminary phytochemical analysis and evaluation of the biological activity of Leonotis nepetifolia (L.) R. Br transformed roots extracts obtained through Rhizobium rhizogenes-mediated transformation. Cells 2021, 10, 1242. [Google Scholar] [CrossRef]
- Kochan, E.; Szymańska, G.; Wielanek, M.; Wiktorowska-Owczarek, A.; Jóźwiak-Bębenista, M.; Grzegorczyk-Karolak, I. The content of triterpene saponins and phenolic compounds in American ginseng hairy root extracts and their antioxidant and cytotoxic properties. Plant Cell Tissue Organ Cult. 2019, 138, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Mitra, A. Accumulation of cell wall-bound phenolic metabolites and their upliftment in hairy root cultures of tomato (Lycopersicon esculentum Mill.). Biotechnol. Lett. 2008, 30, 1253–1258. [Google Scholar] [CrossRef]
- Kumar, M.; Basu, A.; Kumari, P.; Jha, S.; Mitra, A. Tobacco plantlets ameliorate oxidative stress upon expression of a cryptogein gene. Plant Cell Tissue Organ Cult. 2016, 125, 553–570. [Google Scholar] [CrossRef]
- Yousefian, Z.; Golkar, P.; Mirjalili, M.H. Production enhancement of medicinally active coumarin and phenolic compounds in hairy root cultures of Pelargonium sidoides: The effect of elicitation and sucrose. J. Plant Growth Regul. 2021, 40, 628–641. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef] [PubMed]
- Sęczyk, Ł.; Sugier, D.; Dervişoğlu, G.; Özdemir, F.A.; Kołodziej, B. Phytochemical profile, in vitro bioaccessibility, and anticancer potential of golden root (Rhodiola rosea L.) extracts. Food Chem. 2023, 404, 134779. [Google Scholar] [CrossRef]
- Jin, M.; Wang, C.; Xu, Y.; Zhang, Z.; Wu, X.; Ye, R.; Zhang, Q.; Han, D. Pharmacological effects of salidroside on central nervous system diseases. Biomed. Pharmacother. 2022, 156, 113746. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirotani, M.; Yoshikawa, T.; Furuya, T. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry 1997, 44, 83–87. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nishikawa, K.; Furukawa, H.; Fujioka, T.; Fujii, H.; Mihashi, K.; Shimomura, K.; Ishimaru, K. Flavone production in transformed root cultures of Scutellaria baicalensis Georgi. Phytochemistry 1999, 52, 885–890. [Google Scholar] [CrossRef]
- Wilczańska-Barska, A.; Królicka, A.; Głód, D.; Majdan, M.; Kawiak, A.; Krauze-Baranowska, M. Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Wysokińska, H.; Rózga, M. Establishment of transformed root cultures of Paulownia tomentosa for verbascoside production. J. Plant Physiol. 1998, 152, 78–83. [Google Scholar] [CrossRef]
- Wysokińska, H.; Lisowska, K.; Floryanowicz-Czekalska, K. Transformation of Catalpa ovata by Agrobacterium rhizogenes and phenylethanoid glycosides production in transformed root cultures. Z. Naturforsch. 2001, 56, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Fons, F.; Tousch, D.; Rapior, S.; Gueiffier, A.; Roussel, J.L.; Gargadennec, A.; Andary, C. Phenolic profiles of untransformed and hairy root cultures of Plantago lanceolata. Plant Physiol. Biochem. 1999, 37, 291–296. [Google Scholar] [CrossRef]
- Dhakulkar, S.; Ganapathi, T.R.; Bhargava, S.; Bapat, V.A. Induction of hairy roots in Gmelina arborea Roxb. and production of verbascoside in hairy roots. Plant Sci. 2005, 169, 812–818. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Georgiev, M.; Bley, T. Metabolite and hormonal status of hairy root cultures of Devil’s claw (Harpagophytum procumbens) in flasks and in a bubble column bioreactor. Process. Biochem. 2008, 43, 15–23. [Google Scholar] [CrossRef]
- Homova, V.; Weber, J.; Schulze, J.; Alipieva, K.; Bley, T.; Georgiev, M. Devil’s claw hairy root culture in flasks and in a 3-L bioreactor: Bioactive metabolite accumulation and flow cytometry. Z. Naturforsch. 2010, 65c, 472–478. [Google Scholar] [CrossRef]
- Grąbkowska, R.; Królicka, A.; Mielicki, W.; Wielanek, M.; Wysokińska, H. Genetic transformation of Harpagophytum procumbens by Agrobacterium rhizogenes: Iridoid and phenylethanoid glycoside ccumulation in hairy root cultures. Acta Physiol. Plant. 2010, 32, 665–673. [Google Scholar] [CrossRef]
- Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-inflammatory activity of Devil’s claw in vitro systems and their active constituents. Food Chem. 2011, 125, 171–178. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Alipieva, K.; Orhan, I.E. Cholinesterases inhibitory and antioxidant activities of Harpagophytum procumbens from in vitro systems. Phytother. Res. 2012, 26, 313–316. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Wysokińska, H. The influence of methyl jasmonate and salicylic acid on secondary metabolite production in Rehmannia glutinosa Libosch. hairy root culture. Acta Biol. Crac. Ser. Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhi, J.; Zhang, Z.; Wang, L.; Suo, Y.; Xie, C.; Li, M.; Zhang, B.; Du, J.; Gu, L.; et al. Transcriptome analysis of salicylic acid treatment in Rehmannia glutinosa hairy roots using RNA-seq technique for identification of genes involved in acteoside biosynthesis. Front. Plant Sci. 2017, 8, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piątczak, E.; Jeleń, A.; Makówczyńska, J.; Zielińska, S.; Kuźma, Ł.; Balcerczak, E. Establishment of hairy root cultures of Rehmannia elata N.E. Brown ex Prain and production of iridoid and phenylethanoid glycosides. Ind. Crops Prod. 2019, 137, 308–314. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Orhan, I.E.; Cankaya, I.I.T.; Kostadinova, E.P.; Georgiev, M.I. Treasure from garden: Chemical profiling, pharmacology and biotechnology of mulleins. Phytochem. Rev. 2014, 13, 417–444. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Ludwig-Müller, J.; Alipieva, K.; Lippert, A. Sonication-assisted Agrobacterium rhizogenes-mediated transformation of Verbascum xanthophoeniceum Griseb. for bioactive metabolite accumulation. Plant Cell Rep. 2011, 30, 859–866. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Radziszewska, A.; Neumann, M.; Marchev, A.; Alipieva, K.; Ludwig-Müller, J. Metabolic alterations of Verbascum nigrum L. plants and SAArT transformed roots as revealed by NMR-based metabolomics. Plant Cell Tissue Organ Cult. 2015, 123, 349–356. [Google Scholar] [CrossRef]
- Marchev, A.; Yordanova, Z.; Alipieva, K.; Zahmanov, G.; Rusinova-Videva, S.; Kapchina-Toteva, V.; Simova, S.; Popova, M.; Georgiev, M.I. Genetic transformation of rare Verbascum eriophorum Godr. plants and metabolic alterations revealed by NMR-based metabolomics. Biotechnol. Lett. 2016, 38, 1621–1629. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem. Rev. 2015, 14, 657–674. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, J.; Wang, L.; Zhang, C.; Bai, Q.; Lyu, X.; Yang, R. Biosynthesis and biotechnological production of salidroside from Rhodiola genus plants. Phytochem. Rev. 2022, 21, 1605–1626. [Google Scholar] [CrossRef]
- Lütken, H.; Meropi-Antypa, N.; Kemp, O.; Hegelund, J.N.; Müller, R. Hairy Root Cultures of Rhodiola rosea to Increase Valuable Bioactive Compounds. In Production of Plant Derived Natural Compounds through Hairy Root Culture, 1st ed.; Malik, S., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 65–88. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A therapeutic candidate on cardiovascular diseases. Oxidative Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Wang, X.; Liu, B.; Xu, H. Salidroside production by hairy roots of Rhodiola sachalinensis obtained after transformation with Agrobacterium rhizogenes. Biol. Pharm. Bull. 2007, 30, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-S.; Ma, L.-Q.; Zhang, J.-X.; Shi, G.-L.; Hua, Y.-H.; Wang, Y.-N. Characterization of glycosyltransferases responsible for salidroside biosynthesis in Rhodiola sachalinensis. Phytochemistry 2011, 72, 862–870. [Google Scholar] [CrossRef]
- Lan, X.; Chang, K.; Zeng, L.; Liu, X.; Qiu, F.; Zheng, W.; Quan, H.; Liao, Z.; Chen, M.; Huang, W.; et al. Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS ONE 2013, 8, e75459. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Y.; Zeng, J.; Qin, J.; Lin, M.; Chen, M.; Liao, Z.; Lan, X. Biochemical characterization of tyrosine aminotransferase and enhancement of salidroside production by suppressing tyrosine aminotransferase in Rhodiola crenulate. Ind. Crops Prod. 2021, 173, 114075. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Giebułtowicz, J.; Wroczyński, P.; Pietrosiuk, A. Tyrosol glucosyltransferase activity and salidroside production in natural and transformed root cultures of Rhodiola kirilowii (Regel) Regel et Maximowicz. Acta Biol. Crac. Ser. Bot. 2013, 55, 126–133. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Krajewska-Patan, A.; Wyrwał, A.; Pietrosiuk, A. Biotransformation of cinnamyl alcohol to rosavins by non-transformed wild type and hairy root cultures of Rhodiola kirilowii. Biotechnol. Lett. 2014, 36, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, A.; Malunova, M.; Salamaikina, S.; Selimov, R.; Solov’eva, A. Establishment of Rhodiola quadrifida hairy roots and callus culture to produce bioactive compounds. Phyton 2021, 90, 543–552. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.; Jin, G.; Wang, L.; Qi, S.; Cao, Y.; Martin, C.; Zhang, Y. Versatility in acyltransferase activity completes chicoric acid biosynthesis in purple coneflower. Nat. Commun. 2021, 12, 1563. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, L.; Niu, Y.; Liu, Z.; Liu, X. Comparison of chicoric acid, and its metabolites caffeic acid and caftaric acid: In vitro protection of biological macromolecules and inflammatory responses in BV2 microglial cells. Food Sci. Hum. Wellness 2017, 6, 155–166. [Google Scholar] [CrossRef]
- Tanyeli, A.; Akdemir, F.N.E.; Eraslan, E.; Güler, M.C.; Nacar, T. Anti-oxidant and anti-inflamatuar effectiveness of caftaric acid on gastric ulcer induced by indomethacin in rats. Gen. Physiol. Biophys. 2019, 38, 175–181. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. The bioactive effects of chicoric acid as a functional food ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-B.; Wan, M.-Y.; Wang, P.-Y.; Zhang, C.-X.; Xu, D.-Y.; Liao, X.; Sun, H.-J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/ NF-κB/mTOR/P70S6K signaling cascade. Redox Biol. 2018, 14, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Shen, Y.; Teng, L.; Huang, Y.; Yang, Y.; Jian, X.; Fan, S.; Wu, P.; Fu, Q. Chicoric acid attenuates tumor necrosis factor-α-induced inflammation and apoptosis via the Nrf2/HO-1, PI3K/AKT and NF-κB signaling pathways in C28/I2 cells and ameliorates the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2022, 111, 109129. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defense system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-kB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Fang, J.; Chen, P.P.; Die, Y.; Wang, J.; Liu, Z.; Liu, X. Chicoric acid improves neuron survival against inflammation by promoting mitochondrial function and energy metabolism. Food Funct. 2019, 10, 6157–6169. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Saxena, P.K.; Murch, S.J.; Liu, C.-Z. Echinacea biotechnology: Challenges and opportunities. In Vitro Cell. Dev. Biol. Plant 2007, 43, 481–492. [Google Scholar] [CrossRef]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea biotechnology: Advances, commercialization and future considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Murthy, H.N.; Kim, Y.-S.; Park, S.-Y.; Paek, K.-Y. Biotechnological production of caffeic acid derivatives from cell and organ cultures of Echinacea species. Appl. Microbiol. Biotechnol. 2014, 98, 7707–7717. [Google Scholar] [CrossRef]
- Evidente, A.; Masi, M. Natural bioactive cinnamoyltyramine alkylamides and co-metabolites. Biomolecules 2021, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Trypsteen, M.; Van Lijsebettens, M.; Van Severen, R.; Van Montagu, M. Agrobacterium rhizogenes-mediated transformation of Echinacea purpurea. Plant Cell Rep. 1991, 10, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Z.; Abbasi, B.H.; Gao, M.; Murch, S.J.; Saxena, P.K. Caffeic Acid Derivatives Production by Hairy Root Cultures of Echinacea purpurea. J. Agric. Food. Chem. 2006, 54, 8456–8460. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Liu, R.; Saxena, P.K.; Liu, C.-Z. Cichoric acid production from hairy root cultures of Echinacea purpurea grown in amodified airlift bioreactor. J. Chem. Technol. Biotechnol. 2009, 84, 1697–1701. [Google Scholar] [CrossRef]
- Liu, R.; Sun, W.; Liu, C.-Z. Computational fluid dynamics modeling of mass-transfer behavior in a bioreactor for hairy root culture. II. Analysis of ultrasound-intensified process. Biotechnol. Prog. 2011, 27, 1672–1678. [Google Scholar] [CrossRef]
- Liu, R.; Li, W.; Sun, L.-Y.; Liu, C.-Z. Improving root growth and cichoric acid derivatives production in hairy root culture of Echinacea purpurea by ultrasound treatment. Biochem. Eng. J. 2012, 60, 62–66. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Naghdi Badi, H. Influence of KNO3, CaCl2 and MgSO4 concentrations on growth and cichoric acid accumulation in hairy root culture of purple coneflower (Echinacea purpurea L.). J. Med. Plants 2013, 12, 75–84. Available online: http://jmp.ir/article-1-111-en.html (accessed on 31 March 2023).
