Human Exposure to Bisphenols, Parabens, and Benzophenones, and Its Relationship with the Inflammatory Response: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
- Participants: Humans.
- Exposure: Bisphenols [bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), bisphenol A-glycidyl methacrylate (BisGMA), bisphenol A diglycidyl ether (BADGE) and bisphenol F diglycidyl ether (BFDGE)], PBs [methylparaben (MeP), ethylparaben (EtP), propylparaben (PrP) and butylparaben (BuP)] and BPs (BP1-12).
- Comparators: Not applicable.
- Outcomes: Inflammatory biomarkers (cytokines, intracellular adhesion molecules, humoral mediators, C-reactive protein, inflammatory milieu, phagocytic leukocytes, antibodies, complement proteins, receptor activator of nuclear factor-kappa B, prostaglandin-endoperoxide synthases).
2.2. Study Selection and Data Extraction
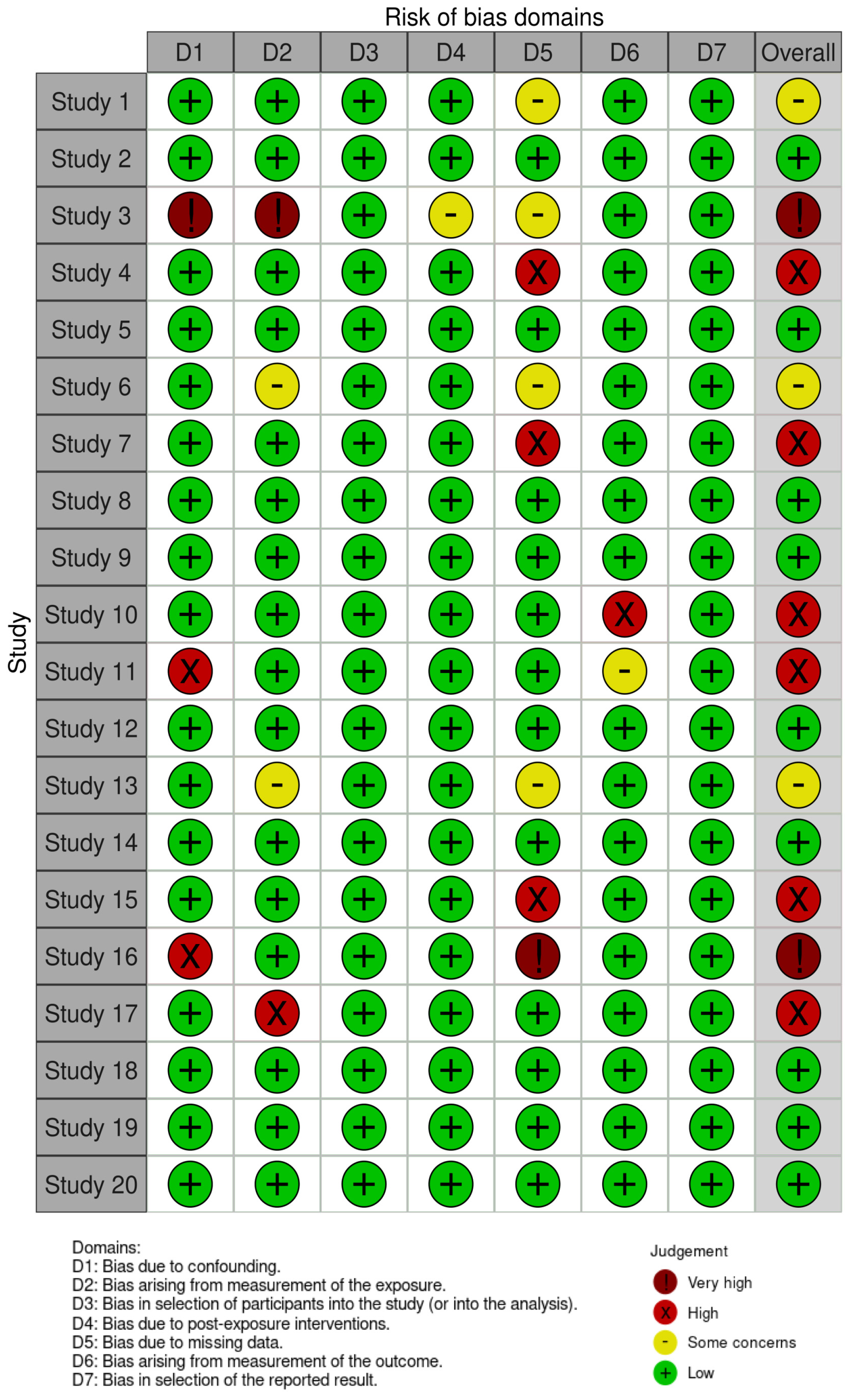
2.3. Assessment of Reporting Quality and Risk of Bias
| Article Number | Reference | Country | Study Design | Period of Sample Collection | Sample Size | Reporting Quality * | |
|---|---|---|---|---|---|---|---|
| For Exposure Assessment | For Outcome Assessment | ||||||
| 1 | Ashley-Martin et al., 2015 [56] | Canada | Cohort | 2008–2011 | 2008–2011 | 1258 | High |
| 2 | Aung et al., 2019 [57] | USA | Cohort | 2006–2008 | 2006–2008 | 482 (1628 samples) | High |
| 3 | Choi et al., 2017 [58] | South Korea | Cohort | 2013 | 2013 | 200 | High |
| 4 | Ferguson et al., 2016 [59] | USA | Case-control | 2006–2008 | 2006–2008 | 482 (1695 samples) | High |
| 5 | Haq et al., 2020 [60] | Pakistan | Cross-sectional | N.R. | N.R. | 400 | High |
| 6 | Huang et al., 2017 [61] | Taiwan | Cohort | 2014–2016 | 2014–2016 | 230 | High |
| 7 | Jain et al., 2020 [62] | India | Cross-sectional | N.R. | N.R. | 300 | Medium |
| 8 | Kelley et al., 2019 [63] | USA | Cohort | 2012–2015 | 2012–2015 | 56 | High |
| 9 | Lang et al., 2008 [64] | USA | Cross-sectional | 2003–2004 | 2003–2004 | 1455 | High |
| 10 | Liang et al., 2020 [65] | China | Cross-sectional | 2015–2016 | 2015–2016 | 111 | High |
| 11 | Linares et al., 2021 [66] | Spain | Prospective Observational | N.R. | N.R. | 200 | Medium |
| 12 | Mohsen et al., 2018 [67] | Egypt | Cross-sectional | N.R. | N.R. | 167 | High |
| 13 | Nalbantoğlu et al., 2021 [68] | Turkey | Case-control | 2018 | 2018 | 280 | High |
| 14 | Qu et al., 2022 [69] | China | Case-control | 2018–2020 | 2018–2020 | 290 | High |
| 15 | Savastano et al., 2015 [70] | Italy | Cross-sectional | N.R. | N.R. | 76 | High |
| 16 | Šimková et al., 2020 [71] | Czech Republic | Case-control | N.R. | N.R. | 39 | Medium |
| 17 | Song et al., 2017 [72] | South Korea | Cross-sectional | 2008–2012 | 2008–2012 | 612 (1141 samples) | Medium |
| 18 | Tsen et al., 2021 [73] | Taiwan | Cross-sectional | N.R. | N.R. | 90 | Medium |
| 19 | Watkins et al., 2015 [43] | Puerto Rico | Cohort | 2010–2012 | 2010–2012 | 106 (238 samples) | High |
| 20 | Yang et al., 2009 [74] | Korea | Cross-sectional | 2005 | 2005 | 485 | High |
3. Results
3.1. Characteristics of Studies
3.2. Exposure of Bisphenols, PBs, and BPs
3.3. Inflammation Assessment
3.4. Association between Exposure to Bisphenols, PBs and BPs, and Inflammation Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Y.; Li, J.; Sun, X.; Liu, H.; Jiang, Y.; Peng, Y.; Zhao, H.; Xia, W.; Li, Y.; et al. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ. Int. 2019, 126, 468–475. [Google Scholar] [CrossRef]
- Engdahl, E.; Rüegg, J. Prenatal Exposure to Endocrine Disrupting Chemicals and Their Effect on Health Later in Life. In Beyond Our Genes: Pathophysiology of Gene and Environment Interaction and Epigenetic Inheritance; Teperino, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–77. [Google Scholar]
- Huo, W.; Cai, P.; Chen, M.; Li, H.; Tang, J.; Xu, C.; Zhu, D.; Tang, W.; Xia, Y. The relationship between prenatal exposure to BP-3 and Hirschsprung’s disease. Chemosphere 2016, 144, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Charisiadis, P.; Arora, M.; van Vliet-Ostaptchouk, J.V.; Makris, K.C. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ. Int. 2015, 85, 352–379. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.S.; Moon, H.-B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef]
- Song, S.; Duan, Y.; Zhang, T.; Zhang, B.; Zhao, Z.; Bai, X.; Xie, L.; He, Y.; Ouyang, J.-P.; Huang, X.; et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose. Ecotoxicol. Environ. Saf. 2019, 169, 822–828. [Google Scholar] [CrossRef]
- Casas, L.; Fernández, M.F.; Llop, S.; Guxens, M.; Ballester, F.; Olea, N.; Irurzun, M.B.; Rodríguez, L.S.; Riaño, I.; Tardón, A.; et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 2011, 37, 858–866. [Google Scholar] [CrossRef]
