Biodegradable Polymers and Polymer Composites with Antibacterial Properties
Abstract
:1. Introduction
2. Mechanisms of Antibacterial Action Used in the Development of Polymers and Polymer Composites with Antibacterial Properties
3. Natural Polymers with Antibacterial and Bacteriostatic Properties
3.1. Chitosan
3.1.1. Factors Influencing the Antimicrobial Activity of Chitosan
3.1.2. Sources and Concentration of Chitosan
3.1.3. Structural Properties—Molecular Weight and Degree of Deacetylation
3.1.4. Effect of pH
3.1.5. Antibacterial Activity of Chitosan and Its Derivatives
- (a)
- HMw chitosan forms a dense polymeric membrane on the surface of the cell, which blocks the exchange of nutrients, leading to metabolic disorders and, consequently, to the death of microbial cells. The deposition of chitosan on the cell surface can be confirmed by SEM observation. This study was conducted by Helander et al. (2001), who observed a thicker appearance of the cell walls of chitosan-treated E. coli and Salmonella typhimurium.
- (b)
- The presence of cationic charges in chitosan is a key requirement for displaying antimicrobial activity. The cationic groups in chitosan are attracted by electrostatic interactions with negatively charged components present on the surface of the bacteria. Quaternary ammonium groups (R-NH3+) in an acidic environment (pH < 6) compete with divalent metal ions such as Ca2+ and Mg2+ present in the bacterial cell wall for bonding to polyanions, leading to an imbalance of surface potential and mutual repulsion of negatively charged particles, and finally rupture of the cell membrane.
- (c)
- As a result of electrostatic interactions between chitosan and the anionic surface of Gram-positive and Gram-negative bacteria, the cell membrane is disrupted. In Gram-positive bacteria, positively charged chitosan can electrostatically interact with negatively charged teichoic acid in peptidoglycan, destroying the cell membrane and resulting in leakage of intracellular components and simultaneous entry of chitosan into the microbial cells. In Gram-negative bacteria, the high negative charge from LPS can be neutralized by the positive charges from chitosan. As a result of this phenomenon, the OM is torn apart, and the chitosan is absorbed into the cell, resulting in the death of the bacterial cell. Several studies have indicated that chitosan can also bind to the phosphorylated mannosyl side in fungi, leading to disruption of the plasma membrane and leakage of intracellular materials.
- (d)
- LMw chitosan and its hydrolysis products can perforate the microbial cell and interact with DNA. By binding to DNA, chitosan prevents DNA transcription and interrupts the synthesis of proteins and mRNA. Studies by Fei Liu et al. (2001) used a confocal laser scanning microscope to determine the antibacterial activity of chitosan oligomers in E. coli cells. Studies have shown the presence of chitosan oligomers within microbial cells, confirming that the likely cause of the antibacterial effect was the prevention of DNA transcription. Xing et al. (2009) analyzed the effect of the concentration of oleyl chitosan (a derivative of the fatty acid chitosan) nanoparticles (OCNP) on changes in the bacterial genome. Studies have shown that negatively charged phosphate groups in DNA/RNA can react with positively charged amino groups in OCNP, thus inhibiting the activity of microorganisms. In their research, Márquez et al. (2013) analyzed the chemical-genetic interactions of low-molecular-weight chitosan with genes from S. cerevisiae. The disruption of protein synthesis by chitosan was supported by an in vivo β-galactosidase expression assay of -galactosidase, suggesting that this is a primary mode of antifungal action. Furthermore, indicated that chitosan has a minor membrane disruption effect [17,18,94,95,97,98,99].
3.2. Chitosan Derivatives
3.2.1. Chitosan Containing Quaternary Ammonium Groups
3.2.2. Carboxymethyl Chitosan
3.2.3. Sulfonated Chitosan
3.2.4. Phosphorylated Chitosan
3.2.5. Chitosan Containing Alkyl and Aromatic Groups
3.2.6. Chitosan Hydrogels
3.2.7. Chitosan-Metal Composites
3.2.8. Chitosan-Metal Oxide Composites
3.3. Carrageenan
3.3.1. Carrageenan Nanocomposites
3.3.2. Carrageenan Hydrogels
3.4. Antimicrobial Peptides (AMPs)
3.4.1. α-Helical AMPs
3.4.2. Cysteine-Rich AMPs
3.4.3. β-Sheet AMPs
3.4.4. AMPs Rich in Regular Amino Acids
3.4.5. AMPs with Rare Modified Amino Acids
3.4.6. Mechanism of Antimicrobial Peptide Action
| Peptide | Source | Active Agent | Mode of Action | References |
|---|---|---|---|---|
| α-Helical AMPs | ||||
| Cecropins | insects (e.g., Hyalophora cecropia, Musca domestica, bacteria (Helicobacter pylori), tunicates, ascarid nematodes, mammals (Ascaris suum) | strong antimicrobial activity against G+ and G− bacteria | Membrane permeabilization; Carpet model | [233,234,235] |
| Magainins | Xenopus laevis | active against G+ and G− bacteria, MIC for E. coli, S. aureus, C. albicans, P. aeruginosa and Trichomonas vaginalis is in the range of 200–300 μg/106 cfu/mL, strong activity against drug-resistant A. baumannii | Membrane permeabilization; Toroidal pore model | [185,191,236,237] |
| Dermaseptins | skin secretions of frogs (Phyllomedusa sauvagii, Phyllomedusa oreades, Phyllomedusa hypochondrialis) | active against G+ and G− bacteria, inhibition of C. albicans biofilm formation at 50 μg mL−1, MIC against E. coli 16 μM, P. aeruginosa 64 μM, S. aureus 32 μM | Membrane permeabilization; Carpet model | [238,239,240] |
| Bombinins | skin secretion of frogs (e.g., Bombina variegata, Bombina orientalis, Bombina maxima) | active against G+ (Bacillus megaterium, S. aureus) and G− (E. coli, Yersinia pseudotuberculosis, Pseudomonas aeruginosa), against Candida albicans; no appreciable hemolytic capacity | Formation of ion channels or pores | [241,242,243,244] |
| Aurein | granular dorsal glands of the Australian frog (Litoria aurea, L. raniformis) | more activity against G+ than G−, MIC for S. aureus for aurein 1.2 = 25 μg/mL, for aurein M.3 = 3.12 μg/mL, MIC for E. coli for aurein 1.2 = 200 μg/mL, for aurein M.3 = 6.25 μg/mL | Membrane permeabilization; aurein 2.2 barrel-stave model, aurein 1.2 carpet model | [186,196,245,246] |
| LL-37 | variety of cells (neutrophils, leukocytes and epithelial cells, Myelocytes, metamyelocytes), tissues, body fluids, and professional phagocytes | strong active against G+ and G− bacteria e.g., against L. monocytogenes MIC = 1.5 μgmL−1, S. aureus MIC = 3.6 μgmL−1, Bacillus subtilis MIC = 2.7 μgmL−1, E. coli MIC = 0.1 μgmL−1, Salmonella typhimurium MIC = 0.4 μgmL−1 | Membrane permeabilization; barrel-stave model and carpet model; Immune modulation | [188,200,247,248,249] |
| Cysteine rich AMPs | ||||
| α-defensins (HNP1—HNP4) | neutrophils, late promyelocytes, bone marrow, some surface epithelial cells, intestinal epithelial cells, female reproductive tract | active against G+ and G− bacteria, e.g., against L. monocytogenes MIC = 39.7 μgmL−1, S. aureus MIC = 2.2 μgmL−1, Bacillus subtilis MIC = 6.4 μgmL−1, E. coli MIC = 1.8 μgmL−1, Salmonella typhimurium MIC = 0.4 μgmL−1 | Mainly membrane permeabilization and disrupt bacterial membranes | [188,200,250] |
| β-defensins (HβD-1—HβD-4) | human (skin, respiratory tract, gastrointestinal tracts, human genomic sequences) | HβD-1—HβD-3 showed antibacterial activities against E. coli and S. aureus in a dose-dependent manner, HβD-3 showed a broad spectrum of antimicrobial activities against S. aureus, E. faecium, P. aeruginosa, K. pneumonia, S. pneumoniae, B. cepacia. HβD-4 showed antibacterial activity against on F.nucleatum, P. gingivalis, P. aeruginosa, E. faecalis | Immune modulation | [247,251,252,253,254] |
| θ-defensins | neutrophils and monocytes of the rhesus monkey | antimicrobial activities against Staphylococcus aureus, Candida albicans, Cryptococcus neoformans, E. coli | Membrane permeabilization | [203,204,255] |
| Gomesin | hemocytes of the spider Acanthoscurria gomesiana | antimicrobial activities against E. coli, P. aeruginosa, S. aureus, K. pneumoniae, B. megaterium | Membrane permeabilization; carpet/detergent mechanisms | [205,256,257] |
| Peneidines | shrimp (Penaeus vannamei, Litopenaeus vannamei) | better antimicrobial activity against G− than G+ bacteria | Binding to superficial membrane and DNA, thereby destroying the bacterial structure and/or interfering with the bacterial proliferation | [258,259] |
| Lysozyme | Insects, plants, chicken egg white, body fluids, and tissues in living organisms | most effective against G+ bacteria | Hydrolysis in microbial cell walls, which results in the rupture of the β(1,4) linkages in their peptidoglycan | [260,261,262] |
| β-sheet AMPs | ||||
| Tachyplesins | hemocytes of horseshoe crabs (Tachypleus tridentatus) | antimicrobial activity against G+ bacteria (MIC = 0.2–0.9 μM) G− bacteria (MIC = 0.3–1 μM) | Membrane permeabilization | [208,263,264] |
| Polyphemusin | hemocyte debris of horseshoe crabs (Limulus polyphemus) | antimicrobial activity against G+ bacteria: S. aureus MIC = 2 μg/mL, S. epidermidis MIC = 1 μg/mL, E. faecalis MIC = 1 μg/mL G-bacteria: E. coli MIC = 0.125–1 μg/mL, S. typhimurium MIC = 0.25–1 μg/mL, P. aeruginosa MIC = 0.25–1 μg/mL | Translocates into cells | [265,266] |