- Demirci, T.; Akçay, U.C.; Baydar, N.G. Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench. Acta Physiol. Plant. 2020, 42, 66. [Google Scholar] [CrossRef]
- Baque, M.A.; Moh, S.-H.; Lee, E.-J.; Zhong, J.-J.; Paek, K.-Y. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Szewczyk, A.; Kisiel, W. Caffeic acid derivatives from a hairy root culture of Lactuca virosa. Acta Physiol. Plant. 2012, 34, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.G.; Park, Y.; Park, J.-E.; Park, N.I. Enhancement of phenolic compounds and antioxidative activities by the combination of culture medium and methyl jasmonate elicitation in hairy root cultures of Lactuca indica L. Nat. Prod. Commun. 2019, 14, 1934578X19861867. [Google Scholar] [CrossRef] [Green Version]
- Abrankó, L.; Clifford, M.N. An unambiguous nomenclature for the acyl-quinic acids commonly known as chlorogenic acids. J. Agric. Food Chem. 2017, 65, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Magaña, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Ciaramelli, C.; Palmioli, A.; De Luigi, A.; Colombo, L.; Sala, G.; Riva, C.; Zoiac, C.P.; Salmona, M.; Airoldi, C. NMR-driven identification of anti-amyloidogenic compounds in green and roasted coffee extracts. Food Chem. 2018, 252, 171–180. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Domínguez-Fernández, M.; Young, P.; Yang, T.; Ludwig, I.A.; Clifford, M.N.; Cid, C.; Rodriguez-Mateos, A. In vivo study of the bioavailability and metabolic profile of (poly)phenols after sous-vide artichoke consumption. Food Chem. 2022, 367, 130620. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Jang, H.; Ahn, H.R.; Jo, H.; Kim, K.-A.; Lee, E.H.; Lee, K.W.; Jung, S.H.; Lee, C.Y. Chlorogenic Acid and Coffee Prevent Hypoxia-Induced Retinal Degeneration. J. Agric. Food Chem. 2014, 62, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, I.-H.; Kim, C.-S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef]
- Skała, E.; Makowczyńska, J.; Wieczfinska, J.; Kowalczyk, T.; Sitarek, P. Caffeoylquinic acids with potential biological activity from plant in vitro cultures as alternative sources of valuable natural products. Curr. Pharm. Des. 2020, 26, 2817–2842. [Google Scholar] [CrossRef]
- Kodoma, M.; Wada, H.; Otani, H.; Kohmoto, K.; Kimura, Y. 3,5-Di-O-caffeoylquinic acid, an infection-inhibiting factor from Pyrus pyrifolia induced by infection with Alternaria alternata. Phytochemistry 1998, 47, 371–373. [Google Scholar] [CrossRef]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Mehrotra, M.; Singh, A.K.; Niranjan, A.; Singh, R.; Sanyal, I.; Lehri, A.; Pande, V.; Amla, D.V. Improvement in Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) by the inhibition of polyphenolics released during wounding of cotyledonary node explants. Protoplasma 2017, 254, 253–269. [Google Scholar] [CrossRef]
- Hook, I. Secondary metabolites in hairy root cultures of Leontopodium alpinum Cass. (Edelweiss). Plant Cell Tissue Org. Cult. 1994, 38, 321–326. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic Transformation and Hairy Root Induction Enhance the Antioxidant Potential of Lactuca serriola L. Oxidative Med. Cell. Longev. 2017, 2017, 5604746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Long-term cultured hairy roots of chicory—A rich source of hydroxycinnamates and 8-deoxylactucin glucoside. Appl. Biochem. Biotechnol. 2013, 171, 1589–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, G.; Alves Dos Santos, H.; Etienne, A.; Samaillie, J.; Neut, C.; Sahpaz, S.; Hilbert, J.-L.; Gagneul, D.; Jullian, N.; Tahrioui, A.; et al. MeJA elicitation of chicory hairy roots promotes efficient increase of 3,5-diCQA accumulation, a potent antioxidant and antibacterial molecule. Antibiotics 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Maciel, G.; Lopes, A.A.; Cantrell, C.L.; de Castro França, S.; Bertoni, B.W.; Lourenço, M.V. Jasmonates promote enhanced production of bioactive caffeoylquinic acid derivative in Eclipta prostrata (L.) L. hairy roots. Plant Cell Tissue Org. Cult. 2022, 149, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Souza, G.R.S.; de Castro França, S.; Lourenço, M.V. Biosynthetic studies through feeding experiments in Eclipta prostrata (L.) L. hairy roots. Plant Cell Tissue Org. Cult. 2022, 151, 215–219. [Google Scholar] [CrossRef]