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, P.; Zhao, N.; Nie, S.; Cui, J.; Zhao, M.; Jin, H. Differences of bisphenol analogue concentrations in indoor dust between rural and urban areas. Chemosphere 2021, 276, 130016. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Snodin, D. Regulatory risk assessments: Is there a need to reduce uncertainty and enhance robustness? Update on propylparaben in relation to its EU regulatory status. Hum. Exp. Toxicol. 2017, 36, 1007–1014. [Google Scholar] [CrossRef]
- Fisher, M.; MacPherson, S.; Braun, J.M.; Hauser, R.; Walker, M.; Feeley, M.; Mallick, R.; Bérubé, R.; Arbuckle, T.E. Paraben Concentrations in Maternal Urine and Breast Milk and Its Association with Personal Care Product Use. Environ. Sci. Technol. 2017, 51, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Chen, Y.; Wu, X.; Xiao, Q.; Li, C.; Li, M.; Hu, W.; Gu, H.; Lu, S. Exposure to parabens and associations with oxidative stress in adults from South China. Sci. Total Environ. 2021, 774, 144917. [Google Scholar] [CrossRef]
- Casas Ferreira, A.M.; Möder, M.; Fernández Laespada, M.E. GC-MS determination of parabens, triclosan and methyl triclosan in water by in situ derivatisation and stir-bar sorptive extraction. Anal. Bioanal. Chem. 2011, 399, 945–953. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Robles-Aguilera, V.; Gálvez-Ontiveros, Y.; Rodrigo, L.; Salcedo-Bellido, I.; Aguilera, M.; Zafra-Gómez, A.; Monteagudo, C.; Rivas, A. Factors Associated with Exposure to Dietary Bisphenols in Adolescents. Nutrients 2021, 13, 1553. [Google Scholar] [CrossRef]
- Morgan, M.K.; Clifton, M.S. Dietary Exposures and Intake Doses to Bisphenol A and Triclosan in 188 Duplicate-Single Solid Food Items Consumed by US Adults. Int. J. Environ. Res. Public Health 2021, 18, 4387. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; Gago-Ferrero, P.; Llorca, M.; Barceló, D. Analysis of UV filters in tap water and other clean waters in Spain. Anal. Bioanal. Chem. 2012, 402, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Benech-Kieffer, F.; Wegrich, P.; Schwarzenbach, R.; Klecak, G.; Weber, T.; Leclaire, J.; Schaefer, H. Percutaneous absorption of sunscreens in vitro: Interspecies comparison, skin models and reproducibility aspects. Ski. Pharmacol. Appl. Ski. Physiol. 2000, 13, 324–335. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Y.; Jiang, J.; Liu, Y.; Luo, X.; Shen, Z.; Chen, X.; Wang, Y.; Dai, Y.; Zhao, J.; et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE 2015, 10, e0127886. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Li, L.; Ying, Y.; Zhang, C.; Wang, W.; Li, Y.; Feng, Y.; Liang, J.; Song, H.; Wang, Y. Bisphenol A exposure and risk of thyroid nodules in Chinese women: A case-control study. Environ. Int. 2019, 126, 321–328. [Google Scholar] [CrossRef]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef]
- Peinado, F.M.; Lendínez, I.; Sotelo, R.; Iribarne-Durán, L.M.; Fernández-Parra, J.; Vela-Soria, F.; Olea, N.; Fernández, M.F.; Freire, C.; León, J.; et al. Association of Urinary Levels of Bisphenols A, F, and S with Endometriosis Risk: Preliminary Results of the EndEA Study. Int. J. Environ. Res. Public Health 2020, 17, 1194. [Google Scholar] [CrossRef]
- Wu, C.; Xia, W.; Li, Y.; Li, J.; Zhang, B.; Zheng, T.; Zhou, A.; Zhao, H.; Huo, W.; Hu, J.; et al. Repeated Measurements of Paraben Exposure during Pregnancy in Relation to Fetal and Early Childhood Growth. Environ. Sci. Technol. 2019, 53, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Johns, L.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int. 2018, 113, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Sramkova, M.; Vitku, J.; Vcelak, J.; Lischkova, O.; Starka, L.; Duskova, M. Parabens and their relation to obesity. Physiol. Res. 2018, 67 (Suppl. 3), S465–S472. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Flaws, J.A. Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertil. Steril. 2016, 106, 827–856. [Google Scholar] [CrossRef] [PubMed]
- Peinado, F.M.; Ocón-Hernández, O.; Iribarne-Durán, L.M.; Vela-Soria, F.; Ubiña, A.; Padilla, C.; Mora, J.C.; Cardona, J.; León, J.; Fernández, M.F.; et al. Cosmetic and personal care product use, urinary levels of parabens and benzophenones, and risk of endometriosis: Results from the EndEA study. Environ. Res. 2021, 196, 110342. [Google Scholar] [CrossRef]
- Perez, P.; Pulgar, R.; Olea-Serrano, F.; Villalobos, M.; Rivas, A.; Metzler, M.; Pedraza, V.; Olea, N. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ. Health Perspect. 1998, 106, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. JAT 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front. Immunol. 2015, 6, 635. [Google Scholar] [CrossRef]