| Thanatin | insect (Podisus maculiventris) | activity against G− bacteria: MIC < 1.2 μM for E. coli, S. typhimurium, K. pneumoniae, E. cloacae; against G+ bacteria: MIC < 5 μM for A. viridans, M. luteus, B. megaterium, B. megate; No activity against S. aureus | Membrane permeabilization | [209,267] |
| Lactoferricin B | bovine, human | antimicrobial activity against G+ bacteria (S. mutans, S. epidermidis) and G− bacteria (E. coli, S. typhimurium, P. aeruginosa, B. cepacia, B. cenocepacia) | Prevents biofilm formation | [268,269] |
| AMPs rich in regular amino acids | ||||
| Histatins | salivary glands | Histatin 5: S. aureus MIC 12.5 μgmL−1, P. aeruginosa MIC 3.1 μgmL−1; MIC > 100 μgmL−1 for B. cepacia, A. xylosoxidans, S. maltophilia | Disruption of the plasma membrane | [188,200] |
| Indolicidin | cytoplasmic granules of bovine neutrophils | S. aureus MIC 2–30 μg/mL, S. hemolyticus MIC 2 μg/mL, S. epidermidis MIC 4–20 μg/mL, B. cereus MIC 12.5 μg/mL L. monocytogenes MIC 3–60 μg/mL, E. coli MIC 5–30 μg/mL, S. enterica MIC 100 μg/mL, P. aeruginosa MIC 100 μg/mL, | Membrane permeabilization; carpet model | [215,217,270,271] |
| Tritrpticin | Human, porcine | MIC 20–30 μg/mL for E. coli MIC 32 μg/mL for S. typhimurim and P. aeruginosa; MIC 8 μg/mL for B. subtilis and S. epidermidis, MIC 10–20 μg/mL for S. aureus | Inhibition of intracellular synthesis of protein, DNA, or RNA | [217,272] |
| Crotalicidin | pit viper | antibacterial activity particularly against G−; P. aeruginosa MIC 0.24–3.8 μM, K. pneumoniae MIC 1.9 μM, E. coli MIC 0.06–3.8 μM, A. baumannii MIC 3.8 μM | Membrane permeabilization | [218,273] |
| Bactenecins (Bac-5, Bac-7) | bovine neutrophils | Bac-5 is active against G− bacteria; Bac-7 has antibacterial activity against E. coli MIC 1–2 μM, S. enterica MIC 1 μM, S. marcescens MIC 1 μM, S. aureus MIC > 128 μM | Older research suggests a permeabilizing mode of action; a recent study showed no strong effect on bacterial membrane integrity | [220,221,274,275] |
| PR-39 | porcine neutrophils | E. coli MIC 1–4 μg/mL, P. aeruginosa MIC > 32 μg/mL, S. typhimurium MIC 4 μg/mL, S. enterica MIC 0.5 μM, A. pleuropneumoniae MIC 4–8 μM, S. aureus MIC > 32 μg/mL, B. cereus MIC > 32 μg/mL | Immune modulation; translocation across the membrane | [276,277,278,279] |
| AMPs with rare modified amino acids | ||||
| Nisin | Lactococcus lactis | highly active against G+ bacteria: L. monocytogenes, S. aureus, B. cereus, L. plantarum, M. luteus, and M. flavus | Membrane permeabilization | [222,280,281,282] |
| Leucocin A | Leuconostoc pseudomesenteroides | active against G+ bacteria, L. monocytogenes MIC 11.7–62.5 μM | Pore formation and ion disruption of the target cell | [283,284] |
| Protein (polypeptide) | ||||
| Sericin | wild silkworms (Antheraea pernyi, Samia cynthia ricini), domestic silkworms (Bombyx mori) | antimicrobial activity against G+ and G- bacteria, ZOI for E. coli and S. aureus 22.6 mm 22.16 mm, highly active against G+ bacteria: B. subtilis, S. aureus, S. epidermidis | no clarity as to the mode of action | [227,228] |
| Fibroin | domestic silkworms (Bombyx mori) | antibacterial effect of silk fibroin-based biomaterials | the mechanism has not yet been fully elucidated | [232] |
3.4.7. Antimicrobial Peptides in Clinical Trials
4. Synthetic Biodegradable Polymers with Antibacterial Properties
4.1. Biomimetic Polymers
4.2. Polycarbonates and Carbonates Copolymers
4.3. Antibacterial Biodegradable Polyesters
4.4. Polyamines and Polyesteramines
5. Biodegradable Polymer Composites with Antibacterial Properties
6. Application of Biodegradable Polymers and Antibacterial Polymer Composites in the Food Industry and Agricultural Technology
6.1. Polysaccharides Composites
6.1.1. Starch
6.1.2. Chitosan
6.1.3. Cellulose
6.1.4. Alginates and Carrageenans
6.2. PLA, PGA, PCL, PBS Composites
6.3. PHA Composites
6.4. PVA Composites
6.5. Proteins
6.6. Lipids and Waxes
7. The Use of Biodegradable Antibacterial Polymers in Biomedical Applications
7.1. Biodegradable and Antibacterial Wound-Dressings
7.2. Biodegradable Drug Release Systems with Antibacterial Properties
8. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, Characterization and Antibacterial Activity of Oxidized κ-Carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.P.C.; Iqbal, Z.; Avis, T.J. Combating Antimicrobial Resistance in Foodborne Microorganisms. J. Food Prot. 2016, 79, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 18 February 2023).
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Saravanan, R.; Li, C.M.; Leong, S.S.J. Antimicrobial Macromolecules: Synthesis Methods and Future Applications. RSC Adv. 2012, 2, 4031–4044. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.S.M.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad Spectrum Antibacterial and Antifungal Polymeric Paint Materials: Synthesis, Structure–Activity Relationship, and Membrane-Active Mode of Action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic Antibiotic Resistance: Mechanisms, Origins, Challenges and Solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs: Current Eye Research: Vol 30, No 7. Available online: https://www.tandfonline.com/doi/full/10.1080/02713680590968637 (accessed on 12 February 2023).
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Zhou, C.-H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Rychter, P.; Rogacz, D.; Lewicka, K.; Lacik, I. Poly(Methylene-Co-Cyanoguanidine) as an Eco-Friendly Nitrogen Fertilizer with Prolonged Activity. J. Polym. Environ. 2019, 27, 1317–1332. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.L.; Tan, J.P.K.; Leong, J.; Voo, Z.X.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial Polycarbonates: Investigating the Impact of Nitrogen-Containing Heterocycles as Quaternizing Agents. Macromolecules 2014, 47, 1285–1291. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Su, Y.; Chen, C.; Jia, G.; Wang, H.; Wu, J.C.G.; Lin, J. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and Characterization of Chitosan Derivatives with Dual-Antibacterial Functional Groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Lagaron, J.M.; Ocio, M.J. Optimization of the Biocide Properties of Chitosan for Its Application in the Design of Active Films of Interest in the Food Area. Food Hydrocoll. 2009, 23, 913–921. [Google Scholar] [CrossRef]
- Engler, A.C.; Tan, J.P.K.; Ong, Z.Y.; Coady, D.J.; Ng, V.W.L.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial Polycarbonates: Investigating the Impact of Balancing Charge and Hydrophobicity Using a Same-Centered Polymer Approach. Biomacromolecules 2013, 14, 4331–4339. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; De Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Liu, R.; Lemke, J.J.; Hayouka, Z.; Welch, R.A.; Weisblum, B.; Masters, K.S.; Gellman, S.H. Effects of Cyclic vs. Acyclic Hydrophobic Subunits on the Chemical Structure and Biological Properties of Nylon-3 Copolymers. ACS Macro Lett. 2013, 2, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.P.; Yao, K.; Wilbon, P.A.; Zhang, W.; Ren, L.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; et al. Robust Antimicrobial Compounds and Polymers Derived from Natural Resin Acids. Chem. Commun. 2011, 48, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, Y.; Riduan, S.N.; Gao, S.; Yang, Y.; Fan, W.; Zhang, Y. Main-Chain Imidazolium Oligomer Material as a Selective Biomimetic Antimicrobial Agent. Biomaterials 2012, 33, 8625–8631. [Google Scholar] [CrossRef]
- Pascual, A.; Tan, J.P.K.; Yuen, A.; Chan, J.M.W.; Coady, D.J.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Broad-Spectrum Antimicrobial Polycarbonate Hydrogels with Fast Degradability. Biomacromolecules 2015, 16, 1169–1178. [Google Scholar] [CrossRef]
- Guo, J.; Qin, J.; Ren, Y.; Wang, B.; Cui, H.; Ding, Y.; Mao, H.; Yan, F. Antibacterial Activity of Cationic Polymers: Side-Chain or Main-Chain Type? Polym. Chem. 2018, 9, 4611–4616. [Google Scholar] [CrossRef]
- Pham, P.; Oliver, S.; Boyer, C. Design of Antimicrobial Polymers. Macromol. Chem. Phys. 2022, 224, 2200226. [Google Scholar] [CrossRef]
- Irzhak, V.I. A Structural Characteristic of Hyperbranched Polymers. Polym. Sci. Ser. B 2009, 51, 143–148. [Google Scholar] [CrossRef]
- Wei, T.; Zhan, W.; Cao, L.; Hu, C.; Qu, Y.; Yu, Q.; Chen, H. Multifunctional and Regenerable Antibacterial Surfaces Fabricated by a Universal Strategy. ACS Appl. Mater. Interfaces 2016, 8, 30048–30057. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A Polycationic Antimicrobial and Biocompatible Hydrogel with Microbe Membrane Suctioning Ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef]
- Malin, J.J.; de Leeuw, E. Therapeutic Compounds Targeting Lipid II for Antibacterial Purposes. Infect. Drug Resist. 2019, 12, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, S.; Shen, J.; Zhu, K. Nonribosomal Antibacterial Peptides That Target Multidrug-Resistant Bacteria. Nat. Prod. Rep. 2019, 36, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Plectasin, a Fungal Defensin, Targets the Bacterial Cell Wall Precursor Lipid II|Science. Available online: https://www.science.org/doi/10.1126/science.1185723 (accessed on 12 February 2023).