- Fu, X.; Yin, Z.-P.; Chen, J.-G.; Shangguan, X.-C.; Wang, X.; Zhang, Q.-F.; Peng, D.-Y. Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J. Agric. Food Chem. 2015, 63, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Skała, E.; Kicel, A.; Olszewska, M.A.; Kiss, A.K.; Wysokińska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. BioMed Res. Int. 2015, 2015, 181098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skała, E.; Sitarek, P.; Toma, M.; Szemraj, J.; Radek, M.; Nieborowska-Skorska, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. Inhibition of human glioma cell proliferation by altered Bax/Bcl-2-p53 expression and apoptosis induction by Rhaponticum carthamoides extracts from transformed and normal roots. J. Pharm. Pharmacol. 2016, 68, 1454–1464. [Google Scholar] [CrossRef]
- Śliwińska, A.; Figat, R.; Zgadzaj, A.; Wileńska, B.; Misicka, A.; Nałęcz-Jawecki, G.; Pietrosiuk, A.; Sykłowska-Baranek, K. Polyscias filicifolia (Araliaceae) hairy roots with antigenotoxic and anti-photogenotoxic activity. Molecules 2022, 27, 186. [Google Scholar] [CrossRef]
- Tusevski, O.; Vinterhalter, B.; Krstić Milošević, D.; Soković, M.; Ćirić, A.; Vinterhalter, D.; Zdravković Korać, S.; Petreska Stanoeva, J.; Stefova, M.; Gadzovska Simic, S. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.-C.; Hong, C.-Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011, 18, 30. Available online: http://www.jbiomedsci.com/content/18/1/30 (accessed on 31 March 2023). [CrossRef] [PubMed] [Green Version]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
- Pandey, V.; Swami, R.K.; Narula, A. Harnessing the potential of roots of traditional power plant: Ocimum. Front. Plant. Sci. 2021, 12, 765024. [Google Scholar] [CrossRef]
- Tada, H.; Murakami, Y.; Omoto, T.; Shimomura, K.; Ishimaru, K. Rosmarinic acid and related phenolics in hairy root cultures of Ocimum basilicum. Phytochemistry 1996, 42, 431–434. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Srivastava, S.; Conlan, X.A.; Adholeya, A.; Cahill, D.M. Elite hairy roots of Ocimum basilicum as a new source of rosmarinic acid and antioxidants. Plant Cell Tissue Organ Cult. 2016, 126, 19–32. [Google Scholar] [CrossRef]
- Srivastava, S.; Conlan, X.A.; Cahill, D.M.; Adholeya, A. Rhizophagus irregularis as an elicitor of rosmarinic acid and antioxidant production by transformed roots of Ocimum basilicum in an in vitro co-culture system. Mycorrhiza 2016, 26, 919–930. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.B.; Kim, J.K.; Park, S.U. Production of rosmarinic acid and correlated gene expression in hairy root cultures of green and purple basil (Ocimum basilicum L.). Prep. Biochem. Biotechnol. 2021, 51, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Wysokińska, H.; Chmiel, A.; Grabias, B. Rosmarinic acid and other phenolic acids in hairy roots of Hyssopus officinalis. Z. Naturforsch. 1999, 54, 11–16. [Google Scholar] [CrossRef]
- Fattahi, M.; Nazeri, V.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R.M.; Zamani, Z.; Palazon, J. A new biotechnological source of rosmarinic acid and surface flavonoids: Hairy root cultures of Dracocephalum kotschyi Boiss. Ind. Crops Prod. 2013, 50, 256–263. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Skała, E.; Olszewska, M.A.; Kiss, A.K.; Balcerczak, E.; Wysokińska, H.; Kicel, A. The identification and quantitative determination of rosmarinic acid and salvianolic acid B in hairy root cultures of Dracocephalum forrestii W.W. Smith. Ind. Crops Prod. 2016, 91, 125–131. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Iron oxide nanoparticles: A novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Coloma, A.; Alegre-Gómez, S.; López-Rodríguez, M.; Amador, L.J.; Díaz, C.E. Bioactive constituents from transformed root cultures of Nepeta teydea. Phytochemistry 2017, 133, 59–68. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, C.Y.; Eom, S.H.; Kim, Y.K.; Park, N.I.; Park, S.U. Rosmarinic acid production from transformed root cultures of Nepeta cataria L. Sci. Res. Essays 2010, 5, 1122–1126. Available online: https://academicjournals.org/journal/SRE/article-full-text-pdf/371F3FF19194 (accessed on 31 March 2023).