- Thompson, P.A.; Khatami, M.; Baglole, C.J.; Sun, J.; Harris, S.A.; Moon, E.Y.; Al-Mulla, F.; Al-Temaimi, R.; Brown, D.G.; Colacci, A.; et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis 2015, 36 (Suppl. 1), S232–S253. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Ferguson, K.K.; Anzalota Del Toro, L.V.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health 2015, 218, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Moutsatsou, P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 2010, 317452. [Google Scholar] [CrossRef] [PubMed]
- Felty, Q.; Yoo, C.; Kennedy, A. Gene expression profile of endothelial cells exposed to estrogenic environmental compounds: Implications to pulmonary vascular lesions. Life Sci. 2010, 86, 919–927. [Google Scholar] [CrossRef]
- Andersson, H.; Garscha, U.; Brittebo, E. Effects of PCB126 and 17β-oestradiol on endothelium-derived vasoactive factors in human endothelial cells. Toxicology 2011, 285, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. Misregulated inflammation as an outcome of early-life exposure to endocrine-disrupting chemicals. Rev. Environ. Health 2012, 27, 117–131. [Google Scholar] [CrossRef]
- Martinez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. Role of IL-10-Producing Natural Killer Cells in the Regulatory Mechanisms of Inflammation during Systemic Infection. Biomolecules 2022, 12, 4. [Google Scholar] [CrossRef]
- Peinado, F.M.; Artacho-Cordón, F.; Barrios-Rodríguez, R.; Arrebola, J.P. Influence of polychlorinated biphenyls and organochlorine pesticides on the inflammatory milieu. A systematic review of in vitro, in vivo and epidemiological studies. Environ. Res. 2020, 186, 109561. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Zhong, K.; Wang, C.; Xu, X. The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2022, 234, 113382. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, M.P.; do Carmo, L.F.; Vasconcelos, C.C.F.; Alvarenga, M.P.; Alvarenga-Filho, H.; de Melo Bento, C.A.; Paiva, C.L.A.; Leyva-Fernández, L.; Fernández, Ó.; Papais-Alvarenga, R.M. Neuromyelitis optica is an HLA associated disease different from Multiple Sclerosis: A systematic review with meta-analysis. Sci. Rep. 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Morgan, R.; Rooney, A.; Taylor, K.; Thayer, K.; Silva, R.; Lemeris, C.; Akl, A.; Arroyave, W.; Batesong, T.; et al. Risk of Bias in Non-randomized Studies-of Exposure (ROBINS-E). Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 5 February 2023).
- Ashley-Martin, J.; Dodds, L.; Levy, A.R.; Platt, R.W.; Marshall, J.S.; Arbuckle, T.E. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ. Res. 2015, 140, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.T.; Ferguson, K.K.; Cantonwine, D.E.; Bakulski, K.M.; Mukherjee, B.; Loch-Caruso, R.; McElrath, T.F.; Meeker, J.D. Associations between maternal plasma measurements of inflammatory markers and urinary levels of phenols and parabens during pregnancy: A repeated measures study. Sci. Total Environ. 2019, 650, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Ha, K.H.; Kim, D.J. Exposure to bisphenol A is directly associated with inflammation in healthy Korean adults. Environ. Sci. Pollut. Res. Int. 2017, 24, 284–290. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod. Toxicol. 2016, 66, 93–98. [Google Scholar] [CrossRef]
- Haq, M.E.U.; Akash, M.S.H.; Sabir, S.; Mahmood, M.H.; Rehman, K. Human exposure to bisphenol A through dietary sources and development of diabetes mellitus: A cross-sectional study in Pakistani population. Environ. Sci. Pollut. Res. Int. 2020, 27, 26262–26275. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lai, C.H.; Yang, W.; Wu, K.Y.; Lu, C.A.; Chen, H.C.; Chen, M.L. Prenatal Nonylphenol and Bisphenol A Exposures and Inflammation Are Determinants of Oxidative/Nitrative Stress: A Taiwanese Cohort Study. Environ. Sci. Technol. 2017, 51, 6422–6429. [Google Scholar] [CrossRef]
- Jain, J.; Gupta, N.; Mathur, R.; Nimesh, S.; Mathur, S.K. A Study on Impact of BPA in the Adipose Tissue Dysfunction (Adiposopathy) in Asian Indian Type 2 Diabetes Mellitus Subjects. Indian J. Clin. Biochem. IJCB 2020, 35, 451–457. [Google Scholar] [CrossRef]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Huo, X.; Wang, W.; Li, Y.; Zhang, J.; Feng, Y.; Wang, Y. Association of bisphenol A or bisphenol S exposure with oxidative stress and immune disturbance among unexplained recurrent spontaneous abortion women. Chemosphere 2020, 257, 127035. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Fernández, M.F.; Gutiérrez, A.; García-Villalba, R.; Suárez, B.; Zapater, P.; Martínez-Blázquez, J.A.; Caparrós, E.; Tomás-Barberán, F.A.; Francés, R. Endocrine disruption in Crohn’s disease: Bisphenol A enhances systemic inflammatory response in patients with gut barrier translocation of dysbiotic microbiota products. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21697. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.A.; Zaki, S.T.; Youssef, M.M.; Salah El-Din, E.M.; AbuShady, M.M.; Hussein, J.; Morsy, S.; Sallam, S.F.; El-alameey, I.R.; AlMenabbawy, K. May detectable urinary Bisphenol A among children be associated with cardiovascular risk factor? Biosci. Res. 2018, 15, 1243–1250. [Google Scholar]