- Scherer, K.M.; Spille, J.-H.; Sahl, H.-G.; Grein, F.; Kubitscheck, U. The Lantibiotic Nisin Induces Lipid II Aggregation, Causing Membrane Instability and Vesicle Budding. Biophys. J. 2015, 108, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Chindera, K.; Mahato, M.; Kumar Sharma, A.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The Antimicrobial Polymer PHMB Enters Cells and Selectively Condenses Bacterial Chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef]
- Chin, W.; Zhong, G.; Pu, Q.; Yang, C.; Lou, W.; De Sessions, P.F.; Periaswamy, B.; Lee, A.; Liang, Z.C.; Ding, X.; et al. A Macromolecular Approach to Eradicate Multidrug Resistant Bacterial Infections While Mitigating Drug Resistance Onset. Nat. Commun. 2018, 9, 917. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-Oxazoline)-Based Functional Peptide Mimics: Eradicating MRSA Infections and Persisters While Alleviating Antimicrobial Resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef]
- Structures of Proline-Rich Peptides Bound to the Ribosome Reveal a Common Mechanism of Protein Synthesis Inhibition|Nucleic Acids Research|Oxford Academic. Available online: https://academic.oup.com/nar/article/44/5/2439/2465042 (accessed on 12 February 2023).
- Fernandes, M.M.; Carvalho, E.O.; Lanceros-Mendez, S. Electroactive Smart Materials: Novel Tools for Tailoring Bacteria Behavior and Fight Antimicrobial Resistance. Front. Bioeng. Biotechnol. 2019, 7, 277. [Google Scholar] [CrossRef]
- Ando, M.; Tamakura, D.; Inoue, T.; Takumi, K.; Yamanaga, T.; Todo, R.; Hosoya, K.; Onishi, O. Electric Antibacterial Effect of Piezoelectric Poly(Lactic Acid) Fabric. Jpn. J. Appl. Phys. 2019, 58, SLLD09. [Google Scholar] [CrossRef]
- Carvalho, E.O.; Fernandes, M.M.; Padrao, J.; Nicolau, A.; Marqués-Marchán, J.; Asenjo, A.; Gama, F.M.; Ribeiro, C.; Lanceros-Mendez, S. Tailoring Bacteria Response by Piezoelectric Stimulation. ACS Appl. Mater. Interfaces 2019, 11, 27297–27305. [Google Scholar] [CrossRef]
- Tan, G.; Wang, S.; Zhu, Y.; Zhou, L.; Yu, P.; Wang, X.; He, T.; Chen, J.; Mao, C.; Ning, C. Surface-Selective Preferential Production of Reactive Oxygen Species on Piezoelectric Ceramics for Bacterial Killing. ACS Appl. Mater. Interfaces 2016, 8, 24306–24309. [Google Scholar] [CrossRef] [PubMed]
- Gorodzha, S.N.; Muslimov, A.R.; Syromotina, D.S.; Timin, A.S.; Tcvetkov, N.Y.; Lepik, K.V.; Petrova, A.V.; Surmeneva, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. A Comparison Study between Electrospun Polycaprolactone and Piezoelectric Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Scaffolds for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 160, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Guerin, S.; Tofail, S.A.M.; Thompson, D. Organic Piezoelectric Materials: Milestones and Potential. NPG Asia Mater. 2019, 11, 10. [Google Scholar] [CrossRef]
- Ando, M.; Takeshima, S.; Ishiura, Y.; Ando, K.; Onishi, O. Piezoelectric Antibacterial Fabric Comprised of Poly(l-Lactic Acid) Yarn. Jpn. J. Appl. Phys. 2017, 56, 10PG01. [Google Scholar] [CrossRef]
- Gazvoda, L.; Nanut, M.P.; Spreitzer, M.; Vukomanović, M. Antimicrobial Activity of Piezoelectric Polymer: Piezoelectricity as the Reason for Damaging Bacterial Membrane. Biomater. Sci. 2022, 10, 4933–4948. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial Polymers: Mechanism of Action, Factors of Activity, and Applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Mozammil Hasnain, S.M.; Hasnain, M.S.; Nayak, A.K. Natural Polysaccharides. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–14. ISBN 978-0-12-817055-7. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. (Eds.) Handbook of Polymers for Pharmaceutical Technologies; John Wiley & Sons: Hoboken, NJ, USA; Scrivener Publishing LLC: Hoboken, NJ, USA; Salem, MA, USA, 2015; ISBN 978-1-119-04135-1. [Google Scholar]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of Ultrasonic Extraction of Polysaccharides from Dried Longan Pulp Using Response Surface Methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Van Griensven, L.J.L.D.; Vrvic, M.; Jakovljevic, D. Polysaccharides of Higher Fungi: Biological Role, Structure, and Antioxidative Activity. Hem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable Polysaccharides for Controlled Drug Delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, C.; Cao, X.; Feng, B.; Li, X. Intestinal Population in Host with Metabolic Syndrome during Administration of Chitosan and Its Derivatives. Molecules 2020, 25, 5857. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural Differences between Chitin and Chitosan Extracted from Three Different Marine Sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Li, P.; Wang, K.; Fang, L.; Ren, F.; Lu, G.; Lu, X. The Interaction of Chitosan and BMP-2 Tuned by Deacetylation Degree and PH Value. J. Biomed. Mater. Res. A 2019, 107, 769–779. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yu, H.; Abdin, Z.u.; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent Progress on Synthesis, Property and Application of Modified Chitosan: An Overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, e1708172. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Qin, Y.; Li, P. Molecular Weight and PH Effects of Aminoethyl Modified Chitosan on Antibacterial Activity in Vitro. Int. J. Biol. Macromol. 2012, 50, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of Acetylation Degree and Molecular Weight of Homogeneous Chitosans on Antibacterial and Antifungal Activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth Inhibitory Effect on Bacteria of Chitosan Membranes Regulated with Deacetylation Degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-Solubility of Chitosan and Its Antimicrobial Activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. PH-Responsive Chitosan-Based Film Incorporated with Alizarin for Intelligent Packaging Applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative Evaluation of Antimicrobial Activity of Oligochitosans against Klebsiella Pneumoniae. Russ. J. Bioorganic Chem. 2015, 41, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Attjioui, M.; Leitão, A.; Moerschbacher, B.M.; Cavalheiro, É.T.G. Characterization, Solubility and Biological Activity of Amphihilic Biopolymeric Schiff Bases Synthesized Using Chitosans. Carbohydr. Polym. 2019, 220, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Aslan, S.; Ulusoy, S.; Oral, A. Natural-Based Polymers for Antibacterial Treatment of Absorbent Materials. J. Appl. Polym. Sci. 2020, 137, 48302. [Google Scholar] [CrossRef]
- Ashry, N.M.; El Bahgy, H.E.K.; Mohamed, A.; Alsubhi, N.H.; Alrefaei, G.I.; Binothman, N.; Alharbi, M.; Selim, S.; Almuhayawi, M.S.; Alharbi, M.T.; et al. Evaluation of Graphene Oxide, Chitosan and Their Complex as Antibacterial Agents and Anticancer Apoptotic Effect on HeLa Cell Line. Front. Microbiol. 2022, 13, 922324. [Google Scholar] [CrossRef] [PubMed]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, Antioxidant, and Antimicrobial Properties of Chitooligosaccharides Produced Using Three Different Enzyme Treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Hmed, A.A.; Sofy, A.R.; Sharaf, A.E.-M.M.A.; El-Dougdoug, K.A. Effectiveness of Chitosan as Naturally-Derived Antimicrobial to Fulfill the Needs of Today’s Consumers Looking for Food without Hazards of Chemical Preservatives. J. Microbiol. Res. 2017, 7, 55–67. [Google Scholar]
- Abdeltwab, W.; Fathy, Y.; Azab, W.; Eldeghedy, M.; Ebid, W. Antimicrobial Effect of Chitosan and Nano-Chitosan against Some Pathogens and Spoilage Microorganisms. J. Adv. Lab. Res. Biol. 2019, 10, 8–15. [Google Scholar]
- Rahimi, M.; Ahmadi, R.; Samadi Kafil, H.; Shafiei-Irannejad, V. A Novel Bioactive Quaternized Chitosan and Its Silver-Containing Nanocomposites as a Potent Antimicrobial Wound Dressing: Structural and Biological Properties. Mater. Sci. Eng. C 2019, 101, 360–369. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial Effect of a Novel Chitosan Derivative and Its Synergistic Effect with Antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef]
- Abdel-Monem, R.A.; Khalil, A.M.; Darwesh, O.M.; Hashim, A.I.; Rabie, S.T. Antibacterial Properties of Carboxymethyl Chitosan Schiff-Base Nanocomposites Loaded with Silver Nanoparticles. J. Macromol. Sci. Part A 2020, 57, 145–155. [Google Scholar] [CrossRef]