- Yang, Y.K.; Lee, S.Y.; Park, W.T.; Park, N.I.; Park, S.U. Exogenous auxins and polyamines enhance growth and rosmarinic acid production in hairy root cultures of Nepeta cataria L. Plant Omics 2010, 3, 190–193. Available online: https://www.pomics.com/park_3_6_2010_190_193.pdf (accessed on 31 March 2023).
- Petersen, M.; Szabo, E.; Meinhard, J.; Karwatzki, B.; Gertlowski, C.; Kempin, B.; FuB, E. Biosynthesis and accumulation of rosmarinic acid in suspension cultures of Coleus blumei. Plant Cell Tissue Org. Cult. 1995, 43, 89–92. [Google Scholar] [CrossRef]
- Li, W.; Koike, K.; Asada, Y.; Yoshikawa, T.; Nikaido, T. Rosmarinic acid production by Coleus forskohlii hairy root cultures. Plant Cell Tissue Organ Cult. 2005, 80, 151–155. [Google Scholar] [CrossRef]
- Bauer, N.; Kiseljak, D.; Jelaska, S. The effect of yeast extract and methyl jasmonate on rosmarinic acid accumulation in Coleus blumei hairy roots. Biol. Plant 2009, 53, 650–656. [Google Scholar] [CrossRef]
- Vuković, R.; Bauer, N.; Ćurkovic-Perica, M. Genetic elicitation by inducible expression of β-cryptogein stimulates secretion of phenolics from Coleus blumei hairy roots. Plant Sci. 2013, 199–200, 18–28. [Google Scholar] [CrossRef]
- Hücherig, S.; Petersen, M. RNAi suppression and overexpression studies of hydroxyphenylpyruvate reductase (HPPR) and rosmarinic acid synthase (RAS) genes related to rosmarinic acid biosynthesis in hairy root cultures of Coleus blumei. Plant Cell Tissue Organ Cult. 2013, 113, 375–385. [Google Scholar] [CrossRef]
- Ru, M.; An, Y.; Wang, K.; Peng, L.; Li, B.; Bai, Z.; Wang, B.; Liang, Z. Prunella vulgaris L. hairy roots: Culture, growth, and elicitation by ethephon and salicylic acid. Eng. Life Sci. 2016, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Ru, M.; Wang, K.; Bai, Z.; Peng, L.; He, S.; Wang, Y.; Liang, Z. A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Sci. Rep. 2017, 7, 4892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, M.; Li, Y.; Guo, M.; Chen, L.; Tan, Y.; Peng, L.; Liang, Z. Increase in rosmarinic acid accumulation and transcriptional responses of synthetic genes in hairy root cultures of Prunella vulgaris induced by methyl jasmonate. Plant Cell Tissue Organ Cult. 2022, 149, 371–379. [Google Scholar] [CrossRef]
- Lee, S.Y.; Xu, H.; Kim, Y.K.; Park, S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbiol. Biotechnol. 2008, 24, 969–972. [Google Scholar] [CrossRef]
- Vergara-Martínez, V.M.; Estrada-Soto, S.E.; Valencia-Díaz, S.; Garcia-Sosa, K.; Peña-Rodríguez, L.M.; Arellano-García, J.J.; Perea-Arango, I. Methyl jasmonate enhances ursolic, oleanolic and rosmarinic acid production and sucrose induced biomass accumulation, in hairy roots of Lepechinia caulescens. PeerJ 2021, 9, e11279. [Google Scholar] [CrossRef]
- Naliwajski, M.R.; Wileńska, B.; Misicka, A.; Pietrosiuk, A.; Sykłowska-Baranek, K. HPLC-PDA-ESI-HRMS-based profiling of secondary metabolites of Rindera graeca anatomical and hairy roots treated with drought and cold stress. Cells 2022, 11, 931. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia officinalis L. hairy root cultures for the production of rosmarinic acid. Z. Naturforsch. 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Matkowski, A.; Wysokińska, H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem. 2007, 104, 536–541. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokińska, H. Antioxidant compounds in Salvia officinalis L. shoot and hairy root cultures in the nutrient sprinkle bioreactor. Acta Soc. Bot. Pol. 2010, 79, 7–10. [Google Scholar] [CrossRef]
