Juglone in Combination with Temozolomide Shows a Promising Epigenetic Therapeutic Effect on the Glioblastoma Cell Line
Abstract
:1. Introduction
2. Results
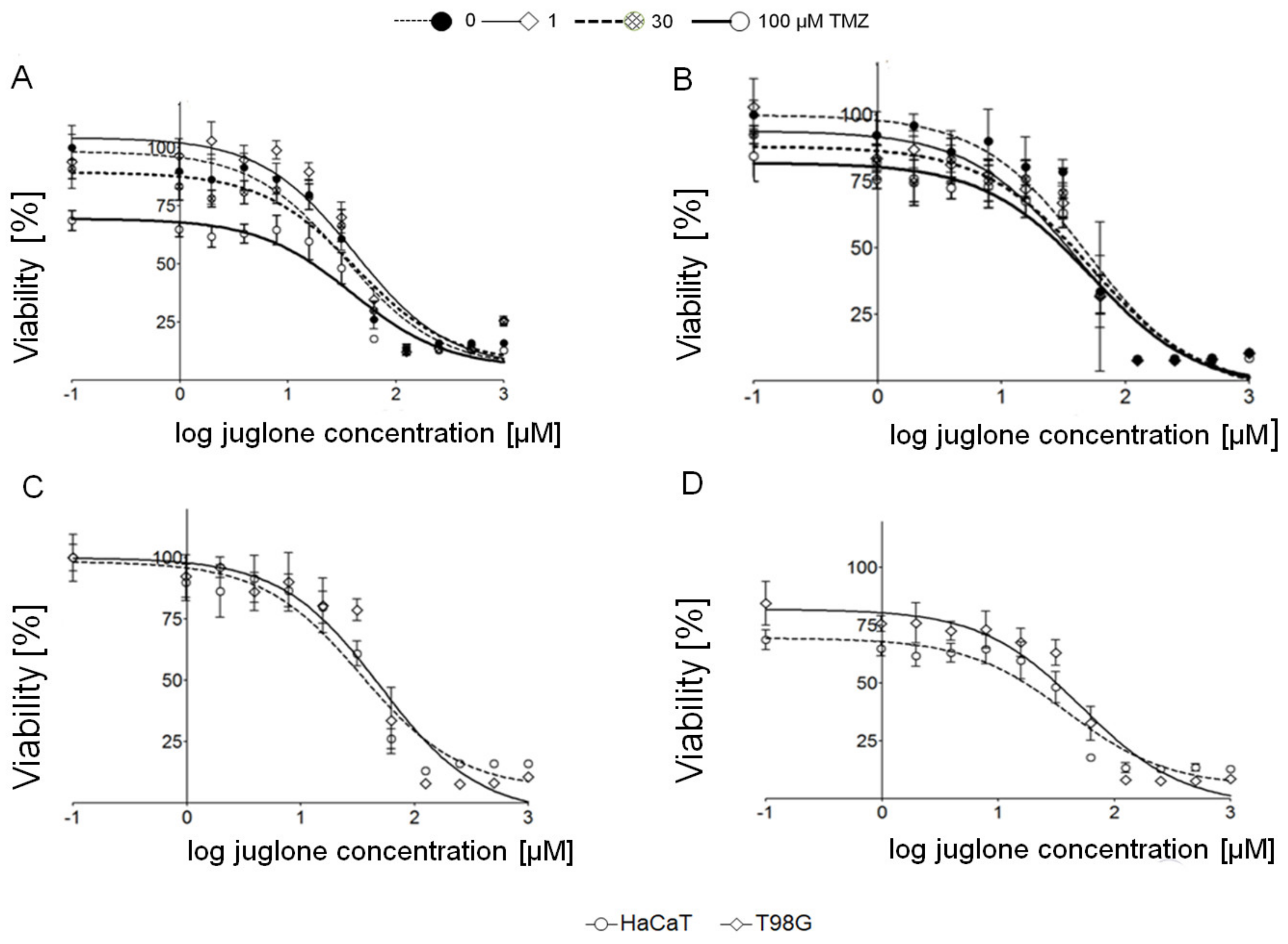
2.1. Cytotoxic Effect of Juglone and Temozolomide in T98G and HaCaT
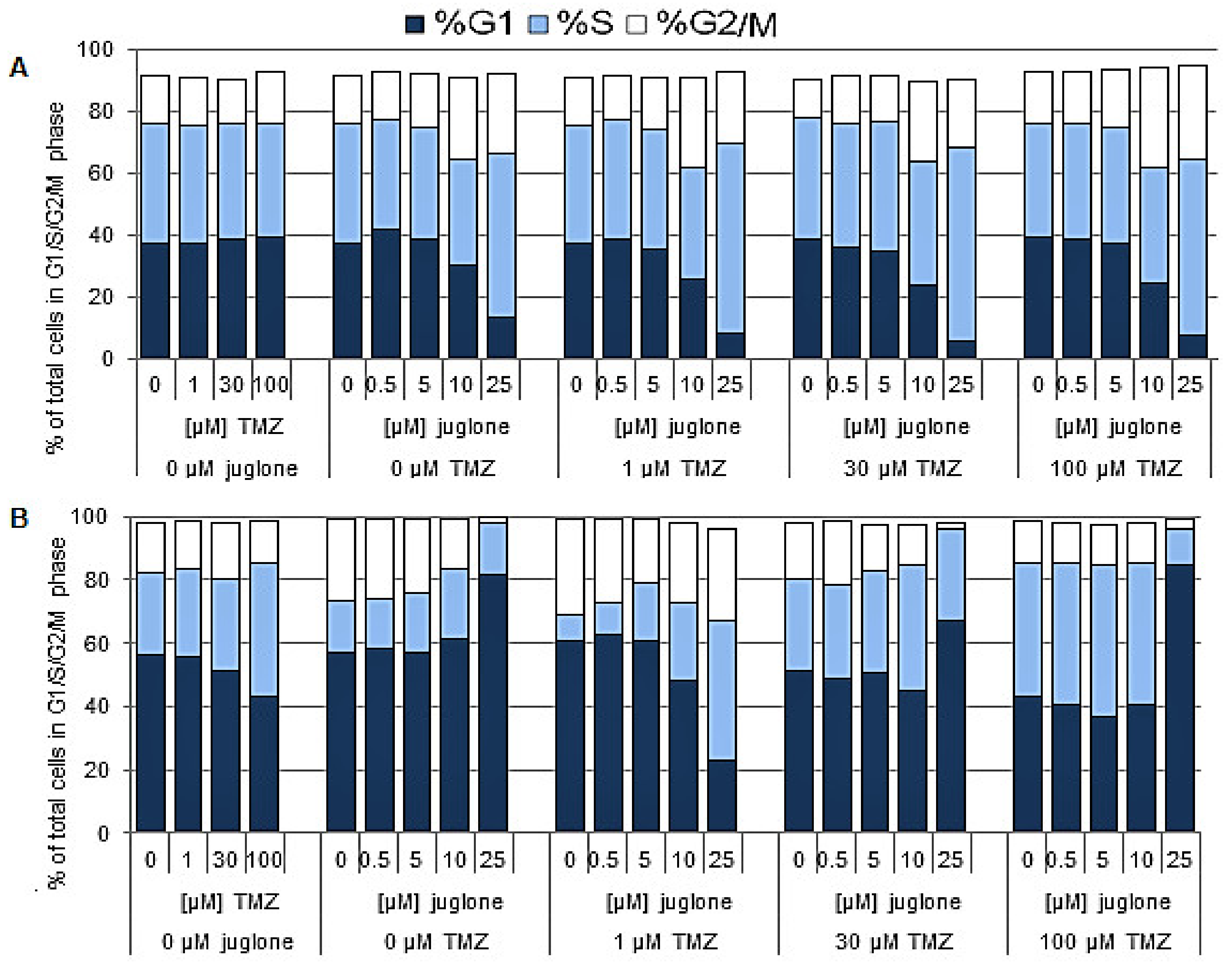
2.2. Cell Cycle Analysis
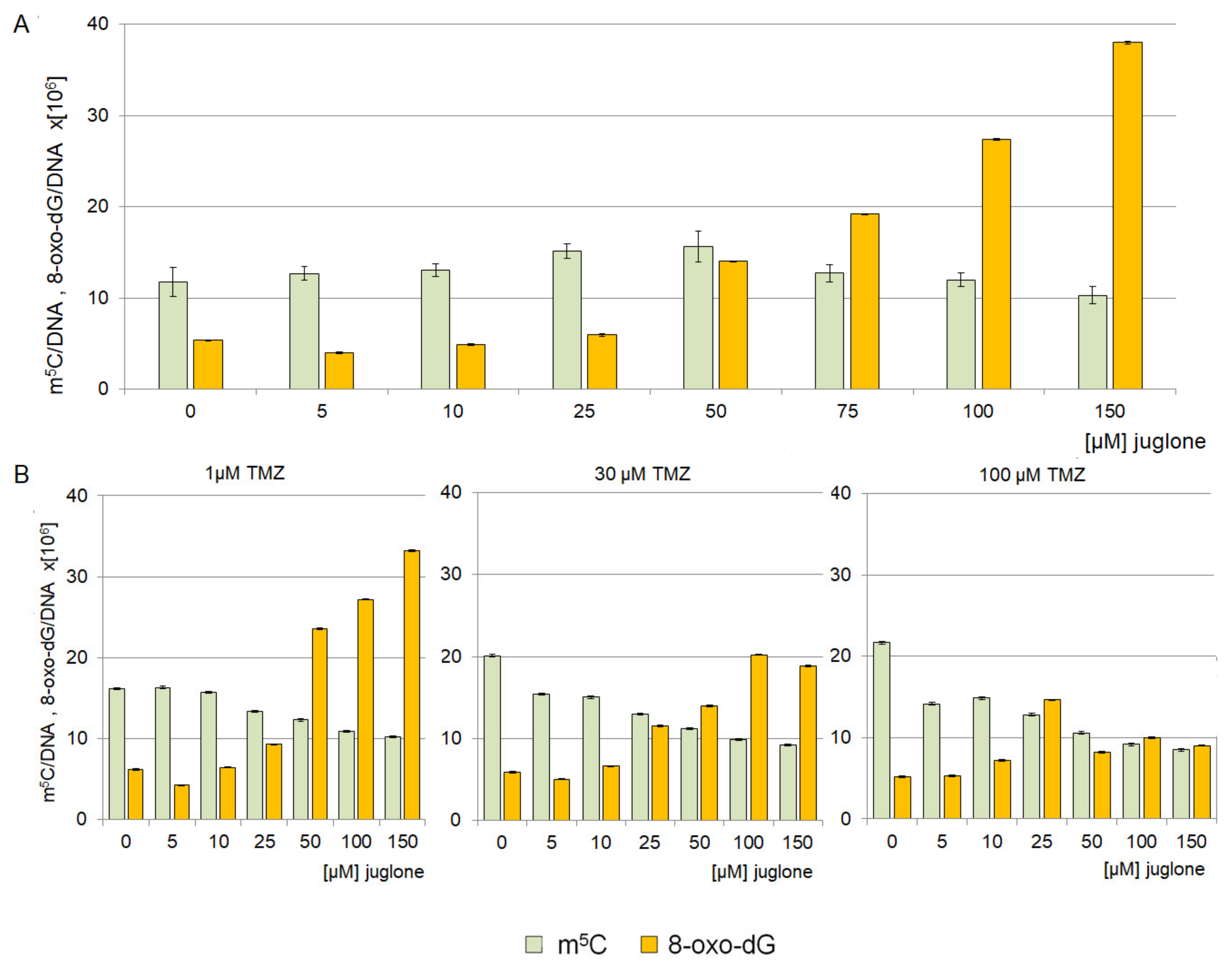
2.3. The Effect of Juglone on Genomic DNA Methylation Level
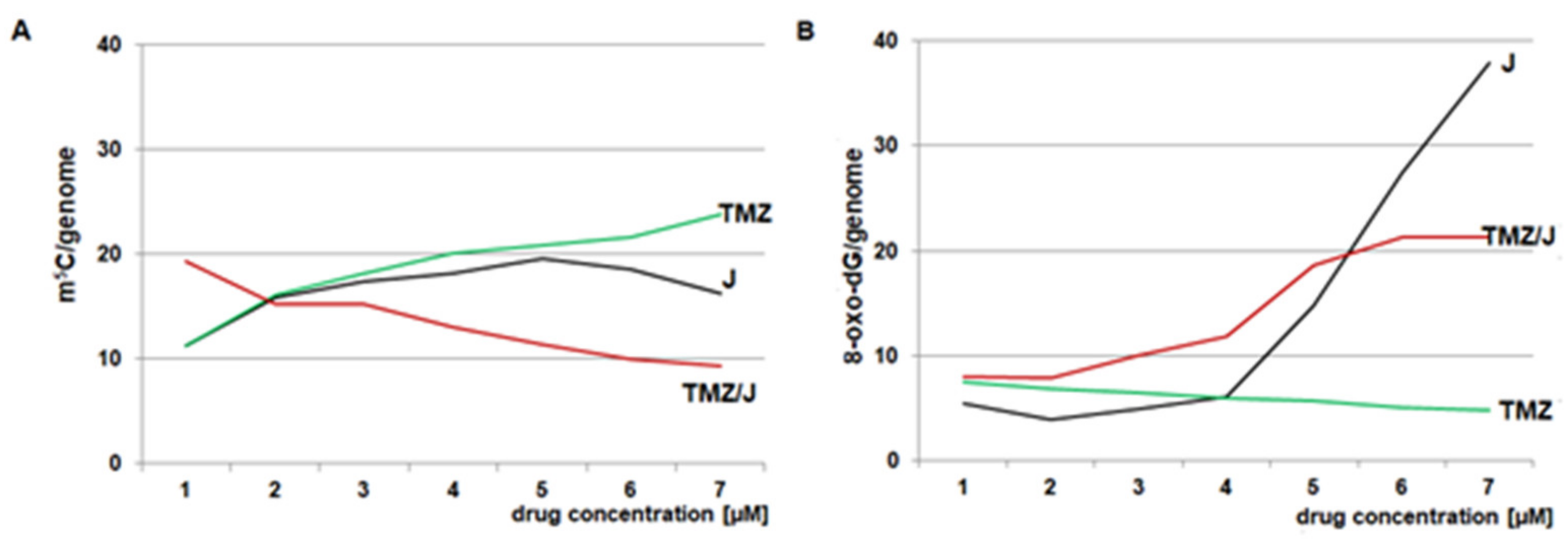
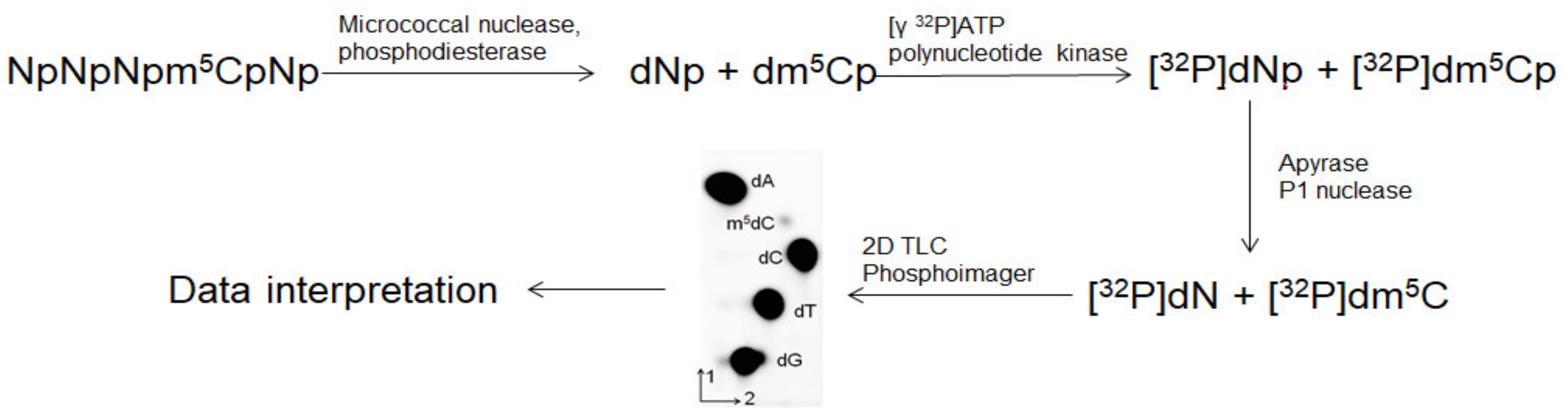
2.4. Analysis of 8-oxo-dG and m5C Contents in the DNA of the T98G Cell Line Treated with Juglone and TMZ
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Line and Culture Conditions
4.3. Cell Viability/Proliferation Assay
4.4. Cell Lines’ Treatment with Juglone
4.5. Cell Lines’ Treatment with the Combination of Juglone and Temozolamide
4.6. Cell Cycle Analysis by Flow Cytometry
4.7. DNA Isolation from Cell Cultures
4.8. Analysis of m5C Contents in DNA
4.9. Analysis of 8-oxo-dG in DNA
4.10. Calculation of the Total Amount of m5C and 8-oxo-dG in Human DNA
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, O.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Bamholtz-Sloan, J.S. CBTRUS Statistical Report Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- McLendon, R.; Friedman, A.; Bigner, D.; van Meir, E.G.; Brat, D.J.; Mastrogianakis, G.M.; Olson, J.J.; Mikkelsen, T.; Lehman, N.; Aldape, K.; et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar]
- Nagarajan, R.P.; Costello, J.F. Epigenetic mechanisms in glioblastoma multiforme. Semin. Cancer Biol. 2009, 19, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome–biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Belter, A.; Barciszewski, J.; Barciszewska, A.M. Revealing the epigenetic effect of temozolomide on glioblastoma cell lines in therapeutic conditions. PLoS ONE 2020, 15, e0229534. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Nam, J.Y.; de Groot, J.F. Treatment of Glioblastoma. J. Oncol. Pract. 2017, 13, 629–638. [Google Scholar] [CrossRef]
- Bady, P.; Kurscheid, S.; Delorenzi, M.; Gorlia, T.; van den Bent, M.J.; Hoang-Xuan, K.; Vauleon, E.; Gijtenbeek, A.; Enting, R.; Thiessen, B.; et al. The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol. 2018, 135, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Bei, R.; Marzocchella, L.; Turriziani, M. The use of temozolomide for the treatment of malignant tumors: Clinical evidence and molecular mechanisms of action. Recent. Pat. Anticancer Drug Discov. 2010, 5, 172–187. [Google Scholar] [CrossRef]
- Li, R.H.; Hou, X.Y.; Yang, C.S.; Liu, W.L.; Tang, J.Q.; Liu, Y.Q.; Jiang, G. Temozolomide for Treating Malignant Melanoma. J. Coll. Physicians Surg. Pak. 2015, 25, 680–688. [Google Scholar]
- Wick, W.; Platten, M.; Weller, M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro-Oncol. 2009, 11, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorribes, I.C.; Handelman, S.K.; Jain, H.V. Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. J. R. Soc. Interface 2020, 17, 20190722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenegro, R.C.; Araújo, A.J.; Molina, M.T.; Filho, J.D.B.M.; Rocha, D.D.; Lopéz-Montero, E.; Goulart, M.O.F.; Bento, E.S.; Alves, A.P.N.N.; Pessoa, C.; et al. Cytotoxic activity of naphthoquinones with special emphasis on juglone and its 5-O-methyl derivative. Chem. Biol. Interact. 2010, 184, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Wang, X.-F.; Sun, D.-J. Juglone reduces growth and migration of U251 glioblastoma cells and disrupts angiogenesis. Oncol. Rep. 2017, 38, 1959–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, S.; Bemonnier, B.L.; Jones, N.; Ficker, C.; Smith, M.L.; Neema, C.; Towers, G.H.N.; Goel, K.; Arnason, J.T. Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. J. Ethnopharm. 2000, 73, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, S.; Jia, J.; Cui, J. Resource efficiency and environmental impact of juglone in Pericarpium Juglandis: A review. Front. Environ. Sci. 2022, 10, 999059. [Google Scholar] [CrossRef]
- Zhang, J.R.; Zhang, J.L.; Zhao, C.B.; Sui, H.; Li, C.F.; Zhong, L.L.; Zhou, Q.; Bai, Y.; An, S.; Du, X.; et al. Green walnut husk extracts proliferation and migration in gastric cancer. J. Cancer 2022, 13, 1130–1144. [Google Scholar] [CrossRef]
- Ji, Y.B.; Xin, G.S.; Qu, Z.Y.; Zou, X.; Yu, M. Mechanism of juglone induced apoptosis of MCF-7 cells by the mitochondrial pathway. Genet. Mol. Res. 2016, 15, gmr.15038785. [Google Scholar] [CrossRef]
- Mahdavi, M.; Azadbakht, M.; Vahdati, A.; Shokrzadeh, M.; Farhadi, A. Cytotoxic effects of juglone and Pterocarya fraxinifolia on prostate cancer cells. J. Pharm. Bioallied Sci. 2019, 11, 195–204. [Google Scholar]
- Seetha, A.; Devaraj, H.; Sudhandiran, G. Indomethacin and juglone inhibit inflammatory molecules to induce apoptosis in colon cancer cells. J. Biochem. Mol. Toxicol. 2020, 34, e22433. [Google Scholar] [CrossRef]
- Narayanan, P.; Farghadani, R.; Nyamathulla, S.; Rajarajeswaran, J.; Thirugnanasampandan, R.; Bhuwaneswari, G. Natural quinones induce ROS-mediated apoptosis and inhibit cell migration in PANC-1 human pancreatic cancer cell line. J. Biochem. Mol. Toxicol. 2022, 36, e23008. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A. Juglone: Atherapeutic phytochemical from Juglans regia L. J. Med. Plants Res. 2011, 22, 5324–5330. [Google Scholar]
- Chao, S.; Greenleaf, A.L.; Price, D.H. Juglone, an inhibitor of the peptidyl-prolyl isomerase Pin1, also directly blocks transcription. Nucleic Acids Res. 2001, 29, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Fila, C. Juglone Inactivates Cysteine-rich Proteins Required for Progression through Mitosis. J. Biol. Chem. 2008, 283, 21714–21724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galas, M.C.; Dourlen, P.; Begard, S.; Ando, K.; Blum, D.; Hamdane, M.; Buee, L. The Peptidylprolyl cis/trans-Isomerase Pin1 Modulates Stress induced Dephosphorylation of Tau in Neurons. Implication in a pathological mechanism related to Alzheimer disease. J. Biol. Chem. 2006, 281, 19296–19304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.T.; Li, Y.; Chu, P.; Ma, X.D.; Tang, Z.Y.; Sun, Z.L. Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development. Biomed. Pharmacother. 2022, 148, 112785. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Kizek, R.; Sladky, Z.; Havel, L. Naphthoquinones as allelochemical triggers of programmed cell death. Environ. Exp. Bot. 2009, 65, 330–337. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, M.; Wu, J.; Fan, F.; Zhang, X.; Li, C.; Yang, H.; Wu, Y.; Yin, Y.; Hua, W. The anti-glioma effect of Juglone derivatives through ROS generation. Front. Pharmacol. 2022, 13, 911760. [Google Scholar] [CrossRef]
- Barciszewska, A.M.; Nowak, S.; Naskręt-Barciszewska, M.Z. The degree of global DNA hypomethylation in peripheral blood correlates with that in matched tumor tissues in several neoplasia. PLoS ONE 2014, 9, e92599. [Google Scholar] [CrossRef]
- Barciszewska, A.M. Global DNA demethylation as an epigenetic marker of human brain metastases. Biosci. Rep. 2018, 38, BSR20180731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barciszewska, A.M.; Murawa, D.; Gawrońska, I.; Murawa, P.; Nowak, S.; Barciszewska, M.Z. Analysis of 5-methylcytosine in DNA of breast and colon cancer tissues. IUBMB Life 2007, 59, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, I.; Wyszko, E.; Barciszewska, A.M.; Nowak, S.; Gawronska, I.; Jablecka, A.; Barciszewska, M.Z. Global DNA methylation changes in blood of patients with essential hypertension. Med. Sci. Monit. 2010, 16, CR149–CR155. [Google Scholar] [PubMed]
- Olszewska, M.; Barciszewska, M.Z.; Fraczek, M.; Huleyuk, N.; Chernykh, V.B.; Zastavna, D.; Barciszewski, J.; Kurpisz, M. Global methylation status of sperm DNA in carriers of chromosome structural aberrations. Asian J. Androl. 2017, 19, 117–124. [Google Scholar]
- Barciszewska, A.M.; Gurda, D.; Głodowicz, P.; Nowak, S.; Naskręt-Barciszewska, M.Z. A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PLoS ONE 2015, 10, e0136669. [Google Scholar] [CrossRef] [Green Version]
- Ovalle, R. A History of the Fenton Reactions. (Fenton Chemistry for Beginners); Reactive Oxygen Species; Ahmad, R., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Barciszewska, A.M.; Giel-Pietraszuk, M.; Perrigue, P.M.; Naskręt-Barciszewska, M. Total DNA Methylation Changes Reflect Random Oxidative DNA Damage in Gliomas. Cells 2019, 8, 1065. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values, ComboSyn Inc., Paramus, (NJ). 2005. Available online: www.combosyn.com (accessed on 15 November 2022).
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Silberman, S.; Hsu, S.H.; Muzyczenko, Z.; Picker, D.; Priebe, W. Design and initiation of an adaptive, randomized, controlled study of berubicin, a topoisomerase 2 poison that crosses the blood brain barrier (BBB), for the treatment of recurrent glioblastoma multiforme (GBM) after first-line therapy. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS2083. [Google Scholar] [CrossRef]
- Babaeenezhad, E.; Sarabi, M.M.; Rajabibazl, M.; Yazdani, S.O.; Karima, S. Global and regional DNA methylation silencing of PPARy associated with glioblastoma multiforme pathogenesis. Mol. Biol. Rep. 2023, 50, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Lacey, M. DNA Hypomethylation and hemimethylation incancer. Adv. Exp. Med. Biol. 2013, 754, 31–56. [Google Scholar] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The complete sequence of a human genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]




| HaCaT | T98G | |
|---|---|---|
| Juglone | 34.0 μM | 49.6 μM |
| Temozolomide | 420 μM | 1430 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barciszewska, A.-M.; Belter, A.; Gawrońska, I.; Giel-Pietraszuk, M.; Naskręt-Barciszewska, M.Z. Juglone in Combination with Temozolomide Shows a Promising Epigenetic Therapeutic Effect on the Glioblastoma Cell Line. Int. J. Mol. Sci. 2023, 24, 6998. https://doi.org/10.3390/ijms24086998
Barciszewska A-M, Belter A, Gawrońska I, Giel-Pietraszuk M, Naskręt-Barciszewska MZ. Juglone in Combination with Temozolomide Shows a Promising Epigenetic Therapeutic Effect on the Glioblastoma Cell Line. International Journal of Molecular Sciences. 2023; 24(8):6998. https://doi.org/10.3390/ijms24086998
Chicago/Turabian StyleBarciszewska, Anna-Maria, Agnieszka Belter, Iwona Gawrońska, Małgorzata Giel-Pietraszuk, and Mirosława Z. Naskręt-Barciszewska. 2023. "Juglone in Combination with Temozolomide Shows a Promising Epigenetic Therapeutic Effect on the Glioblastoma Cell Line" International Journal of Molecular Sciences 24, no. 8: 6998. https://doi.org/10.3390/ijms24086998