- de Britto, D.; Celi Goy, R.; Campana Filho, S.P.; Assis, O.B.G. Quaternary Salts of Chitosan: History, Antimicrobial Features, and Prospects. Int. J. Carbohydr. Chem. 2011, 2011, e312539. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, Characterization, and Antimicrobial Activities of Sulfonated Chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Kathiresan, K.; Nayak, L. Preparation, Characterization and Antibacterial Activity of Chitosan and Phosphorylated Chitosan from Cuttlebone of Sepia Kobiensis (Hoyle, 1885). Biotechnol. Rep. 2015, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Haj, N.Q.; Mohammed, M.O.; Mohammood, L.E. Synthesis and Biological Evaluation of Three New Chitosan Schiff Base Derivatives. ACS Omega 2020, 5, 13948–13954. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial Activity of Chitosan Tripolyphosphate Nanoparticles Loaded with Various Metal Ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Khalid, S.; Piggot, T.J.; Samsudin, F. Atomistic and Coarse Grain Simulations of the Cell Envelope of Gram-Negative Bacteria: What Have We Learned? Acc. Chem. Res. 2019, 52, 180–188. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-Negative Bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J. Oleoyl-Chitosan Nanoparticles Inhibits Escherichia Coli and Staphylococcus aureus by Damaging the Cell Membrane and Putative Binding to Extracellular or Intracellular Targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Galván Márquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of Protein Synthesis as Antifungal Mode of Action by Chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan Kills Escherichia Coli through Damage to Be of Cell Membrane Mechanism. Carbohydr. Polym. 2010, 3, 493–499. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials Based on N,N,N-Trimethyl Chitosan Fibers in Wound Dressing Applications. Int. J. Biol. Macromol. 2016, 89, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Elella, M.H.A.; Sabaa, M.W. Synthesis, Characterization and Applications of N-Quaternized Chitosan/Poly(Vinyl Alcohol) Hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, A.M.; Fouda, M.M.G.; Knittel, D.; Schollmeyer, E. Antibacterial Activity of Cationically Modified Cotton Fabric with Carboxymethyl Chitosan. J. Appl. Polym. Sci. 2008, 110, 1289–1296. [Google Scholar] [CrossRef]
- Yin, M.; Lin, X.; Ren, T.; Li, Z.; Ren, X.; Huang, T.-S. Cytocompatible Quaternized Carboxymethyl Chitosan/Poly(Vinyl Alcohol) Blend Film Loaded Copper for Antibacterial Application. Int. J. Biol. Macromol. 2018, 120, 992–998. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Zhang, Y.; Yu, Z.; Qi, C. Quaternized Carboxymethyl Chitosan-Based Silver Nanoparticles Hybrid: Microwave-Assisted Synthesis, Characterization and Antibacterial Activity. Nanomaterials 2016, 6, 118. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in Vitro Antioxidant Activities of 6-Amino-6-Deoxychitosan and Its Sulfonated Derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Dimassi, S.; Tabary, N.; Leclercq, L.; Degoutin, S.; Chai, F.; Pierlot, C.; Cazaux, F.; Ung, A.; Staelens, J.-N.; et al. Synthesis and Characterization of Polyampholytic Aryl-Sulfonated Chitosans and Their in Vitro Anticoagulant Activity. Carbohydr. Polym. 2018, 196, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of Cellulose and Chitin/Chitosan Derivatives and Composites as Antibacterial Materials: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wang, H.; Wang, Y.; Chen, G.; Yuan, L.; Chen, H. Recyclable Antibacterial Material: Silicon Grafted with 3,6-O-Sulfated Chitosan and Specifically Bound by Lysozyme. J. Mater. Chem. B 2014, 2, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Yang, L.; Zhou, F. Synthesis of Sulfonated Chitosan and Its Antibiofilm Formation Activity against E. coli and S. aureus. Int. J. Biol. Macromol. 2019, 129, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Suchyta, D.J.; Soto, R.J.; Schoenfisch, M.H. Selective Monophosphorylation of Chitosan via Phosphorus Oxychloride. Polym. Chem. 2017, 8, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, Characterization and Anticoagulant Activity of Chitosan Derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef]
- Soliman, E.A.; El-Kousy, S.M.; Abd-Elbary, H.M.; Abou-zeid, A.R. Low Molecular Weight Chitosan-Based Schiff Bases: Synthesis, Characterization and Antibacterial Activity. Am. J. Food Technol. 2012, 8, 17–30. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, Physicochemical Characterization and Antimicrobial Activities of Novel Two Phenolic Chitosan Schiff Base Derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Baset, W.M.A.; Hassan, M.E.; El-Shafeey, M.E.A.; Eldin, M.S.M. Synthesis, Characterization and Antimicrobial Evaluation of Two Aromatic Chitosan Schiff Base Derivatives. Process Biochem. 2016, 51, 1721–1730. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Omer, A.M.; Baset, W.M.A.; Abbas, E.; Mohy-Eldin, M.S. Therapeutic Potential of Two Formulated Novel Chitosan Derivatives with Prominent Antimicrobial Activities against Virulent Microorganisms and Safe Profiles toward Fibroblast Cells. Int. J. Pharm. 2023, 634, 122649. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; ElTantawy, M.M.; Brussevich, A.; Nebalueva, A.; Novikov, A.; Moskalenko, I.V.; Abu-Serie, M.M.; Hassan, M.A.; Ulasevich, S.; Skorb, E.V. Functionalization of Chitosan with Poly Aromatic Hydroxyl Molecules for Improving Its Antibacterial and Antioxidant Properties: Practical and Theoretical Studies. Int. J. Biol. Macromol. 2023, 234, 123687. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Moaddel, H. Structural Modification of Chitosan Biopolymer as a Novel Polyelectrolyte Membrane for Green Power Generation. Polym. Adv. Technol. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of Hydrogels Based on Chitosan/Alginate for the Delivery of Lysozyme and Their Antibacterial Activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial Poly (Ethylene Glycol) Diacrylate/Chitosan Hydrogels Enhance Mechanical Adhesiveness and Promote Skin Regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Hu, H.; Ye, B.; Lv, Y.; Zhang, Q. Preparing Antibacterial and In-Situ Formable Double Crosslinking Chitosan/Hyaluronan Composite Hydrogels. Mater. Lett. 2019, 254, 17–20. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Radetić, T.; Vukašinović-Sekulić, M.; Kojić, V.; Živković, L.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-Based Hydrogel Wound Dressings with Electrochemically Incorporated Silver Nanoparticles—In Vitro Study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Jiang, M.; Li, S.; Ming, P.; Guo, Y.; Yuan, L.; Jiang, X.; Liu, Y.; Chen, J.; Xia, D.; He, Y.; et al. Rational Design of Porous Structure-Based Sodium Alginate/Chitosan Sponges Loaded with Green Synthesized Hybrid Antibacterial Agents for Infected Wound Healing. Int. J. Biol. Macromol. 2023, 237, 123944. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; Kim, D.; Seo, J. Microwave Assisted Antibacterial Chitosan-Silver Nanocomposite Films. Int. J. Biol. Macromol. 2016, 84, 281–288. [Google Scholar] [CrossRef]
- An, J.; Ji, Z.; Wang, D.; Luo, Q.; Li, X. Preparation and Characterization of Uniform-Sized Chitosan/Silver Microspheres with Antibacterial Activities. Mater. Sci. Eng. C 2014, 36, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lu, F.; Ran, L.; Yu, K.; Xiao, Y.; Li, Z.; Dai, F.; Wu, D.; Lan, G. Imidazole-Molecule-Capped Chitosan–Gold Nanocomposites with Enhanced Antimicrobial Activity for Treating Biofilm-Related Infections. J. Colloid Interface Sci. 2018, 531, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Mehmood, S.; Shafiq, M.; Yasin, T.; Akhter, Z.; Ahmad, S. Structural and Antimicrobial Properties of Irradiated Chitosan and Its Complexes with Zinc. Radiat. Phys. Chem. 2013, 91, 138–142. [Google Scholar] [CrossRef]
- Mallick, S.; Sharma, S.; Banerjee, M.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Iodine-Stabilized Cu Nanoparticle Chitosan Composite for Antibacterial Applications. ACS Appl. Mater. Interfaces 2012, 4, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sanpui, P.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Synthesis, Characterization and Enhanced Bactericidal Action of a Chitosan Supported Core–Shell Copper–Silver Nanoparticle Composite. RSC Adv. 2015, 5, 12268–12276. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef]
- Khan, S.B.; Ali, F.; Kamal, T.; Anwar, Y.; Asiri, A.M.; Seo, J. CuO Embedded Chitosan Spheres as Antibacterial Adsorbent for Dyes. Int. J. Biol. Macromol. 2016, 88, 113–119. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO Nanoparticles with Chitosan as Stabilizing Agent and Their Antibacterial Properties against Gram-Positive and Gram-Negative Bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and Chitosan–ZnO-Based Complex Nanoparticles: Formation, Characterization, and Antibacterial Activity. J. Mater. Chem. B 2013, 1, 1968–1976. [Google Scholar] [CrossRef]
- Tran, C.D.; Makuvaza, J.; Munson, E.; Bennett, B. Biocompatible Copper Oxide Nanoparticle Composites from Cellulose and Chitosan: Facile Synthesis, Unique Structure, and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2017, 9, 42503–42515. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO Nanofluids—A Potential Antibacterial Agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Souza Cruz, R.; Alves Medeiros, E.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Wrońska, N.; Katir, N.; Miłowska, K.; Hammi, N.; Nowak, M.; Kędzierska, M.; Anouar, A.; Zawadzka, K.; Bryszewska, M.; El Kadib, A.; et al. Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria. Int. J. Mol. Sci. 2021, 22, 5839. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb Rahman, P.; Abdul Mujeeb, V.M.; Muraleedharan, K.; Thomas, S.K. Chitosan/Nano ZnO Composite Films: Enhanced Mechanical, Antimicrobial and Dielectric Properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Ali, M.; Mujahid, M. A Critical Review of Algal Biomass: A Versatile Platform of Bio-Based Polyesters from Renewable Resources. Int. J. Biol. Macromol. 2016, 86, 937–949. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Varela, P.; Fiszman, S.M. Hydrocolloids in Fried Foods. A Review. Food Hydrocoll. 2011, 25, 1801–1812. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent Advances in Carrageenan-Based Films for Food Packaging Applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and In Vivo. Antitumor Activity of κ-Carrageenan Oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and Antitumor Activity of κ-Carrageenan Oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Liu, S. Enhanced Immunostimulatory and Antitumor Activity of Different Derivatives of κ-Carrageenan Oligosaccharides from Kappaphycus Striatum. J. Appl. Phycol. 2011, 23, 59–65. [Google Scholar] [CrossRef]
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Immunomodulatory Properties of Photopolymerizable Fucoidan and Carrageenans. Carbohydr. Polym. 2020, 230, 115691. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of Sulfate Group in Red Seaweed Polysaccharides on Anticoagulant Activity and Cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Ma, Z.; Rao, Z.; Zheng, X.; Tang, K. Soluble Soybean Polysaccharide/Carrageenan Antibacterial Nanocomposite Films Containing Green Synthesized Silver Nanoparticles. ACS Appl. Polym. Mater. 2022, 4, 5608–5618. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Wang, L.-F. Preparation and Characterization of Carrageenan-Based Nanocomposite Films Reinforced with Clay Mineral and Silver Nanoparticles. Appl. Clay Sci. 2014, 97–98, 174–181. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-Mediated Synthesis of Silver Nanoparticle and Its Use for the Preparation of Carrageenan-Based Antibacterial Films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.-W. Preparation of Carrageenan-Based Nanocomposite Films Incorporated with Functionalized Halloysite Using AgNP and Sodium Dodecyl Sulfate. Food Hydrocoll. 2020, 106, 105934. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bang, Y.-J.; Yoon, K.S.; Priyadarshi, R.; Rhim, J.-W. Pine Needle (Pinus densiflora) Extract-Mediated Synthesis of Silver Nanoparticles and the Preparation of Carrageenan-Based Antimicrobial Packaging Films. J. Nanomater. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Gün Gök, Z.; Karayel, M.; Yiğitoğlu, M. Synthesis of Carrageenan Coated Silver Nanoparticles by an Easy Green Method and Their Characterization and Antimicrobial Activities. Res. Chem. Intermed. 2021, 47, 1843–1864. [Google Scholar] [CrossRef]
- Pramesti, E.S.; Febriani, N.A.; ‘Aisy, R.N.; Salsabila, S.; Rachmawati, V.P.N.; Susilowati, E. Synthesis of Silver/K-Caragenan Nanocomposites Assisted with Microwave Iradiation as a Potential Antibacterial Material. J. Phys. Conf. Ser. 2022, 2190, 012004. [Google Scholar] [CrossRef]
- Bahadoran, A.; Najafizadeh, M.; Liu, Q.; De Lile, J.R.; Zhang, D.; Masudy-Panah, S.; Ramakrishna, S.; Fakhri, A.; Gupta, V.K. Co-Doping Silver and Iron on Graphitic Carbon Nitride-Carrageenan Nanocomposite for the Photocatalytic Process, Rapidly Colorimetric Detection and Antibacterial Properties. Surf. Interfaces 2021, 26, 101279. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Silver Ion Loaded 3-Aminopropyl Trimethoxysilane-Modified Fe3O4 Nanoparticles for the Fabrication of Carrageenan-Based Active Packaging Films. Colloids Surf. B Biointerfaces 2021, 208, 112085. [Google Scholar] [CrossRef] [PubMed]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Carrageenan-Based Antimicrobial Films Integrated with Sulfur-Coated Iron Oxide Nanoparticles (Fe3O4 @SNP). ACS Appl. Polym. Mater. 2021, 3, 4913–4923. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of Copper Sulfide Nanoparticles and Limonene Incorporated Pullulan/Carrageenan-Based Film with Improved Mechanical and Antibacterial Properties. Polymers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhe, T.; Guo, Z.; Sun, X.; Wang, Q.; Wang, L. Copper Sulfide Nanoparticle-Carrageenan Films for Packaging Application. Food Hydrocoll. 2020, 109, 106094. [Google Scholar] [CrossRef]
- Ezati, P.; Riahi, Z.; Rhim, J.-W. Carrageenan-Based Functional Films Integrated with CuO-Doped Titanium Nanotubes for Active Food-Packaging Applications. ACS Sustain. Chem. Eng. 2021, 9, 9300–9307. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Venkatnarayanan, S.; Dharani, G. Fabrication and Characterization of Bio-Nanocomposite Films Using κ-Carrageenan and Kappaphycus Alvarezii Seaweed for Multiple Industrial Applications. Int. J. Biol. Macromol. 2022, 219, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Esmkhani, M.; Zallaghi, M.; Nezafat, Z.; Javanshir, S. Biomedical and Environmental Applications of Carrageenan-Based Hydrogels: A Review. J. Polym. Environ. 2023, 31, 1679–1705. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W.; Hahm, D.-H. Lignin-Mediated Green Synthesis of AgNPs in Carrageenan Matrix for Wound Dressing Applications. Int. J. Biol. Macromol. 2020, 159, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; El-Naggar, M.E.; Elsherbiny, D.A.; Ali, S.; Abdel-Aziz, M.S.; Abdel-Monem, Y.K. Antibacterial Carrageenan/Cellulose Nanocrystal System Loaded with Silver Nanoparticles, Prepared via Solid-State Technique. J. Environ. Chem. Eng. 2020, 8, 104276. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Pavithra, U.; Sivaranjani, V.; Balasubramanian, N.; Sakthivel, K.M.; Pruncu, C.I. A Novel Ag/Carrageenan–Gelatin Hybrid Hydrogel Nanocomposite and Its Biological Applications: Preparation and Characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104257. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Carrageenan-Based Hydrogels and Films: Effect of ZnO and CuO Nanoparticles on the Physical, Mechanical, and Antimicrobial Properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Patwa, R.; Zandraa, O.; Saha, N.; Saha, P. Preparation and Characterization of Injectable Self-Antibacterial Gelatin/Carrageenan/Bacterial Cellulose Hydrogel Scaffolds for Wound Healing Application. J. Drug Deliv. Sci. Technol. 2021, 63, 102415. [Google Scholar] [CrossRef]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpur, A.; Talebi, A.; Moshtaghian, J. A Multifunctional Nanocomposite Spray Dressing of Kappa-Carrageenan-Polydopamine Modified ZnO/L-Glutamic Acid for Diabetic Wounds. Mater. Sci. Eng. C 2020, 111, 110837. [Google Scholar] [CrossRef]
- Khodaei, T.; Nourmohammadi, J.; Ghaee, A.; Khodaii, Z. An Antibacterial and Self-Healing Hydrogel from Aldehyde-Carrageenan for Wound Healing Applications. Carbohydr. Polym. 2023, 302, 120371. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic Effects of Antibiotics Loaded Cellulose Nanofiber and κ-Carrageenan Oligosaccharide Composite Hydrogels for Periodontitis Treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar] [CrossRef] [PubMed]
- Özbaş, Z.; Özkahraman, B.; Bayrak, G.; Kılıç Süloğlu, A.; Perçin, I.; Boran, F.; Tamahkar, E. Poly(Vinyl Alcohol)/(Hyaluronic Acid-g-Kappa-Carrageenan) Hydrogel as Antibiotic-Releasing Wound Dressing. Chem. Pap. 2021, 75, 6591–6600. [Google Scholar] [CrossRef]
- Alinavaz, S.; Mahdavinia, G.R.; Jafari, H.; Hazrati, M.; Akbari, A. Hydroxyapatite (HA)-Based Hybrid Bionanocomposite Hydrogels: Ciprofloxacin Delivery, Release Kinetics and Antibacterial Activity. J. Mol. Struct. 2021, 1225, 129095. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Q.; Liu, J.; Zhu, Z.; Shao, W. Antibacterial Performance of Berberine Loaded Carrageenan/Konjac Glucomannan Hydrogels. Mater. Express 2021, 11, 1516–1522. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W. Carrageenan-Based Functional Hydrogel Film Reinforced with Sulfur Nanoparticles and Grapefruit Seed Extract for Wound Healing Application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible PH-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Polat, T.G.; Duman, O.; Tunç, S. Agar/κ-Carrageenan/Montmorillonite Nanocomposite Hydrogels for Wound Dressing Applications. Int. J. Biol. Macromol. 2020, 164, 4591–4602. [Google Scholar] [CrossRef]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial Peptides: Premises and Promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Seyedi, S.; Hoseini Goki, N.; Khameneh, B. Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms. Int. J. Pept. Res. Ther. 2021, 27, 801–816. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Lehrer, R. Cationic Peptides: A New Source of Antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Cociancich, S.; Bulet, P.; Hetru, C.; Hoffmann, J.A. The Inducible Antibacterial Peptides of Insects. Parasitol. Today 1994, 10, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.G. Antimicrobial Peptides (AMPs) with Dual Mechanisms: Membrane Disruption and Apoptosis. J. Microbiol. Biotechnol. 2015, 25, 759–764. [Google Scholar] [CrossRef]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor Activity of the Antimicrobial Peptide Magainin II against Bladder Cancer Cell Lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef]
- Lorin, C.; Saidi, H.; Belaid, A.; Zairi, A.; Baleux, F.; Hocini, H.; Bélec, L.; Hani, K.; Tangy, F. The Antimicrobial Peptide Dermaseptin S4 Inhibits HIV-1 Infectivity in Vitro. Virology 2005, 334, 264–275. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial Peptides from Ranid Frogs: Taxonomic and Phylogenetic Markers and a Potential Source of New Therapeutic Agents. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2004, 1696, 1–14. [Google Scholar] [CrossRef]
- Lai, R.; Liu, H.; Lee, W.H.; Zhang, Y. An Anionic Antimicrobial Peptide from Toad Bombina Maxima. Biochem. Biophys. Res. Commun. 2002, 295, 796–799. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The Antibiotic and Anticancer Active Aurein Peptides from the Australian Bell Frogs Litoria Aurea and Litoria Raniformis: The Solution Structure of Aurein 1.2. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef]
- Ogan, A.; Yüce-Dursun, B.; Abdullah, D.; Beyler-Çiğil, A.; Kahraman, M.V.; Çağlayan, P.; Birbir, M.; Mutlu, Ö.; Gülsoy, N. Preparation and Antimicrobial Properties of LL-37 Peptide Immobilized Lignin/Caprolactone Polymer Film. Polym. Adv. Technol. 2020, 31, 2222–2228. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide Antibiotics and Their Role in Innate Immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- De Smet, K.; Contreras, R. Human Antimicrobial Peptides: Defensins, Cathelicidins and Histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Matsumoto, K.; Saito, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; et al. Antimicrobial Peptides Human β-Defensin (HBD)-3 and HBD-4 Activate Mast Cells and Increase Skin Vascular Permeability. Eur. J. Immunol. 2007, 37, 434–444. [Google Scholar] [CrossRef]
- Skeate, J.G.; Segerink, W.H.; Garcia, M.D.; Fernandez, D.J.; Prins, R.; Lühen, K.P.; Voss, F.O.; Da Silva, D.M.; Kast, W.M. Theta-Defensins Inhibit High-Risk Human Papillomavirus Infection Through Charge-Driven Capsid Clustering. Front. Immunol. 2020, 11, 561843. [Google Scholar] [CrossRef]
- Selsted, M.E. θ-Defensins: Cyclic Antimicrobial Peptides Produced by Binary Ligation of Truncated α-Defensins. Curr. Protein Pept. Sci. 2004, 5, 365–371. [Google Scholar] [CrossRef]
- Garcia, A.E.; Ösapay, G.; Tran, P.A.; Yuan, J.; Selsted, M.E. Isolation, Synthesis, and Antimicrobial Activities of Naturally Occurring θ-Defensin Isoforms from Baboon Leukocytes. Infect. Immun. 2008, 76, 5883–5891. [Google Scholar] [CrossRef]
- Tanner, J.; Deplazes, E.; Mancera, R. The Biological and Biophysical Properties of the Spider Peptide Gomesin. Molecules 2018, 23, 1733. [Google Scholar] [CrossRef]
- Dimarcq, J.; Bulet, P.; Hetru, C.; Hoffmann, J. Cysteine-rich Antimicrobial Peptides in Invertebrates. Pept. Sci. 1998, 47, 465–477. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An Overview of Antimicrobial Activity of Lysozyme and Its Functionality in Cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Blaskovich, M.A.T.; Cooper, M.A. Structure-Activity and-Toxicity Relationships of the Antimicrobial Peptide Tachyplesin-1. ACS Infect. Dis. 2017, 3, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L. Antibacterial Peptides and Proteins with Multiple Cellular Targets. J. Pept. Sci. 2005, 11, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine Lactoferrin: Benefits and Mechanism of Action against Infections. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial Peptides: Basic Facts and Emerging Concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Infante, V.V.; Miranda-Olvera, A.D.; De Leon-Rodriguez, L.M.; Anaya-Velazquez, F.; Rodriguez, M.C.; Avila, E.E. Effect of the Antimicrobial Peptide Tritrpticin on the In Vitro Viability and Growth of Trichomonas Vaginalis. Curr. Microbiol. 2011, 62, 301–306. [Google Scholar] [CrossRef]
- Batista Araujo, J.; Sastre de Souza, G.; Lorenzon, E.N. Indolicidin Revisited: Biological Activity, Potential Applications and Perspectives of an Antimicrobial Peptide Not yet Fully Explored. World J. Microbiol. Biotechnol. 2022, 38, 39. [Google Scholar] [CrossRef]
- Wang, G. (Ed.) Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Advances in molecular and cellular microbiology; CABI: Wallingford, UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-657-0. [Google Scholar]
- Yang, S.-T.; Yub Shin, S.; Kim, Y.-C.; Kim, Y.; Hahm, K.-S.; Kim, J.I. Conformation-Dependent Antibiotic Activity of Tritrpticin, a Cathelicidin-Derived Antimicrobial Peptide. Biochem. Biophys. Res. Commun. 2002, 296, 1044–1050. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Dias, S.A.; Domingues, M.M.; Benfield, A.H.; Freire, J.M.; Rádis-Baptista, G.; Gaspar, D.; Castanho, M.A.R.B.; Craik, D.J.; Henriques, S.T.; et al. Mechanisms of Bacterial Membrane Permeabilization by Crotalicidin (Ctn) and Its Fragment Ctn(15–34), Antimicrobial Peptides from Rattlesnake Venom. J. Biol. Chem. 2018, 293, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; De Menezes, R.R.P.P.B.; Sampaio, T.L.; Falcão, C.B.; Rádis-Baptista, G.; Martins, A.M.C. Antichagasic Effect of Crotalicidin, a Cathelicidin-like Vipericidin, Found in Crotalus durissus terrificus Rattlesnake’s Venom Gland. Parasitology 2018, 145, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Price, R.L.; Bugeon, L.; Mostowy, S.; Makendi, C.; Wren, B.W.; Williams, H.D.; Willcocks, S.J. In Vitro and in Vivo Properties of the Bovine Antimicrobial Peptide, Bactenecin 5. PLoS ONE 2019, 14, e0210508. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-H.; Shah, P.; Chen, Y.-W.; Chen, C.-S. Systematic Analysis of Intracellular-Targeting Antimicrobial Peptides, Bactenecin 7, Hybrid of Pleurocidin and Dermaseptin, Proline–Arginine-Rich Peptide, and Lactoferricin B, by Using Escherichia coli Proteome Microarrays. Mol. Cell. Proteom. 2016, 15, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Ni, L.; Ling, J. Antibacterial Peptide Nisin: A Potential Role in the Inhibition of Oral Pathogenic Bacteria. Peptides 2014, 60, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Chow, L.N.Y.; Mookherjee, N. Cationic Host Defence Peptides: Multifaceted Role in Immune Modulation and Inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of Silk Sericin from Cocoons of Three Southern African Wild Silk Moths with a Focus on Their Antimicrobial and Antioxidant Properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef]
- Ahamad, S. Extraction and Evaluation of Antimicrobial Potential of Antheraea Mylitta Silk Sericin. Int. Jouinal Recent Sci. Res. 2018, 9, 23019–23022. [Google Scholar]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of Antibacterial Sericin Based Hydrogel as an Injectable and Mouldable Wound Dressing. Mater. Sci. Eng. C 2021, 119, 111597. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.; Zhang, Q.; Liang, C.; Qin, H.; Liu, P.; Wang, K.; Zhang, X.; Chen, L.; Wei, Y. Shape Changes and Interaction Mechanism of Escherichia coli Cells Treated with Sericin and Use of a Sericin-Based Hydrogel for Wound Healing. Appl. Environ. Microbiol. 2016, 82, 4663–4672. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Seo, S.; Yang, I.-J.; Nguyen, L.T.H.; Shin, H.-S.; Patra, J.K. Synthesis of Biogenic Gold Nanoparticles by Using Sericin Protein from Bombyx mori Silk Cocoon and Investigation of Its Wound Healing, Antioxidant, and Antibacterial Potentials. Int. J. Nanomed. 2023, 18, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Ghalei, S.; Handa, H. A Review on Antibacterial Silk Fibroin-Based Biomaterials: Current State and Prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef]
- Peng, J.; Wu, Z.; Liu, W.; Long, H.; Zhu, G.; Guo, G.; Wu, J. Antimicrobial Functional Divergence of the Cecropin Antibacterial Peptide Gene Family in Musca Domestica. Parasit. Vectors 2019, 12, 537. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, P.; Wu, X.; Wang, Y.; Rehman, T.; Yao, X.; Luo, Y.; Yang, Z. Expression of Antimicrobial Peptide Cecropin P1 in Saccharomyces cerevisiae and Its Antibacterial and Antiviral Activity in Vitro. Electron. J. Biotechnol. 2021, 50, 16–22. [Google Scholar] [CrossRef]
- Wang, M.; Lin, J.; Sun, Q.; Zheng, K.; Ma, Y.; Wang, J. Design, Expression, and Characterization of a Novel Cecropin A-Derived Peptide with High Antibacterial Activity. Appl. Microbiol. Biotechnol. 2019, 103, 1765–1775. [Google Scholar] [CrossRef]
- Maloy, W.L.; Kari, U.P. Structure-Activity Studies on Magainins and Other Host Defense Peptides. Biopolymers 1995, 37, 105–122. [Google Scholar] [CrossRef]
- Kim, M.; Kang, N.; Ko, S.; Park, J.; Park, E.; Shin, D.; Kim, S.; Lee, S.; Lee, J.; Lee, S.; et al. Antibacterial and Antibiofilm Activity and Mode of Action of Magainin 2 against Drug-Resistant Acinetobacter Baumannii. Int. J. Mol. Sci. 2018, 19, 3041. [Google Scholar] [CrossRef]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 Decreases Candida albicans Growth, Biofilm Formation and the Expression of Hyphal Wall Protein 1 and Aspartic Protease Genes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis. Molecules 2017, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, Multifunctional Antimicrobial Peptides: A Review of Their Pharmacology, Effectivity, Mechanism of Action, and Possible Future Directions. Front. Pharmacol. 2019, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Kreil, G.; Barra, D. Bombinins, Antimicrobial Peptides from Bombina Species. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1551–1555. [Google Scholar] [CrossRef]
- Coccia, C.; Rinaldi, A.C.; Luca, V.; Barra, D.; Bozzi, A.; Di Giulio, A.; Veerman, E.C.I.; Mangoni, M.L. Membrane Interaction and Antibacterial Properties of Two Mildly Cationic Peptide Diastereomers, Bombinins H2 and H4, Isolated from Bombina Skin. Eur. Biophys. J. 2011, 40, 577–588. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Grovale, N.; Giorgi, A.; Mignogna, G.; Simmaco, M.; Barra, D. Structure-Function Relationships in Bombinins H, Antimicrobial Peptides from Bombina Skin Secretions☆. Peptides 2000, 21, 1673–1679. [Google Scholar] [CrossRef]