- Khoshsokhan, F.; Babalar, M.; Salami, S.A.; Sheikhakbari-Mehr, R.; Mirjalili, M.H. Rosmarinic acid production in hairy root cultures of Salvia nemorosa L. (Lamiaceae). Biocatal. Agric. Biotechnol. 2022, 45, 102494. [Google Scholar] [CrossRef]
- Dowom, S.A.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Elicitor-induced phenolic acids accumulation in Salvia virgata Jacq. hairy root cultures. Plant Cell Tissue Organ Cult. 2022, 148, 107–117. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Luo, L.; Cao, F.; Yang, B.; Gao, J.; Yan, Y.; Zhang, G.; Peng, L.; Hu, B. Increased phenolic acid and tanshinone production and transcriptional responses of biosynthetic genes in hairy root cultures of Salvia przewalskii Maxim. treated with methyl jasmonate and salicylic acid. Mol. Biol. Rep. 2020, 47, 8565–8578. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crops Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I. Optimization of culture conditions and cultivation phase for the growth of Salvia viridis transformed roots and polyphenolic compound production. Plant Cell Tissue Organ Cult. 2020, 142, 571–581. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Gonciarz, W.; Chmiela, M.; Olszewska, M.A.; Grzegorczyk-Karolak, I. The antioxidant, cytotoxic and antimicrobial potential of phenolic acids-enriched extract of elicited hairy roots of Salvia bulleyana. Molecules 2022, 27, 992. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Olszewska, M.A.; Grzegorczyk-Karolak, I. In vitro strategy for the enhancement of the production of bioactive polyphenols in transformed roots of Salvia bulleyana. Int. J. Mol. Sci. 2022, 23, 7771. [Google Scholar] [CrossRef]


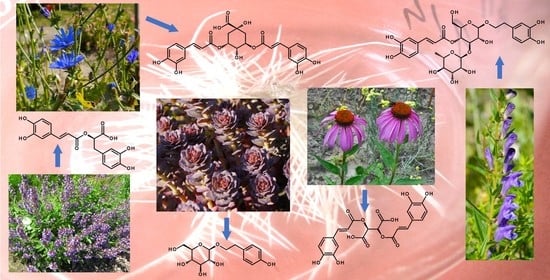
| Plant Species | Hairy Roots | Natural Product | Maximum Content in the Biomass | Literature |
|---|---|---|---|---|
| Datura stramonium L. | Expressing pmHCHL | p-Hydroxybenzoic acid O-β-D-glucoside p-Hydroxybenzyl alcohol O-β-D-glucoside | 4.4 mg/g FW 2.8 mg/g FW | [32] |
| Beta vulgaris L. | Expressing pmHCHL | p-Hydroxybenzoic acid (4-Hydroxybenzoic acid) glucose ester | 140 mg/g DW | [33] |
| Momordica charantia L. | Wild type | Gentisic acid Salicylic acid | 5.5 mg/g DW 2.5 mg/g DW | [45] |
| Polygonum multiflorum Thunb. | Wild type | Pyrogallol | 1.4 mg/g DW | [48] |
| Ficus carica L. cv. Sabz | Wild type | Gallic acid Coumaric acid | 7.5 mg/g DW 0.9 mg/g DW | [52] |
| Leonurus sibirica L. | Expressing AtPAP1 | Chlorogenic acid Caffeic acid | 19.4 mg/g DW 11.4 mg/g DW | [56] |
| Leonotis nepetifolia (L.) R. Br. | Wild type | p-Coumaric acid m-Coumaric acid | 4.9 mg/g DW 2.1 mg/g DW | [58] |
| Plant Species | Hairy Roots | Natural Product | Maximum Content in the Biomass | Literature |
|---|---|---|---|---|
| Paulownia tomentosa Steud. | Wild type | Verbascoside | 94.9 mg/g DW | [73] |
| Plantago lanceolata L. | Wild type | Plantamajoside | 30–80 mg/g DW | [75] |
| Verbascum xanthophoeniceum Griseb. | Wild type | Verbascoside | 23.3 mg/g DW | [86] |