- Nalbantoğlu, A.; Çelikkol, A.; Samancı, N.; Günaydın, N.C.; Nalbantoğlu, B. Bisphenol A as a risk factor for allergic rhinitis in children. Hum. Exp. Toxicol. 2021, 40, 395–402. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, Y.; Zhao, M.; Wu, P.; Xue, J.; Jin, H. Human serum paraben levels and their associations with rheumatoid arthritis: A case-control study from Hangzhou, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 7198–7206. [Google Scholar] [CrossRef]
- Savastano, S.; Tarantino, G.; D′Esposito, V.; Passaretti, F.; Cabaro, S.; Liotti, A.; Liguoro, D.; Perruolo, G.; Ariemma, F.; Finelli, C.; et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: A cross-sectional study on adult male population. J. Transl. Med. 2015, 13, 169. [Google Scholar] [CrossRef]
- Šimková, M.; Vítků, J.; Kolátorová, L.; Vrbíková, J.; Vosátková, M.; Včelák, J.; Dušková, M. Endocrine disruptors, obesity, and cytokines-how relevant are they to PCOS? Physiol. Res. 2020, 69 (Suppl. 2), S279–S293. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, J.; Bui, P.T.C.; Choi, K.; Gye, M.C.; Hong, Y.C.; Kim, J.H.; Lee, Y.J. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ. Res. 2017, 158, 490–498. [Google Scholar] [CrossRef]
- Tsen, C.M.; Liu, J.H.; Yang, D.P.; Chao, H.R.; Chen, J.L.; Chou, W.C.; Ho, Y.C.; Chuang, C.Y. Study on the correlation of bisphenol A exposure, pro-inflammatory gene expression, and C-reactive protein with potential cardiovascular disease symptoms in young adults. Environ. Sci. Pollut. Res. Int. 2021, 28, 32580–32591. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hong, Y.C.; Oh, S.Y.; Park, M.S.; Kim, H.; Leem, J.H.; Ha, E.H. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res. 2009, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Luo, D.; Zhai, L.; Bai, Y.; Wei, W.; Sun, Q.; Jia, L. Bisphenol A increases TLR4-mediated inflammatory response by up-regulation of autophagy-related protein in lung of adolescent mice. Chemosphere 2021, 268, 128837. [Google Scholar] [CrossRef] [PubMed]
- Priego, A.R.; Parra, E.G.; Mas, S.; Morgado-Pascual, J.L.; Ruiz-Ortega, M.; Rayego-Mateos, S. Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice. Int. J. Mol. Sci. 2021, 22, 7189. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, M.; Wu, C.; Zhou, C.; Zhang, J.; Zhu, Q.; Shen, T. Bisphenol A promotes macrophage proinflammatory subtype polarization via upregulation of IRF5 expression in vitro. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 60, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.F.; Coden, M.E.; Berdnikovs, S. Endocrine Disruptor Bisphenol A (BPA) Triggers Systemic Para-Inflammation and is Sufficient to Induce Airway Allergic Sensitization in Mice. Nutrients 2020, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Inderbinen, S.G.; Kley, M.; Zogg, M.; Sellner, M.; Fischer, A.; Kędzierski, J.; Boudon, S.; Jetten, A.M.; Smieško, M.; Odermatt, A. Activation of retinoic acid-related orphan receptor γ(t) by parabens and benzophenone UV-filters. Toxicology 2022, 471, 153159. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L. UV filters, ingredients with a recognized anti-inflammatory effect. PLoS ONE 2012, 7, e46187. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Amaya, E.; Grimaldi, M.; Sáenz, J.M.; Real, M.; Fernández, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Artacho-Cordón, F.; Ríos-Arrabal, S.; León, J.; Frederiksen, H.; Sáenz, J.M.; Martín-Olmedo, P.; Fernández, M.F.; Olea, N.; Arrebola, J.P. Adipose tissue concentrations of non-persistent environmental phenols and local redox balance in adults from Southern Spain. Environ. Int. 2019, 133, 105118. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: Mechanisms and implications for immunity. Horm. Behav. 2012, 62, 254–262. [Google Scholar] [CrossRef]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef]
- Dietert, R.R. Role of developmental immunotoxicity and immune dysfunction in chronic disease and cancer. Reprod. Toxicol. 2011, 31, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110 (Suppl. 1), 25–42. [Google Scholar] [CrossRef]
- Yuan, M.; Bai, M.Z.; Huang, X.F.; Zhang, Y.; Liu, J.; Hu, M.H.; Zheng, W.Q.; Jin, F. Preimplantation Exposure to Bisphenol A and Triclosan May Lead to Implantation Failure in Humans. BioMed. Res. Int. 2015, 2015, 184845. [Google Scholar] [CrossRef]
- Holsapple, M.P.; Paustenbach, D.J.; Charnley, G.; West, L.J.; Luster, M.I.; Dietert, R.R.; Burns-Naas, L.A. Symposium summary: Children’s health risk—What’s so special about the developing immune system? Toxicol. Appl. Pharmacol. 2004, 199, 61–70. [Google Scholar] [CrossRef]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.D.; Jarabek, A.M.; Landreth, K.; Peden, D.B.; Pinkerton, K.; Smialowicz, R.J.; et al. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000, 108 (Suppl. 3), 483–490. [Google Scholar]
- Shakil, S.S.; Temu, T.M.; Kityo, C.; Nazzinda, R.; Erem, G.; Kentoffio, K.; Bittencourt, M.; Ntusi, N.A.B.; Zanni, M.V.; Longenecker, C.T. Sex modulates the association between inflammation and coronary atherosclerosis among older Ugandan adults with and without HIV. AIDS 2022, 37, 579–586. [Google Scholar] [CrossRef]


| Article Number | Reference | EDC Family | Compounds | Matrix | Frequency of Detection (%) | Unit | Concentrations |
|---|---|---|---|---|---|---|---|
| 1 | Ashley-Martin et al., 2015 [56] | Bisphenols | BPA | Urine | 86.6 | µg/L | N.R. |
| 2 | Aung et al., 2019 [57] | Bisphenols | BPS | Urine | 20.6 | ng/mL | P50: 0.38 |
| PBs | MeP | 99.9 | P50: 186 | ||||
| EtP | 59.5 | P50: 2.15 | |||||
| PrP | 99.0 | P50: 45.60 | |||||
| BuP | 68.4 | P50: 0.85 | |||||
| BPs | BP-3 | 99.7 | P50: 42.60 | ||||
| 3 | Choi et al., 2017 [58] | Bisphenols | BPA | Urine | N.R. | µg/L | N.R. |
| 4 | Ferguson et al., 2016 [59] | Bisphenols | BPA | Urine | 83.4 | ng/mL | GM: 1.32–1.38 |
| 5 | Haq et al., 2020 [60] | Bisphenols | BPA | Urine | N.R. | ng/mL | Diabetic: 3.44 ± 1.82 * |
| Healthy: 1.70 ± 0.43 * | |||||||
| 6 | Huang et al., 2017 [61] | Bisphenols | BPA | Urine | 82.2 | ng/mL | P50: 1.77 |
| 7 | Jain et al., 2020 [62] | Bisphenols | BPA | Serum | N.R. | N.R. | N.R. |
| 8 | Kelley et al., 2019 [63] | Bisphenols | BPA | Urine | N.R. | N.R. | N.R. |
| BPS | |||||||
| BPF | |||||||
| PBs | MeP | ||||||
| EtP | |||||||
| PrP | |||||||
| BuP | |||||||
| BPs | BP-3 | ||||||
| 9 | Lang et al., 2008 [64] | Bisphenols | BPA | Urine | N.R. | ng/mL | Men, weighted mean: 4.53 |
| Women, weighted mean: 4.66 | |||||||
| 10 | Liang et al., 2020 [65] | Bisphenols | BPA | Urine | 99.1 | ng/mL | P50: 0.95 |
| BPS | 41.4 | P50: <LOD | |||||
| 11 | Linares et al., 2021 [66] | Bisphenols | BPA | Serum | N.R. | µM | In remission: 5.57 ± 8.29 * |
| Active disease: 11.98 ± 20.25 * | |||||||
| PBs | MeP | In remission: 3.67 ± 5.72 * | |||||
| Active disease: 3.26 ± 5.50 * | |||||||
| EtP | In remission: 0.90 ± 2.79 * | ||||||
| Active disease: 0.31 ± 0.55 * | |||||||
| PrP | In remission: 0.32 ± 0.82 * | ||||||
| Active disease: 0.15 ± 0.25 * | |||||||
| BuP | In remission: 0.07 ± 0.40 * | ||||||
| Active disease: 0.04 ± 0.13 * | |||||||
| BP-1 | In remission: 0.10 ± 0.52 * | ||||||
| BPs | Active disease: 0.05 ± 0.14 * | ||||||
| BP-3 | In remission: 0.21 ± 0.46 * | ||||||
| Active disease: 0.03 ± 0.09 * | |||||||
| 12 | Mohsen et al., 2018 [67] | Bisphenols | BPA free | Urine | N.R. | ng/mL | P50 Boys: 0.20 P50 Girls: 0.21 P50 Boys: 0.25 P50 Girls: 0.33 P50 Boys: 0.60 P50 Girls: 0.67 |
| BPA conjugated | |||||||
| BPA total | |||||||
| 13 | Nalbantoğlu et al., 2021 [68] | Bisphenols | BPA | Serum | N.R. | µg/L | Healthy: 445.38 ± 329.14 * |
| Allergic rhinitis: 2225.83 ± 1321.75 * | |||||||
| 14 | Qu et al., 2022 [69] | PBs | MeP | Serum | Healthy: 97.0, | ng/mL | P50 Healthy: 2.60 |
| Rheumatoid arthritis: 100.0 | P50 Rheumatoid arthritis: 4.70 | ||||||
| EtP | Healthy: 50.0, | P50 Healthy: 0.33 | |||||
| Rheumatoid arthritis: 63.0 | P50 Rheumatoid arthritis: 0.96 | ||||||
| PrP | Healthy: 53.0, | P50 Healthy: 0.49 | |||||
| Rheumatoid arthritis: 71.0 | P50 Rheumatoid arthritis: 0.74 | ||||||
| BuP | Healthy: 43.0, | P50 Healthy: <LOD | |||||
| Rheumatoid arthritis: 55.0 | P50 Rheumatoid arthritis: 0.98 | ||||||
| 15 | Savastano et al., 2015 [70] | Bisphenols | BPA | Plasma | N.R. | ng/mL | 1.04 ± 0.77 * |
| 16 | Šimková et al., 2020 [71] | Bisphenols | BPA | Blood | Controls: 70.0 | nM/L | P50 Controls: 0.13 |
| Normal weight PCOS: 100.0 | P50 Normal weight PCOS: 0.28 | ||||||
| Obesity PCOS: 90.0 | P50 Obesity PCOS: 0.13 | ||||||
| BPS | Controls: 25.0 | P50 Controls: 0.00 | |||||
| Normal weight PCOS: 33.0 | P50 Normal weight PCOS: 0.00 | ||||||
| Obesity PCOS: 40.0 | P50 Obesity PCOS: 0.00 | ||||||
| BPF | N.R. | N.R. | |||||
| BPAF | N.R. | N.R. | |||||
| PBs | MeP | N.R. | N.R. | ||||
| EtP | N.R. | N.R. | |||||
| PrP | N.R. | N.R. | |||||
| BuP | N.R. | N.R. | |||||
| BzP | N.R. | N.R. | |||||
| Total PBs | Controls: 30.0 | P50 Controls: 0.00 | |||||
| Normal weight PCOS: 56.0 | P50 Normal weight PCOS: 0.49 | ||||||
| Obesity PCOS: 10.0 | P50 Obesity PCOS: 0.00 | ||||||
| 17 | Song et al., 2017 [72] | Bisphenols | BPA free | Urine | N.R. | µg/L | N.R. |
| BPA conjugated | |||||||
| 18 | Tsen et al., 2021 [73] | Bisphenols | BPA | Plasma | 100.0 | ng/mL | 4.50 ± 2.00 * |
| 19 | Watkins et al., 2015 [43] | Bisphenols | BPA | Urine | 98.7 | ng/mL | P50: 2.67 |
| PBs | MeP | 100.0 | P50: 152.00 | ||||
| PrP | 100.0 | P50: 45.40 | |||||
| BuP | 75.6 | P50: 0.60 | |||||
| BPs | BP-3 | 100.0 | P50: 34.50 | ||||
| 20 | Yang et al., 2009 [74] | Bisphenols | BPA | Urine | 76.0 | µg/L | P50: 0.64 |
| Article Number | Reference | Inflammation Parameter | Matrix | Unit | Concentrations |
|---|---|---|---|---|---|
| 1 | Ashley-Martin et al., 2015 [56] | IL-33 | Cord blood | pg/mL | GM: 0.90 |
| GM: 0.90 | |||||
| 2 | Aung et al., 2019 [57] | CRP | Plasma | µg/mL | P50: 5.26 |
| IL-10 | pg/mL | P50: 13.20 | |||
| IL-6 | P50: 1.33 | ||||
| TNF-α | P50: 2.99 | ||||
| IL-1β | P50: 0.26 | ||||
| 3 | Choi et al., 2017 [58] | CRP | Serum | mg/L | 0.63–4.57 |
| 4 | Ferguson et al., 2016 [59] | CRP | Plasma | N.R. | N.R. |
| IL-1β | |||||
| IL-6 | |||||
| IL-10 | |||||
| TNF-α | |||||
| 5 | Haq et al., 2020 [60] | CRP | Blood | ng/mL | Diabetic BPA detected: Mean:10.63 |
| Diabetic BPA non detected: Mean: 7.50 | |||||
| Non-diabetic BPA detected: Mean: 5.29 | |||||
| Non-diabetic BPA non detected: Mean: 2.63 | |||||
| IL-6 | pg/mL | Diabetic BPA detected: Mean: 14.87 | |||
| Diabetic BPA non detected: Mean: 10.49 | |||||
| Non-diabetic BPA detected: Mean: 4.62 | |||||
| Non-diabetic BPA non detected: Mean: 2.75 | |||||
| 6 | Huang et al., 2017 [61] | CRP | Plasma and cord serum | µg/mL | P50 Plasma: 2.60 |
| P50 Cord serum: N.R. | |||||
| IL-6 | pg/mL | P50 Plasma: 6.26 | |||
| P50 Cord serum: 3.70 | |||||
| TNF-α | pg/mL | P50 Plasma: 3.65 | |||
| P50 Cord serum: 5.47 | |||||
| 7 | Jain et al., 2020 [62] | TNF-α | Serum | pg/mL | Diabetic population: 87.88 ± 26.77 * |
| Control: 82.12 ± 27.45 * | |||||
| IL-6 | Diabetic population: 103.89 ± 16.83 * | ||||
| Control: 101.76 ± 13.37 * | |||||
| IL-1α | Diabetic population: 62.42 ± 10.53 * | ||||
| Control: 60.15 ± 7.73 * | |||||
| 8 | Kelley et al., 2019 [63] | GM-CSF | Blood and cord blood | pg/mL | N.R. |
| IFN-γ | |||||
| MCP-1 | |||||
| MCP-3 | |||||
| MIP-1α | |||||
| MIP-1β | |||||
| TNFα | |||||
| VEGF | |||||
| IL-1β | |||||
| IL-6 | |||||
| IL-8 | |||||
| IL-17A | |||||
| 9 | Lang et al., 2008 [64] | CRP | Serum | N.R. | N.R. |
| 10 | Liang et al., 2020 [65] | IL-1β | Serum | ng/mL | P50: 0.08 |
| IL-2 | P50: <LOD | ||||
| IL-4 | P50: <LOD | ||||
| IL-6 | P50: 0.70 | ||||
| IL-8 | µg/mL | P50: 0.06 | |||
| IL-10 | ng/mL | P50: 0.17 | |||
| IL-12p70 | P50: 0.01 | ||||
| IL-13 | P50: 0.24 | ||||
| TNF-α | P50: 1.82 | ||||
| TGF-β | µg/mL | P50: 17.13 | |||
| IFN-γ | ng/mL | P50: 5.54 | |||
| 11 | Linares et al., 2021 [66] | IL-12 | Serum | µg/mL | In remission: 38.60 ± 17.20 * |
| Active disease: 42.50 ± 16.90 * | |||||
| IFN-γ | In remission: 21.10 ± 10.90 * | ||||
| Active disease: 26.13 ± 11.50 * | |||||
| IL-6 | In remission: 28.90 ± 16.30 * | ||||
| Active disease: 27.70 ± 13.50 * | |||||
| IL-23 | In remission: 12.60 ± 10.40 * | ||||
| Active disease: 16.50 ± 8.90 * | |||||
| IL-17A | In remission: 26.6 ± 11.60 * | ||||
| Active disease: 32.0 ± 16.60 * | |||||
| 12 | Mohsen et al., 2018 [67] | CRP | Serum | ng/mL | Boys: 5.17 ± 7.01 * |
| Girls: 4.13 ± 5.75 * | |||||
| 13 | Nalbantoğlu et al., 2021 [68] | IL-4 | Serum | µg/mL | Healthy: 14.28 ± 10.17 * |
| Allergic rhinitis: 32.03 ± 26.45 * | |||||
| IL-13 | Healthy: 9.09 ± 5.13 * | ||||
| Allergic rhinitis: 9.27 ± 5.44 * | |||||
| IFN-γ | Healthy: 5.12 ± 3.79 * | ||||
| Allergic rhinitis: 5.79 ± 4.13 * | |||||
| 14 | Qu et al., 2022 [69] | CRP | Serum | mg/L | P25-P75 Controls: 1.60–2.40 |
| P25-P75 Cases: 4.30–55.30 | |||||
| 15 | Savastano et al., 2015 [70] | MCP1 | Plasma | µg/mL | 27.40 ± 23.50 * |
| IL-6 | P50: 0.77 | ||||
| TNF-α | P50: 1.90 | ||||
| 16 | Šimková et al., 2020 [71] | FGF basic | Plasma | pg/mL | N.R. |
| Eotaxin | N.R. | ||||
| GM-CSF | N.R. | ||||
| IFN-γ | P50 Controls: 19.90 | ||||
| P50 Normal weight PCOS: 13.40 | |||||
| P50 Obesity PCOS: 32.80 | |||||
| IL-1β | N.R. | ||||
| IL-1ra | N.R. | ||||
| IL-2 | P50 Controls: 18.00 | ||||
| P50 Normal weight PCOS: 12.50 | |||||
| P50 Obesity PCOS: 22.20 | |||||
| IL-4 | N.R. | ||||
| IL-5 | N.R. | ||||
| IL-6 | P50 Controls: 23.10 | ||||
| P50 Normal weight PCOS: 56.70 | |||||
| P50 Obesity PCOS: 82.10 | |||||
| IL-7 | N.R. | ||||
| IL-8 | N.R. | ||||
| IL-9 | N.R. | ||||
| IL-10 | N.R. | ||||
| IL-12 (p70) | N.R. | ||||
| IL-13 | P50 Controls: 7.38 | ||||
| P50 Normal weight PCOS: 5.82 | |||||
| P50 Obesity PCOS: 8.85 | |||||
| IL-15 | N.R. | ||||
| IL-17A | N.R. | ||||
| IP-10 | N.R. | ||||
| MCP-1 | N.R. | ||||
| MIP-1α | N.R. | ||||
| MIP-1β | N.R. | ||||
| PDGF-BB | P50 Controls: 216.00 | ||||
| P50 Normal weight PCOS: 328.00 | |||||
| P50 Obesity PCOS: 291.00 | |||||
| RANTES | N.R. | ||||
| TNF-α | N.R. | ||||
| VEGF | P50 Controls: 459.00 | ||||
| P50 Normal weight PCOS: 1028.00 | |||||
| P50 Obesity PCOS: 1120.00 | |||||
| 17 | Song et al., 2017 [72] | CRP | Blood and serum | N.R. | N.R. |
| IL-10 | |||||
| ALT | |||||
| AST | |||||
| γ-GTP | |||||
| 18 | Tsen et al., 2021 [73] | CRP | Plasma | ng/mL | 678.00 ± 918.10 * |
| 19 | Watkins et al., 2015 [43] | CRP | Serum | N.R. | N.R. |
| IL-1β | |||||
| IL-6 | |||||
| IL-10 | |||||
| TNF-α | |||||
| 20 | Yang et al., 2009 [74] | CRP | Serum | mL/dL | Men: 0.08 ± 2.45 * |
| Premenopausal women: 0.06 ± 3.63 * | |||||
| Postmenopausal women: 0.08 ± 3.00 * |
| Article Number | Reference | Exposure-Inflammation Biomarkers | Statistical Test | Magnitude of the Association | p-Value |
|---|---|---|---|---|---|
| 1 | Ashley-Martin et al., 2015 [56] | BPA-IL-33 | Bayesian hierarchical logistic regression models [OR (95% CI)] | 1.00 (0.70–1.30) | 0.050 |
| 2 | Aung et al., 2019 [57] | MeP-CRP | Percent change (95% CI) | 5.56 (−1.49–13.1) | 0.130 |
| EtP-CRP | 3.36 (−4.31–11.6) | 0.400 | |||
| PrP-CRP | 6.40 (−0.25–13.5) | 0.060 | |||
| BuP-CRP | 7.17 (−2.22–17.5) | 0.140 | |||
| BP-3-CRP | 0.79 (−6.44–8.59) | 0.840 | |||
| MeP-IL-1β | −0.15 (−6.37–6.48) | 0.960 | |||
| EtP-IL-1β | −7.70 (−14.1–−0.86) | 0.030 | |||
| PrP-IL-1β | −2.36 (−8.01–3.63) | 0.430 | |||
| BuP-IL-1β | −6.28 (−13.9–2.04) | 0.130 | |||
| BP-3-IL-1β | 1.05 (−5.83–8.43) | 0.770 | |||
| MeP-IL-6 | 6.69 (0.02–13.8) | 0.049 | |||
| EtP-IL-6 | −4.20 (−10.9–2.95) | 0.240 | |||
| PrP-IL-6 | 2.94 (−3.05–9.30) | 0.340 | |||
| BuP-IL-6 | −3.59 (−11.5–5.03) | 0.400 | |||
| BP-3-IL-6 | −1.60 (−8.32–5.61) | 0.650 | |||
| MeP-IL-10 | 0.34 (−4.38–5.29) | 0.890 | |||
| EtP-IL-10 | −3.33 (−8.37–2.00) | 0.220 | |||
| PrP-IL-10 | −1.53 (−5.82–2.97) | 0.500 | |||
| BuP-IL-10 | 0.80 (−5.42–7.44) | 0.800 | |||
| BP-3-IL-10 | −0.34 (−5.47–5.07) | 0.900 | |||
| MeP-TNF-α | 1.42 (−1.85–4.80) | 0.400 | |||
| EtP-TNF-α | −3.14 (−6.61–0.46) | 0.090 | |||
| PrP-TNF-α | −0.05 (−3.05–3.03) | 0.970 | |||
| BuP-TNF-α | −0.42 (−4.66–4.00) | 0.850 | |||
| BP-3-TNF-α | −3.69 (−7.09–−0.17) | 0.040 | |||
| 3 | Choi et al., 2017 [58] | BPA-CRP | Multiple logistic regression [OR (95% CI)] | 2.85 (1.16–6.97) | 0.022 |
| 4 | Ferguson et al., 2016 [59] | BPA-CRP | Percent change (95% CI) | −1.64 (−8.63–5.88) | 0.660 |
| BPA-IL-1β | 3.36 (−3.41–10.60) | 0.340 | |||
| BPA-IL-6 | 8.95 (1.81–16.60) | 0.010 | |||
| BPA-IL-10 | 3.05 (−1.98–8.35) | 0.240 | |||
| BPA-TNF-α | 0.30 (−3.18–3.91) | 0.860 | |||
| 5 | Haq et al., 2020 [60] | BPA Detected-CRP | Two-tailed Student’s t test (mean ± SEM). | Diabetes: 10.63 ± 0.66 | <0.05 |
| BPA Non-detected-CRP | Diabetic: 7.50 ± 1.51 | ||||
| BPA Detected-CRP | Non-diabetic: 5.29 ± 0.59 | ||||
| BPA Non-detected-CRP | Non-diabetic: 2.63 ± 0.34 | ||||
| BPA Detected-IL-6 | Diabetes: 14.84 ± 0.63 | <0.001 | |||
| BPA Non-detected-IL-6 | Diabetic: 10.49 ± 0.76 | ||||
| BPA Detected-IL-6 | Non-diabetic: 4.62 ± 0.37 | ||||
| BPA Non-detected-IL-6 | Non-diabetic: 2.75 ± 0.21 | ||||
| 6 | Huang et al., 2017 [61] | BPA-CRP (plasma) | Multivariate linear regression [β (SE)] | −0.06 (0.10) | 0.570 |
| BPA-CRP (cord serum) | N.R. | N.R. | |||
| BPA-Il-6 (plasma) | −0.82 (0.98) | 0.400 | |||
| BPA-Il-6 (cord serum) | −0.74 (2.30) | 0.750 | |||
| BPA-TNF-α (plasma) | −0.16 (0.32) | 0.620 | |||
| BPA-TNF-α (cord serum) | −0.14 (0.26) | 0.590 | |||
| 7 | Jain et al., 2020 [62] | BPA-TNF-α (control population) | Spearman correlation (Sρ) | −0.07 | 0.940 |
| BPA-TNF-α (diabetes population) | −0.05 | 0.560 | |||
| BPA-IL-6 (control population) | −0.11 | 0.180 | |||
| BPA-IL−6 (diabetes population) | −0.04 | 0.660 | |||
| BPA-IL-1α (control population) | −0.05 | 0.510 | |||
| BPA-IL-1α (diabetes population) | 0.04 | 0.660 | |||
| 8 | Kelley et al., 2019 [63] | BuP-IL-6 | Linear regression. Effect size (standard deviation) | −0.32 (0.11) | 0.097 |
| BPA-MCP-1 | 0.82 (0.21) | 0.019 | |||
| BPA, BPS, BPF, MeP, EtP, PrP, BuP, BP-3-GM-CSF, IFN-γ, MCP-1, MCP-3, MIP-1α, MIP-1β, TNFα, VEGF, IL-1β, IL-6, IL-8, and IL-17A | No significant correlations | N.R. | |||
| 9 | Lang et al., 2008 [64] | BPA-CRP | Multivariate linear regression [β (95% CI)] | 0.09 (0.02–0.15) | 0.020 |
| 10 | Liang et al., 2020 [65] | BPA-IL-1β | Multivariate Linear regression [β (95% CI)] | 0.31 (−0.48–1.10) | 0.439 |
| BPA-IL-2 | N.R. | N.R. | |||
| BPA-IL-4 | N.R. | N.R. | |||
| BPA-IL-6 | 0.15 (−0.14–0.44) | 0.314 | |||
| BPA-IL-8 | 0.06 (−0.31–0.46) | 0.776 | |||
| BPA-IL-10 | 0.03 (−0.18–0.23) | 0.801 | |||
| BPA-IL-12p70 | −0.09 (−0.40–0.22) | 0.573 | |||
| BPA-IL-13 | 0.26 (−0.17–0.69) | 0.225 | |||
| BPA-TNF-α | 0.00 (−0.16–0.16) | 0.996 | |||
| BPA-TGF-β | −0.00 (−0.07–0.07) | 0.981 | |||
| BPA-IFN-γ | 0.18 (0.00–0.36) | 0.045 | |||
| BPS-IL-1β | 0.17 (−0.27–0.61) | 0.433 | |||