- Lipscomb, T.H.; Middleton, E.R. Towards Finding the Antimicrobial Mechanism of Action of Bombina Maxima’s Maximin 3, Using GROMACS. Ph.D. Thesis, University of New York, New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Manzo, G.; Ferguson, P.M.; Hind, C.K.; Clifford, M.; Gustilo, V.B.; Ali, H.; Bansal, S.S.; Bui, T.T.; Drake, A.F.; Atkinson, R.A.; et al. Temporin L and Aurein 2.5 Have Identical Conformations but Subtly Distinct Membrane and Antibacterial Activities. Sci. Rep. 2019, 9, 10934. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Saeedi, N.; Mesbahfar, E.; Farrokh, P.; Salimi, F.; Rezaei, A. Design and Characterization of New Antimicrobial Peptides Derived from Aurein 1.2 with Enhanced Antibacterial Activity. Biochimie 2021, 181, 42–51. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic Effect of Antibacterial Agents Human β-Defensins, Cathelicidin LL-37 and Lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Noore, J.; Noore, A.; Li, B. Cationic Antimicrobial Peptide LL-37 Is Effective against Both Extra- and Intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J. Roles of Antimicrobial Peptides Such as Defensins in Innate and Adaptive Immunity. Ann. Rheum. Dis. 2003, 62, ii17–ii21. [Google Scholar] [CrossRef] [PubMed]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The Human Beta-Defensin-3, an Antibacterial Peptide with Multiple Biological Functions. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Inthanachai, T.; Thammahong, A.; Edwards, S.W.; Virakul, S.; Kiatsurayanon, C.; Chiewchengchol, D. The Inhibitory Effect of Human Beta-Defensin-3 on Candida glabrata Isolated from Patients with Candidiasis. Immunol. Investig. 2021, 50, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human β-Defensins. Cell. Mol. Life Sci. CMLS 2006, 63, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, Y.; Rao, N.; Li, J.; Li, X.; Fang, T.; Zhao, Y.; Ge, L. Activation and Biological Properties of Human β Defensin 4 in Stem Cells Derived from Human Exfoliated Deciduous Teeth. Front. Physiol. 2019, 10, 1304. [Google Scholar] [CrossRef]
- Tran, D.; Tran, P.A.; Tang, Y.-Q.; Yuan, J.; Cole, T.; Selsted, M.E. Homodimeric θ-Defensins from Rhesus MacaqueLeukocytes. J. Biol. Chem. 2002, 277, 3079–3084. [Google Scholar] [CrossRef]
- Fázio, M.A.; Oliveira, V.X.; Bulet, P.; Miranda, M.T.M.; Daffre, S.; Miranda, A. Structure-Activity Relationship Studies of Gomesin: Importance of the Disulfide Bridges for Conformation, Bioactivities, and Serum Stability. Biopolymers 2006, 84, 205–218. [Google Scholar] [CrossRef]
- Moraes, L.G.M.; Fázio, M.A.; Vieira, R.F.F.; Nakaie, C.R.; Miranda, M.T.M.; Schreier, S.; Daffre, S.; Miranda, A. Conformational and Functional Studies of Gomesin Analogues by CD, EPR and Fluorescence Spectroscopies. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 52–58. [Google Scholar] [CrossRef]
- Xiao, B.; Liao, X.; Wang, H.; He, J.; Li, C. BigPEN, an Antimicrobial Peptide of Penaeidin Family from Shrimp Litopenaeus vannamei with Membrane Permeable and DNA Binding Activity. Fish Shellfish Immunol. Rep. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Cuthbertson, B.J.; Deterding, L.J.; Williams, J.G.; Tomer, K.B.; Etienne, K.; Blackshear, P.J.; Büllesbach, E.E.; Gross, P.S. Diversity in Penaeidin Antimicrobial Peptide Form and Function. Dev. Comp. Immunol. 2008, 32, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Leśnierowski, G.; Yang, T. Lysozyme and Its Modified Forms: A Critical Appraisal of Selected Properties and Potential. Trends Food Sci. Technol. 2021, 107, 333–342. [Google Scholar] [CrossRef]
- Branen, J.K.; Davidson, P.M. Enhancement of Nisin, Lysozyme, and Monolaurin Antimicrobial Activities by Ethylenediaminetetraacetic Acid and Lactoferrin. Int. J. Food Microbiol. 2004, 90, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Masschalck, B.; Michiels, C.W. Antimicrobial Properties of Lysozyme in Relation to Foodborne Vegetative Bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; So, L.-Y.; Chen, F.; Liang, J.; Chow, H.-Y.; Wong, K.-Y.; Wan, S.; Jiang, T.; Yu, R. Influences of Disulfide Connectivity on Structure and Antimicrobial Activity of Tachyplesin I. J. Pept. Sci. 2018, 24, e3087. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.-A.; Yang, J.-L. Marked Increase in Membranolytic Selectivity of Novel Cyclic Tachyplesins Constrained with an Antiparallel Two-β Strand Cystine Knot Framework. Biochem. Biophys. Res. Commun. 2000, 267, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.-P.S.; Rozek, A.; Hancock, R.E.W. Structure–Activity Relationships for the β-Hairpin Cationic Antimicrobial Peptide Polyphemusin I. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2004, 1698, 239–250. [Google Scholar] [CrossRef]
- Zhang, L.; Scott, M.G.; Yan, H.; Mayer, L.D.; Hancock, R.E.W. Interaction of Polyphemusin I and Structural Analogs with Bacterial Membranes, Lipopolysaccharide, and Lipid Monolayers. Biochemistry 2000, 39, 14504–14514. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-Activity Analysis of Thanatin, a 21-Residue Inducible Insect Defense Peptide with Sequence Homology to Frog Skin Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R. Antimicrobial Properties of Lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent Antibacterial Peptides Generated by Pepsin Digestion of Bovine Lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Vasilchenko, A.V.; Pashkova, T.M.; Smirnova, M.P.; Kolodkin, N.I.; Manukhov, I.V.; Zavilgelsky, G.B.; Sizova, E.A.; Kartashova, O.L.; Simbirtsev, A.S.; et al. Antimicrobial Activity of the Indolicidin-Derived Novel Synthetic Peptide In-58: Novel Synthetic Antimicrobial Peptide. J. Pept. Sci. 2017, 23, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Nguyen, L.T.; Kernaghan, S.D.; Rekdal, Ø.; Vogel, H.J. Structure-Function Analysis of Tritrpticin Analogs: Potential Relationships between Antimicrobial Activities, Model Membrane Interactions, and Their Micelle-Bound NMR Structures. Biophys. J. 2006, 91, 4413–4426. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; Pérez-Peinado, C.; de la Torre, B.G.; Mayol, X.; Zamora-Carreras, H.; Jiménez, M.Á.; Rádis-Baptista, G.; Andreu, D. Structural Dissection of Crotalicidin, a Rattlesnake Venom Cathelicidin, Retrieves a Fragment with Antimicrobial and Antitumor Activity. J. Med. Chem. 2015, 58, 8553–8563. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Scocchi, M.; Podda, E.; Skerlavaj, B.; Dolzani, L.; Gennaro, R. Antimicrobial Activity of Bac7 Fragments against Drug-Resistant Clinical Isolates. Peptides 2004, 25, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Barrière, Q.; Timchenko, T.; Müller, C.; Pacor, S.; Mergaert, P.; Scocchi, M.; Wilson, D.N. Fragments of the Nonlytic Proline-Rich Antimicrobial Peptide Bac5 Kill Escherichia coli Cells by Inhibiting Protein Synthesis. Antimicrob. Agents Chemother. 2018, 62, e00534-18. [Google Scholar] [CrossRef]
- Fan, F.; Wu, Y.; Liu, J. Expression and Purification of Two Different Antimicrobial Peptides, PR-39 and Protegrin-1 in Escherichia coli. Protein Expr. Purif. 2010, 73, 147–151. [Google Scholar] [CrossRef]
- Jeon, H.; Le, M.T.; Ahn, B.; Cho, H.; Le, V.C.Q.; Yum, J.; Hong, K.; Kim, J.-H.; Song, H.; Park, C. Copy Number Variation of PR-39 Cathelicidin, and Identification of PR-35, a Natural Variant of PR-39 with Reduced Mammalian Cytotoxicity. Gene 2019, 692, 88–93. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Schneider, V.A.F.; Agustiandari, H.; van Dijk, A.; Tjeerdsma-van Bokhoven, J.L.M.; Bikker, F.J.; Haagsman, H.P. Antimicrobial and Immunomodulatory Activities of PR-39 Derived Peptides. PLoS ONE 2014, 9, e95939. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Li, G.; Liu, S.; Cui, N.; Liu, S.; Langford, P.R.; Wang, C. The SapA Protein Is Involved in Resistance to Antimicrobial Peptide PR-39 and Virulence of Actinobacillus Pleuropneumoniae. Front. Microbiol. 2017, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Hampikyan, H. Efficacy of Nisin against Staphylococcus aureus in Experimentally Contaminated Sucuk, a Turkish-Type Fermented Sausage. J. Food Prot. 2009, 72, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Periago, P.M.; Moezelaar, R. Combined Effect of Nisin and Carvacrol at Different PH and Temperature Levels on the Viability of Different Strains of Bacillus cereus. Int. J. Food Microbiol. 2001, 68, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of Nisin Resistance in Gram-Positive Bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Etayash, H.; Norman, L.; Thundat, T.; Stiles, M.; Kaur, K. Surface-Conjugated Antimicrobial Peptide Leucocin a Displays High Binding to Pathogenic Gram-Positive Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 1131–1138. [Google Scholar] [CrossRef]
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of Leucocin A for Use on Wieners to Inhibit Listeria Monocytogenes in the Presence of Spoilage Organisms. Int. J. Food Microbiol. 2017, 255, 25–31. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial Peptides under Clinical Investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Zhang, S.W. 1-Biodegradable Medical Polymers: Fundamental Sciences. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Zhang, X., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2017; ISBN 978-0-08-100372-5. [Google Scholar]
- Vert, M. Bioresorbable Polymers for Temporary Therapeutic Applications. Angew. Makromol. Chem. 1989, 166, 155–168. [Google Scholar] [CrossRef]
- Vert, M. Degradable, Biodegradable, and Bioresorbable Polymers for Time-Limited Therapy. In Bioresorbable Scaffolds; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-38062-9. [Google Scholar]
- Science and Principles of Biodegradable and Bioresorbable Medical Polymers-1st Edition. Available online: https://www.elsevier.com/books/science-and-principles-of-biodegradable-and-bioresorbable-medical-polymers/zhang/978-0-08-100372-5 (accessed on 10 February 2023).