| Rhodiola sachalinensis Boriss. | Overexpressing UGT72B14 | Salidroside | 19.8 mg/g DW | [94] |
| Plant Species | Hairy Roots | Natural Product | Maximum Content in the Biomass | Literature |
|---|---|---|---|---|
| Echinacea purpurea (L.) Moench | Wild type | Chicoric acid | 27 mg/g DW | [117] |
| Echinacea purpurea (L.) Moench | Wild type, supplemented with 0.025 μM GA3 | Chicoric acid Caftaric acid | 36 mg/g DW 7.5 mg/g DW | [118] |
| Echinacea purpurea (L.) Moench | Wild type, supplemented with 1 mg/L of 24-epibrassinolide | Chicoric acid Caftaric acid Echinacoside | 24.1 mg/g DW 6.9 mg/g DW 4.3 mg/g DW | [124] |
| Echinacea purpurea (L.) Moench | Wild type, supplemented with 500 μM L-phenylalanine | Chicoric acid Caftaric acid Echinacoside | 17.4 mg/g DW 6.3 mg/g DW 5.4 mg/g DW | [124] |
| Plant Species | Hairy Roots | Natural Product | Maximum Content in the Biomass | Literature |
|---|---|---|---|---|
| Lactuca virosa L. | Wild type | 3,5-di-O-caffeoylquinic acid | 25.8 mg/g DW | [125] |
| Cichorium intybus L. | Wild type | 3,5-di-O-caffeoylquinic acid 5-O-caffeoylquinic acid | 55.7 mg/g DW 9.4 mg/g DW | [145] |
| Cichorium intybus L. var Orchies | Wild type, elicited with 150 μM MeJa | 3,5-di-O-caffeoylquinic acid | 120 mg/g DW | [146] |
| Eclipta prostrata (L.) L. | Wild type, elicited with 100 μM of jasmonic acid | 3,5-di-O-caffeoylquinic acid | 44.7 mg/g DW | [147] |
| Eclipta prostrata (L.) L. | Wild type, elicited with 140 μM MeJa | 3,5-di-O-caffeoylquinic acid | 41.6 mg/g DW | [147] |
| Stevia rebaudiana Bertoni var. FengNong 3 | Wild type | 5-O-caffeoylquinic acid 3,5-di-O-caffeoylquinic acid | 39.4 mg/g DW 48.1 mg/g DW | [149] |
| Plant Species | Hairy Roots | Natural Product | Maximum Content in the Biomass | Literature |
|---|---|---|---|---|
| Ocimum basilicum L. | Wild type | Rosmarinic acid | 120 mg/g DW | [158] |
| Ocimum basilicum L. | Wild type, elicited with 2% of Phytophthora cinnamoni cell wall extract | Rosmarinic acid | 81 mg/g FW | [159] |
| Ocimum basilicum L. | Wild type, cocultivated with Rhizophagus irregularis | Rosmarinic acid | 140.5 mg/g DW | [161] |
| Agastache rugosa Kuntze | Wild type | Rosmarinic acid | 116 mg/g DW | [178] |
| Rindera graeca (A.DC.) Boiss. & Heldr. | Wild type | Lithospermic acid B | 106.1 mg/g DW | [180] |
| Salvia przewalskii Maxim. | Wild type | Rosmarinic acid Lithospermic acid B | 67.1 mg/g DW 21.4 mg/g DW | [186] |
| Salvia bulleyana Diels | Wild type | Rosmarinic acid | 110.2 mg/g DW | [190] |
| Salvia bulleyana Diels | Wild type | Rosmarinic acid Salvianolic acid K | 90 mg/g DW 10 mg/g DW | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malarz, J.; Yudina, Y.V.; Stojakowska, A. Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates. Int. J. Mol. Sci. 2023, 24, 6920. https://doi.org/10.3390/ijms24086920
Malarz J, Yudina YV, Stojakowska A. Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates. International Journal of Molecular Sciences. 2023; 24(8):6920. https://doi.org/10.3390/ijms24086920
Chicago/Turabian StyleMalarz, Janusz, Yulia V. Yudina, and Anna Stojakowska. 2023. "Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates" International Journal of Molecular Sciences 24, no. 8: 6920. https://doi.org/10.3390/ijms24086920