| BPS-IL-2 | N.R. | N.R. | |||
| BPS-IL-4 | N.R. | N.R. | |||
| BPS-IL-6 | 0.03 (−0.13–0.19) | 0.724 | |||
| BPS-IL-8 | 0.05 (−0.17–0.27) | 0.644 | |||
| BPS-IL-10 | 0.06 (−0.06–0.17) | 0.328 | |||
| BPS-IL-12p70 | 0.08 (−0.09–0.25) | 0.340 | |||
| BPS-IL-13 | 0.07 (−0.17–0.31) | 0.572 | |||
| BPS-TNF-α | −0.00 (−0.09–0.09) | 0.984 | |||
| BPS-TGF-β | 0.01 (−0.03–0.05) | 0.658 | |||
| BPS-IFN-γ | −0.01 (−0.11–0.09) | 0.890 | |||
| 11 | Linares et al., 2021 [66] | BPA IL-23 | Multivariate linear regression [β (95% CI)] | 1.69 (1.60–1.77) | 0.001 |
| BPA IL-17A | 1.15 (1.00–1.29) | 0.001 | |||
| MeP, EtP, PrP, BuP, BP-1, BP-3-IL-12, IFN-γ, IL-6, IL-23, IL-17A | N.R. | N.R. | N.R. | ||
| 12 | Mohsen et al., 2018 [67] | BPA-CRP | Spearman correlation coefficients (Sρ) | N.R. | N.R. |
| 13 | Nalbantoğlu et al., 2021 [68] | BPA-IL-4 | Multivariate linear regression [β (95% CI)] | 0.31 (3.47–7.40) | 0.000 |
| BPA-IL-13 | N.R. | N.R. | |||
| BPA-IFN-γ | N.R. | N.R. | |||
| 14 | Qu et al., 2022 [69] | MeP-CRP | Multivariate linear regression [β (95% CI)] | 0.15 (0.04–0.28) | <0.05 |
| EtP-CRP | 0.23 (−0.11–0.56) | >0.05 | |||
| PrP-CRP | 0.20 (0.10–0.32) | <0.05 | |||
| BuP-CRP | 0.27 (−0.10–0.80) | >0.05 | |||
| 15 | Savastano et al., 2015 [70] | BPA-MCP-1 | Multivariate linear regression (β) | N.R. | N.R. |
| BPA-IL-6 | 0.24 | 0.037 | |||
| BPA-TNF-α | N.R. | N.R. | |||
| 16 | Šimková et al., 2020 [71] | BPA, BPS, BPF, BPAF, Mep, EtP, PrP, BuP, BzP, total PBs-FGF basic, eotaxin, GM-CSF, IFN-γ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, VEGF | Spearman correlation coefficients (Sρ) | No significant correlations | N.R. |
| 17 | Song et al., 2017 [72] | BPA-CRP | Linear mixed-effect model and a generalized additive mixed model (GAMM) | Positive non-linear association | 0.081 |
| BPA-IL-10 | Negative non-linear association | 0.083 | |||
| BPA-ALT | Positive non-linear association | 0.001 | |||
| BPA-AST | Positive non-linear association | 0.056 | |||
| BPA-γ-GTP | Positive non-linear association | 0.018 | |||
| 18 | Tsen et al., 2021 [73] | BPA-CRP | Multiple logistic regression [OR (95% CI)] | 1.82 (0.58–5.36) | 0.283 |
| 19 | Watkins et al., 2015 [43] | BPA-CRP | Percent change (95% CI) | 5.10 (−7.47–19.40) | 0.440 |
| BPA-IL-1β | 4.65 (−7.91–18.90) | 0.480 | |||
| BPA-IL-6 | 12.50 (−2.50–29.70) | 0.110 | |||
| BPA-IL-10 | −1.20 (−13.40–12.70) | 0.850 | |||
| BPA-TNF-α | 4.85 (−1.70–11.80) | 0.150 | |||
| MeP-CRP | −6.75 (−19.00–7.38) | 0.330 | |||
| MeP-IL-1β | −3.63 (−17.10–12.10) | 0.630 | |||
| MeP-IL-6 | 4.90 (−11.20–23.90) | 0.570 | |||
| MeP-IL-10 | 6.66 (−8.62–24.50) | 0.410 | |||
| MeP-TNF-α | 2.00 (−6.18–10.90) | 0.640 | |||
| PrP-CRP | −13.60 (−25.80–0.50) | 0.060 | |||
| PrP-IL-1β | −1.76 (−16.50–15.60) | 0.830 | |||
| PrP-IL-6 | 3.70 (−13.40–24.20) | 0.690 | |||
| PrP-IL-10 | −0.09 (−15.40–18.00) | 0.990 | |||
| PrP-TNF-α | −0.83 (−9.29–8.42) | 0.850 | |||
| BuP-CRP | −17.50 (−30.30–−2.27) | 0.030 | |||
| BuP-IL-1β | 10.50 (−8.11–32.80) | 0.290 | |||
| BuP-IL-6 | 15.80 (−5.47–41.70) | 0.150 | |||
| BuP-IL-10 | 5.28 (−12.90–27.20) | 0.590 | |||
| BuP-TNF-α | 5.69 (−4.67–17.20) | 0.290 | |||
| BP-3-CRP | −16.30 (−27.50–−3.42) | 0.020 | |||
| BP-3-IL-1β | −0.75 (−15.10–16.10) | 0.920 | |||
| BP-3-IL-6 | −4.81 (−19.90–13.10) | 0.570 | |||
| BP-3-IL-10 | −3.88 (−18.10–12.80) | 0.620 | |||
| BP-3-TNF-α | −2.13 (−10.30–6.77) | 0.620 | |||
| 20 | Yang et al., 2009 [74] | BPA-CRP | Multivariate linear regression (β) | Men: −0.02 | 0.418 |
| BPA-CRP | Premenopausal women: 0.09 | 0.268 | |||
| BPA-CRP | Postmenopausal women: 0.11 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peinado, F.M.; Iribarne-Durán, L.M.; Artacho-Cordón, F. Human Exposure to Bisphenols, Parabens, and Benzophenones, and Its Relationship with the Inflammatory Response: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7325. https://doi.org/10.3390/ijms24087325
Peinado FM, Iribarne-Durán LM, Artacho-Cordón F. Human Exposure to Bisphenols, Parabens, and Benzophenones, and Its Relationship with the Inflammatory Response: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(8):7325. https://doi.org/10.3390/ijms24087325
Chicago/Turabian StylePeinado, Francisco Manuel, Luz María Iribarne-Durán, and Francisco Artacho-Cordón. 2023. "Human Exposure to Bisphenols, Parabens, and Benzophenones, and Its Relationship with the Inflammatory Response: A Systematic Review" International Journal of Molecular Sciences 24, no. 8: 7325. https://doi.org/10.3390/ijms24087325
APA StylePeinado, F. M., Iribarne-Durán, L. M., & Artacho-Cordón, F. (2023). Human Exposure to Bisphenols, Parabens, and Benzophenones, and Its Relationship with the Inflammatory Response: A Systematic Review. International Journal of Molecular Sciences, 24(8), 7325. https://doi.org/10.3390/ijms24087325