- Vert, M. Bioabsorbable Polymers in Medicine: An Overview. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2009, 5 (Suppl. SF), F9–F14. [Google Scholar] [CrossRef]
- Casalini, T.; Perale, G. 1-Types of Bioresorbable Polymers for Medical Applications. In Durability and Reliability of Medical Polymers; Jenkins, M., Stamboulis, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 3–29. ISBN 978-1-84569-929-1. [Google Scholar]
- Winnacker, M.; Rieger, B. Poly(Ester Amide)s: Recent Insights into Synthesis, Stability and Biomedical Applications. Polym. Chem. 2016, 7, 7039–7046. [Google Scholar] [CrossRef]
- Fukushima, K. Biodegradable Functional Biomaterials Exploiting Substituted Trimethylene Carbonates and Organocatalytic Transesterification. Polym. J. 2016, 48, 1103–1114. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Do Bioresorbable Polyesters Have Antimicrobial Properties? J. Mater. Sci. Mater. Med. 2018, 29, 18. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Biswal, A.K.; Dhingra, S.; Gupta, A.; Saha, S. Antibacterial Response of Polylactide Surfaces Modified with Hydrophilic Polymer Brushes. Iran. Polym. J. 2019, 28, 493–504. [Google Scholar] [CrossRef]
- Xu, F.J.; Wang, Z.H.; Yang, W.T. Surface Functionalization of Polycaprolactone Films via Surface-Initiated Atom Transfer Radical Polymerization for Covalently Coupling Cell-Adhesive Biomolecules. Biomaterials 2010, 31, 3139–3147. [Google Scholar] [CrossRef]
- Xu, F.J.; Yang, X.C.; Li, C.Y.; Yang, W.T. Functionalized Polylactide Film Surfaces via Surface-Initiated ATRP. Macromolecules 2011, 44, 2371–2377. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive Surfaces of Polylactide and Silver Nanoparticles for the Prevention of Microbial Contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Alfredo, N.V.; Rodríguez-Hernández, J. Chapter 4—Antimicrobial Polymeric Nanostructures. In Nanostructures for Antimicrobial Therapy; Micro and Nano Technologies; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 85–115. ISBN 978-0-323-46152-8. [Google Scholar]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Hicks, R.P.; Abercrombie, J.J.; Wong, R.K.; Leung, K.P. Antimicrobial Peptides Containing Unnatural Amino Acid Exhibit Potent Bactericidal Activity against ESKAPE Pathogens. Bioorg. Med. Chem. 2013, 21, 205–214. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides with Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, H.; Yang, L.; Li, Q.; Huang, X. Simulating the Antimicrobial Mechanism of Human β-Defensin-3 with Coarse-Grained Molecular Dynamics. J. Biomol. Struct. Dyn. 2015, 33, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The Interaction of Antimicrobial Peptides with Membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Synthesis and Antimicrobial Activity of Amino Acid and Peptide Derivatives of Mycophenolic Acid|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0223523417309972?token=40F6BB8FA8A5684D35CC75FC3A54BA5A943246912D373AD05F43A49D56E3D4D1DD33358A4C6DB3B57EB00957429F1204&originRegion=eu-west-1&originCreation=20230210173536 (accessed on 10 February 2023).
- Li, H.; Fu, S.; Wang, Y.; Yuan, X.; Liu, L.; Dong, H.; Wang, Q.; Zhang, Z. Antimicrobial and Antitumor Activity of Peptidomimetics Synthesized from Amino Acids. Bioorganic Chem. 2021, 106, 104506. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic Antimicrobial Peptides: Characteristics, Design, and Potential as Alternative Molecules to Overcome Microbial Resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Tarazi, S.; Abu-Alhaijaa, A.; Altall, Y.; Alshar’i, N.; Bodoor, K.; Al-Balas, Q. Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals 2014, 7, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Andersson, L.; Blomberg, L.; Flegel, M.; Lepsa, L.; Nilsson, B.; Verlander, M. Large-Scale Synthesis of Peptides. Pept. Sci. 2000, 55, 227–250. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, X.; Li, P.; Chen, W.N.; Mouad, L.; Chang, M.W.; Leong, S.S.J.; Chan-Park, M.B. High Potency and Broad-Spectrum Antimicrobial Peptides Synthesized via Ring-Opening Polymerization of α-Aminoacid-N-Carboxyanhydrides. Biomacromolecules 2010, 11, 60–67. [Google Scholar] [CrossRef]
- Song, Z.; Tan, Z.; Cheng, J. Recent Advances and Future Perspectives of Synthetic Polypeptides from N-Carboxyanhydrides. Macromolecules 2019, 52, 8521–8539. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Wu, X.; Liu, L.; Zhang, W.; Ding, Y.; Liu, S.; Zhou, M.; Shao, N.; Ji, Z.; et al. Superfast and Water-Insensitive Polymerization on α-Amino Acid N-Carboxyanhydrides to Prepare Polypeptides Using Tetraalkylammonium Carboxylate as the Initiator. Angew. Chem. Int. Ed. 2021, 60, 26063–26071. [Google Scholar] [CrossRef]
- Mazo, A.R.; Allison-Logan, S.; Karimi, F.; Chan, N.J.-A.; Qiu, W.; Duan, W.; O′Brien-Simpson, N.M.; Qiao, G.G. Ring Opening Polymerization of α-Amino Acids: Advances in Synthesis, Architecture and Applications of Polypeptides and Their Hybrids. Chem. Soc. Rev. 2020, 49, 4737–4834. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.C.; Shukla, A.; Puranam, S.; Buss, H.G.; Jreige, N.; Hammond, P.T. Effects of Side Group Functionality and Molecular Weight on the Activity of Synthetic Antimicrobial Polypeptides. Biomacromolecules 2011, 12, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Phuong, P.T.; Oliver, S.; He, J.; Wong, E.H.H.; Mathers, R.T.; Boyer, C. Effect of Hydrophobic Groups on Antimicrobial and Hemolytic Activity: Developing a Predictive Tool for Ternary Antimicrobial Polymers. Biomacromolecules 2020, 21, 5241–5255. [Google Scholar] [CrossRef]
- Xia, Y.; Song, Z.; Tan, Z.; Xue, T.; Wei, S.; Zhu, L.; Yang, Y.; Fu, H.; Jiang, Y.; Lin, Y.; et al. Accelerated Polymerization of N-Carboxyanhydrides Catalyzed by Crown Ether. Nat. Commun. 2021, 12, 732. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Zhang, Z.; Wang, S.; Lu, H. A Moisture-Tolerant Route to Unprotected α/β-Amino Acid N-Carboxyanhydrides and Facile Synthesis of Hyperbranched Polypeptides. Nat. Commun. 2021, 12, 5810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Zhang, W.-W.; Zhou, R.-Y.; Chen, Q.; Xie, C.-Y.; Xiang, H.-X.; Sun, B.; Zhu, M.-F.; Liu, R.-H. Facile Synthesis of High Molecular Weight Polypeptides via Fast and Moisture Insensitive Polymerization of α-Amino Acid N-Carboxyanhydrides. Chin. J. Polym. Sci. 2020, 38, 1131–1140. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, Y.; Gao, J.; Xiao, Y.; Du, J. Dual Corona Vesicles with Intrinsic Antibacterial and Enhanced Antibiotic Delivery Capabilities for Effective Treatment of Biofilm-Induced Periodontitis. ACS Nano 2019, 13, 13645–13657. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Ring-Opening Polymerization of Lactams. Living Anionic Polymerization and Its Applications. Prog. Polym. Sci. 2000, 25, 1411–1462. [Google Scholar] [CrossRef]
- Zhang, J.; Kissounko, D.A.; Lee, S.E.; Gellman, S.H.; Stahl, S.S. Access to Poly-β-Peptides with Functionalized Side Chains and End Groups via Controlled Ring-Opening Polymerization of β-Lactams. J. Am. Chem. Soc. 2009, 131, 1589–1597. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, P.; Xie, J.; Zhang, S.; Xiao, X.; Qiao, Z.; Shao, N.; Zhou, M.; Zhang, W.; Dai, C.; et al. Host Defense Peptide Mimicking Poly-β-Peptides with Fast, Potent and Broad Spectrum Antibacterial Activities. Biomater. Sci. 2019, 7, 2144–2151. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Structural Determinants of Antimicrobial Activity in Polymers Which Mimic Host Defense Peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Palermo, E.F. Antimicrobial Synthetic Polymers: An Update on Structure-Activity Relationships. Curr. Pharm. Des. 2018, 24, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; DeGrado, W.F. Amphiphilic Polymethacrylate Derivatives as Antimicrobial Agents. J. Am. Chem. Soc. 2005, 127, 4128–4129. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Palermo, E.F.; Thoma, L.M.; Satoh, K.; Kamigaito, M.; Kuroda, K. Design and Synthesis of Self-Degradable Antibacterial Polymers by Simultaneous Chain- and Step-Growth Radical Copolymerization. Biomacromolecules 2012, 13, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Nederberg, F.; Zhang, Y.; Tan, J.P.K.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable Nanostructures with Selective Lysis of Microbial Membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Coady, D.J.; Tan, J.P.K.; Li, Y.; Chan, J.M.W.; Yang, Y.Y.; Hedrick, J.L. Design and Synthesis of Biodegradable Grafted Cationic Polycarbonates as Broad Spectrum Antimicrobial Agents. J. Polym. Sci. Part Polym. Chem. 2016, 54, 1029–1035. [Google Scholar] [CrossRef]
- Hae Cho, C.A.; Liang, C.; Perera, J.; Liu, J.; Varnava, K.G.; Sarojini, V.; Cooney, R.P.; McGillivray, D.J.; Brimble, M.A.; Swift, S.; et al. Molecular Weight and Charge Density Effects of Guanidinylated Biodegradable Polycarbonates on Antimicrobial Activity and Selectivity. Biomacromolecules 2018, 19, 1389–1401. [Google Scholar] [CrossRef]
- Cho, C.A.; Liang, C.; Perera, J.; Brimble, M.A.; Swift, S.; Jin, J. Guanidinylated Amphiphilic Polycarbonates with Enhanced Antimicrobial Activity by Extending the Length of the Spacer Arm and Micelle Self-Assembly. Macromol. Biosci. 2020, 20, 2000065. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lou, W.; Zhong, G.; Lee, A.; Leong, J.; Chin, W.; Ding, B.; Bao, C.; Tan, J.P.K.; Pu, Q.; et al. Degradable Antimicrobial Polycarbonates with Unexpected Activity and Selectivity for Treating Multidrug-Resistant Klebsiella Pneumoniae Lung Infection in Mice. Acta Biomater. 2019, 94, 268–280. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Wang, C.; Liu, W.; Zhang, Y.; Deng, L.; Zhang, J.; Huang, P.; Wang, W.; Dong, A. Combating Drug-Resistant Bacterial Infection Using Biodegradable Nanoparticles Assembled from Comb-like Polycarbonates Grafted with Amphiphilic Polyquaternium. J. Mater. Chem. B 2021, 9, 357–365. [Google Scholar] [CrossRef]
- Nimmagadda, A.; Liu, X.; Teng, P.; Su, M.; Li, Y.; Qiao, Q.; Khadka, N.K.; Sun, X.; Pan, J.; Xu, H.; et al. Polycarbonates with Potent and Selective Antimicrobial Activity toward Gram-Positive Bacteria. Biomacromolecules 2017, 18, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, X.; Chen, L.; Xiong, Z.; Xiong, C.; Chen, D. Synthesis of New Aliphatic Poly(Ester-Carbonate)s Bearing Amino Groups Based on Photolabile Protecting Group and Evaluation of Antibacterial Property. