Improved Simulated-Daylight Photodynamic Therapy and Possible Mechanism of Ag-Modified TiO2 on Melanoma
Abstract
:1. Introduction
2. Results
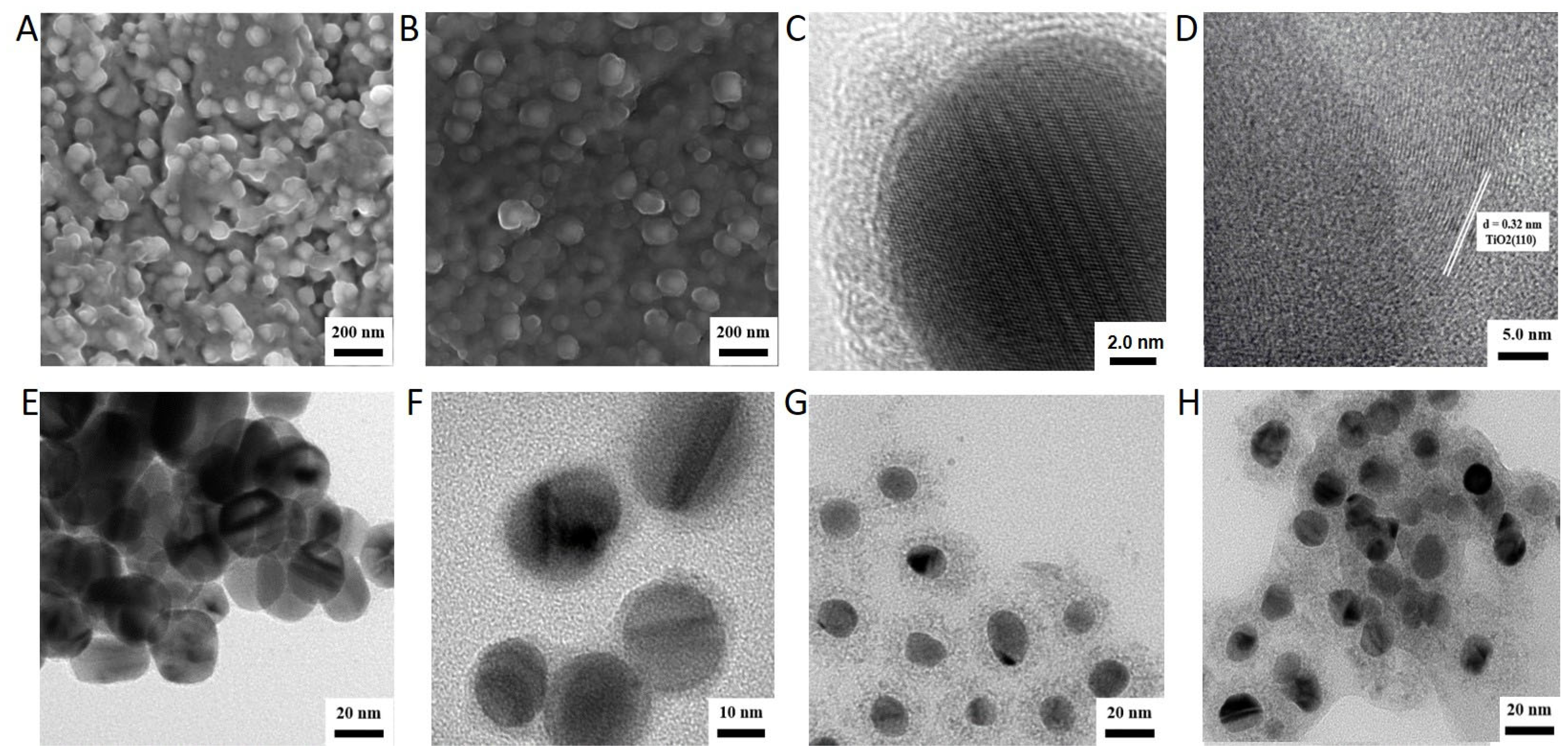
2.1. Synthesis and Characterization of Ag-Modified TiO2 with Different Structure
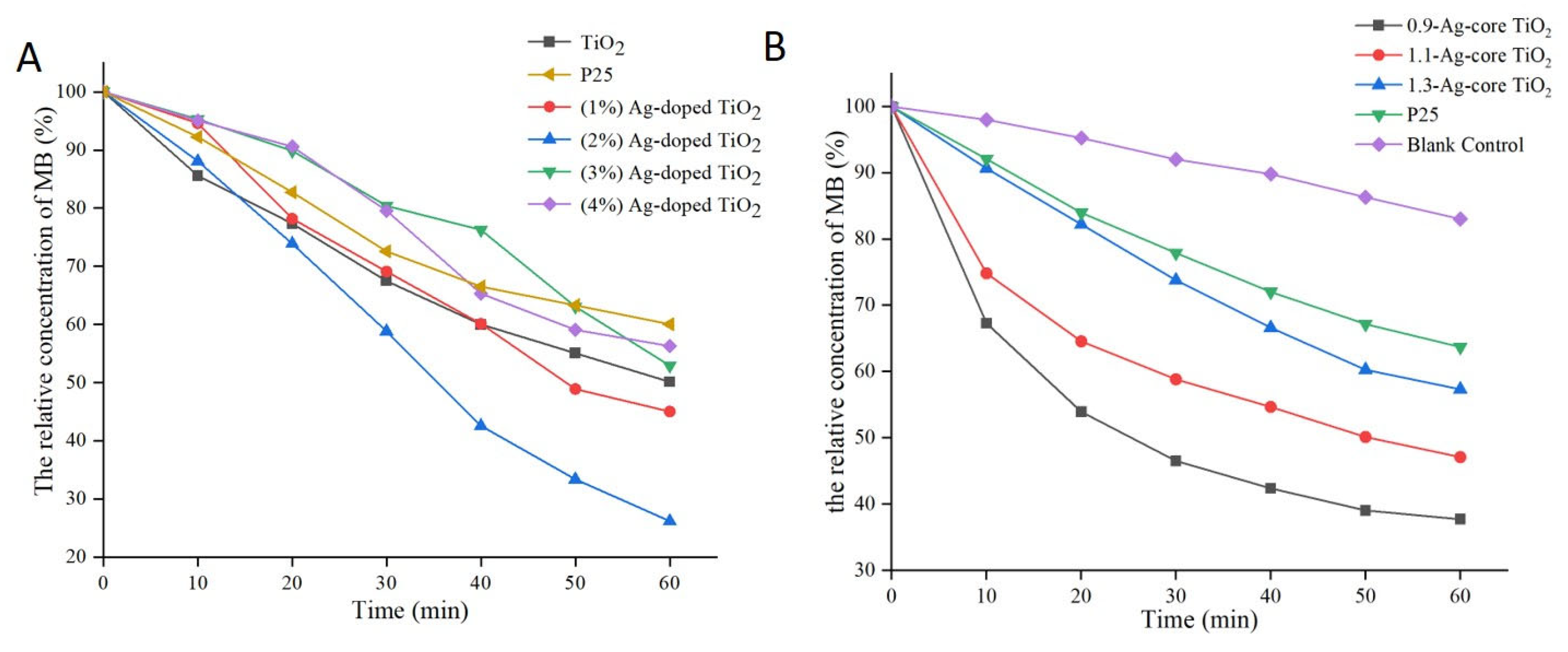
2.2. Comparative Analysis of the Photocatalytic Activity of Ag-Modified TiO2 with Different Structures
2.3. Comparative Analysis of the Cytotoxicity Assay and Phototoxicity of Ag-Modified TiO2 with Different Structures
2.4. Comparative Analysis of ROS Generation by Ag-Modified TiO2 with Different Structures
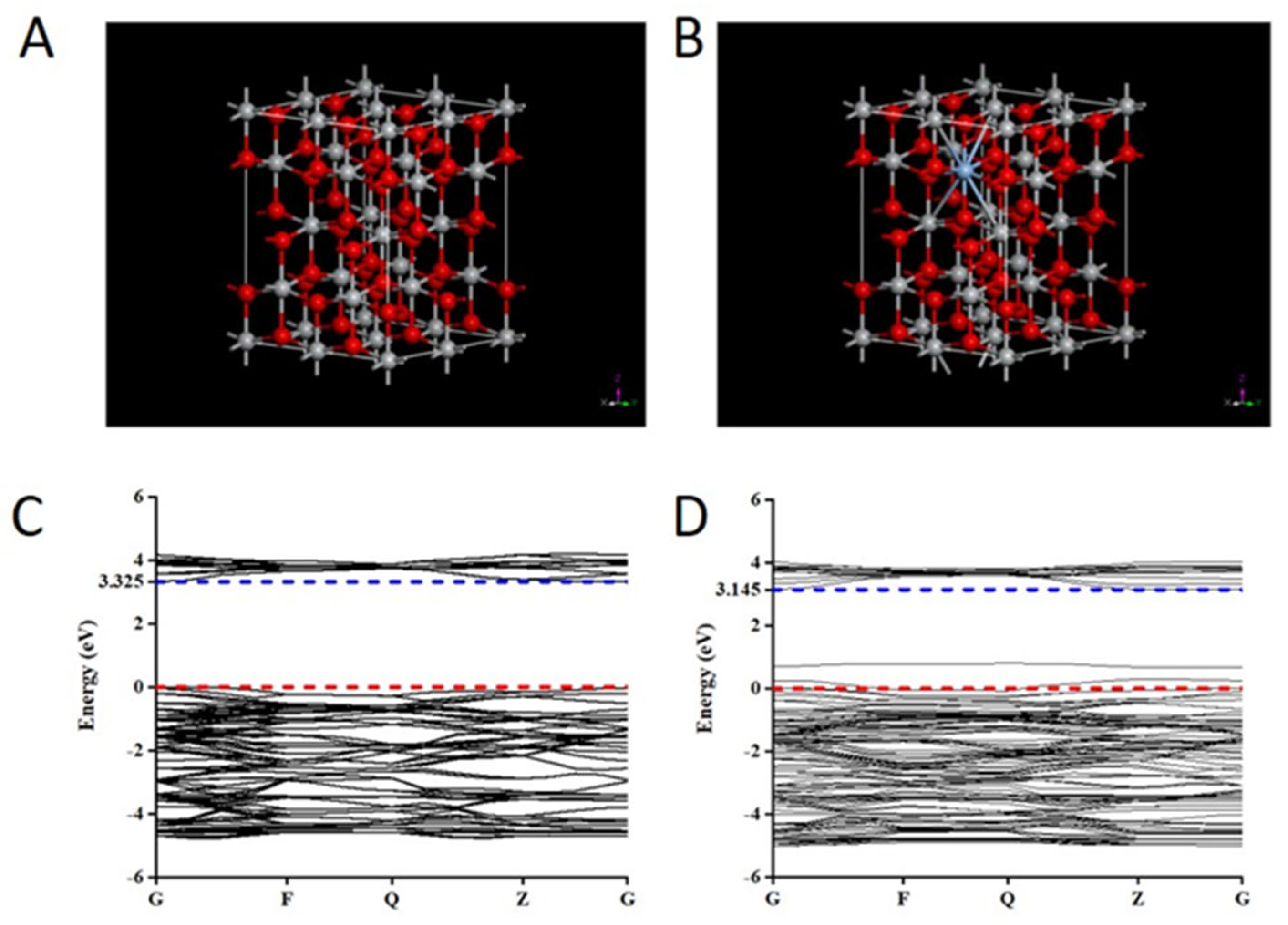
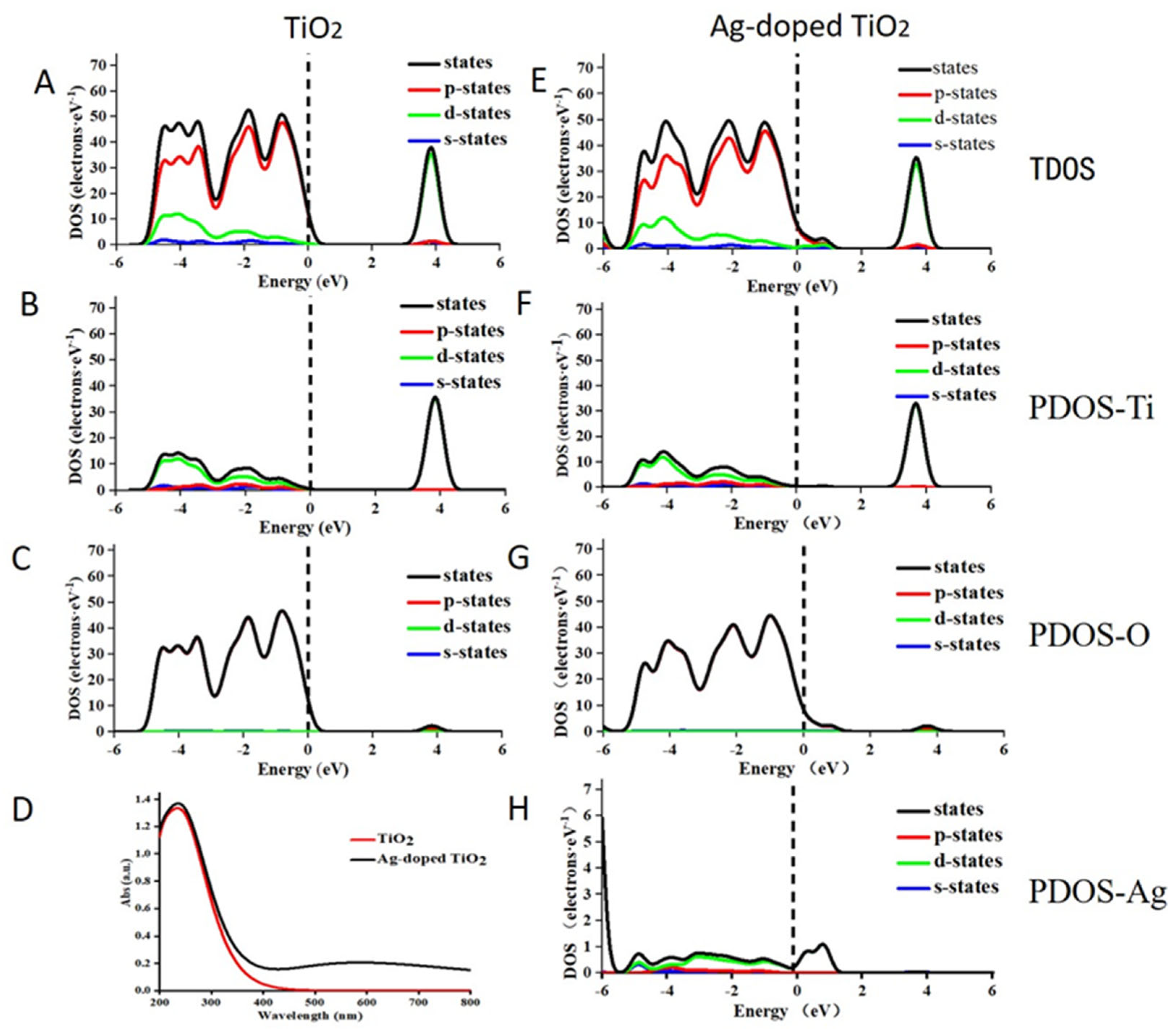
2.5. The Theoretical Mechanistic Analysis
3. Discussion
4. Material and Methods
4.1. Reagents and the Cell Lines
4.2. Synthesis and Modification of TiO2
4.3. Characterization of Ag-Doped TiO2 and Ag-Core TiO2
4.4. Cell Viability Analysis
4.5. Generation of ROS
4.6. First-Principles Analysis for Ag-Doped TiO2
4.7. Discrete Dipole Approximation for Ag-Core TiO2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Aladowicz, E.; Ferro, L.; Vitali, G.C.; Venditti, E.; Fornasari, L.; Lanfrancone, L. Molecular networks in melanoma invasion and metastasis. Future Oncol. 2013, 9, 713–726. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.A. Mechanism of drug sensitivity and resistance in melanoma. Curr. Cancer Drug Targets 2009, 9, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Invest. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Wang, Y.Y.; Liu, Y.C.; Sun, H.W.; Guo, D.S. Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications. Coordin. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, e2006742. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. The history of PDT in Norway Part one: Identification of basic mechanisms of general PDT. Photodiagn. Photodyn. Ther. 2007, 4, 3–11. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425, x. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Correia, J.H. Enhanced Photodynamic Therapy: A Review of Combined Energy Sources. Cells 2022, 11, 3995. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drugs Dermatol. 2006, 5, 353–356. [Google Scholar]
- Anand, S.; Yasinchak, A.; Govande, M.; Shakya, S.; Maytin, E.V. Painless versus conventional photodynamic therapy for treatment of actinic keratosis: Comparison of cell death and immune response in a murine model. Proc. SPIE Int. Soc. Opt. Eng. 2019, 10860, 61–70. [Google Scholar]
- Beiki, D.; Eggleston, I.M.; Pourzand, C. Daylight-PDT: Everything under the sun. Biochem. Soc. Trans. 2022, 50, 975–985. [Google Scholar] [CrossRef]
- Garcia-Gil, M.F.; Gracia-Cazana, T.; Cerro-Munoz, P.; Bernal-Masferrer, L.; Navarro-Bielsa, A.; Gilaberte, Y. Fully home-based methyl aminolevulinate daylight photodynamic therapy for actinic keratosis of the face or scalp: A real life open study. Dermatol. Ther. 2022, 35, e15879. [Google Scholar] [CrossRef]
- Karrer, S.; Szeimies, R.M.; Philipp-Dormston, W.G.; Gerber, P.A.; Prager, W.; Datz, E.; Zeman, F.; Muller, K.; Koller, M. Repetitive Daylight Photodynamic Therapy versus Cryosurgery for Prevention of Actinic Keratoses in Photodamaged Facial Skin: A Prospective, Randomized Controlled Multicentre Two-armed Study. Acta Derm. Venereol. 2021, 101, adv00355. [Google Scholar] [CrossRef]
- O’Mahoney, P.; Khazova, M.; Eadie, E.; Ibbotson, S. Measuring Daylight: A Review of Dosimetry in Daylight Photodynamic Therapy. Pharmaceuticals 2019, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Sjoholm, A.; Claeson, M.; Paoli, J. Measurements of illuminance in simulated daylight photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2022, 38, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Galvao, L.E.; Goncalves, H.S.; Botelho, K.P.; Caldas, J.C. Daylight photodynamic therapy—Experience and safety in treatment of actinic keratoses of the face and scalp in low latitude and high brightness region. An. Bras. Dermatol. 2017, 92, 142–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Liu, P.; Wu, J.; Ma, L.; Zheng, H.; Antosh, M.P.; Zhang, H.; Wang, B.; Chen, W.; Wang, X. The effectiveness and safety of X-PDT for cutaneous squamous cell carcinoma and melanoma. Nanomedicine 2019, 14, 2027–2043. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Goldmann, W.H. Insights into Theranostic Properties of Titanium Dioxide for Nanomedicine. Nano-Micro Lett. 2020, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Rahman, M.A.; Bin Sharif, S.; Kim, J.H.; Siddiqui, S.E.T.; Hossain, M.A. TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Y.; He, H.; Xu, L.; Li, W.; Zhao, D. Recent advances in the synthesis of hierarchically mesoporous TiO(2) materials for energy and environmental applications. Natl. Sci. Rev. 2020, 7, 1702–1725. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical Sterilization of Microbial-Cells by Semiconductor Powders. Fems. Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.W.; Zhang, Z.X.; Wang, S.J.; Yao, C.P. Influence of Parameters on Photodynamic Therapy of Au@TiO2-HMME Core-Shell Nanostructures. Nanomaterials 2022, 12, 1358. [Google Scholar] [CrossRef]
- Yang, C.C.; Tsai, M.H.; Li, K.Y.; Hou, C.H.; Lin, F.H. Carbon-Doped TiO(2) Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. Int. J. Mol. Sci. 2019, 20, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.L.; Wang, S.J.; Zhang, L.W.; Xin, J.; Wang, J.; Yao, C.P.; Zhang, Z.X.; Yang, C.C. Sensitized TiO2 nanocomposites through HMME linkage for photodynamic effects. J. Biomed. Opt. 2016, 21, 128001. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, H.; Zhu, X.; Zhang, X.; Chen, Q.; Chen, J.; Hou, L.; Zhang, Z. Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2+ liberating and artemisinin delivery. Oncotarget 2017, 8, 58738–58753. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Yu, Y.; Wang, E.; Wang, J.; Yao, J.; Cao, Y. Structure of nitrogen and zirconium co-doped titania with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 4622–4629. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO(2) nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Yadav, S.; Dutta, S.; Kale, H.B.; Warkad, I.R.; Zboril, R.; Varma, R.S.; Gawande, M.B. Silver nanomaterials: Synthesis and (electro/photo) catalytic applications. Chem. Soc. Rev. 2021, 50, 11293–11380. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Fu, L.; Cui, J.; Yang, Y. Global Trends and Research Progress of Photodynamic Therapy in Skin Cancer: A Bibliometric Analysis and Literature Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 479–498. [Google Scholar] [CrossRef]
- Lerche, C.M.; Heerfordt, I.M.; Heydenreich, J.; Wulf, H.C. Alternatives to Outdoor Daylight Illumination for Photodynamic Therapy--Use of Greenhouses and Artificial Light Sources. Int. J. Mol. Sci. 2016, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- O’Mahoney, P.; Khazova, M.; Higlett, M.; Lister, T.; Ibbotson, S.; Eadie, E. Use of illuminance as a guide to effective light delivery during daylight photodynamic therapy in the U.K. Br. J. Dermatol. 2017, 176, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Arisi, M.; Galli, B.; Pisani, E.G.; La Rosa, G.; Licata, G.; Rovaris, S.; Tomasi, C.; Rossi, M.; Venturini, M.; Spiazzi, L.; et al. Randomized Clinical Trial of Conventional versus Indoor Daylight Photodynamic Therapy for Treatment of Actinic Cheilitis. Dermatol. Ther. 2022, 12, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pei, X.; Mei, M.; Li, Z.; Lu, S. Study on Photocatalytic Performance of Ag/TiO(2) Modified Cement Mortar. Materials 2022, 15, 4031. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Ag-doping regulates the cytotoxicity of TiO(2) nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017, 7, 17662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, Z. Bonding and Anti-bonding Modes of Plasmon Coupling Effects in TiO2-Ag Core-shell Dimers. Sci. Rep. 2016, 6, 19433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent progress on Ag/TiO(2) photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. Int. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Y.; Yu, X.; Zhang, H.; Zhu, J.; Wang, C.; Chen, F.; Liu, C.; Wang, J.; Zhu, H. Cuprous oxide nanoparticle-inhibited melanoma progress by targeting melanoma stem cells. Int. J. Nanomed. 2017, 12, 2553–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R. Density Functional Theory Study of Optical and Electronic Properties of (TiO2) (n = 5,8,68) Clusters for Application in Solar Cells. Molecules 2021, 26, 955. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Du, Y.; Wu, F.; Cai, Y.; Zhou, T. Review on LSPR assisted photocatalysis: Effects of physical fields and opportunities in multifield decoupling. Nanoscale Adv. 2022, 4, 2608–2631. [Google Scholar] [CrossRef]
- DePrince, A.E.; Hinde, R.J. Accurate Computation of Electric Field Enhancement Factors for Metallic Nanoparticles Using the Discrete Dipole Approximation. Nanoscale Res. Lett. 2010, 5, 592–596. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, J.; Wang, J.; Yao, Y.; Wang, S.; Zhang, Z.; Yao, C. Improved Simulated-Daylight Photodynamic Therapy and Possible Mechanism of Ag-Modified TiO2 on Melanoma. Int. J. Mol. Sci. 2023, 24, 7061. https://doi.org/10.3390/ijms24087061
Xin J, Wang J, Yao Y, Wang S, Zhang Z, Yao C. Improved Simulated-Daylight Photodynamic Therapy and Possible Mechanism of Ag-Modified TiO2 on Melanoma. International Journal of Molecular Sciences. 2023; 24(8):7061. https://doi.org/10.3390/ijms24087061
Chicago/Turabian StyleXin, Jing, Jing Wang, Yuanping Yao, Sijia Wang, Zhenxi Zhang, and Cuiping Yao. 2023. "Improved Simulated-Daylight Photodynamic Therapy and Possible Mechanism of Ag-Modified TiO2 on Melanoma" International Journal of Molecular Sciences 24, no. 8: 7061. https://doi.org/10.3390/ijms24087061





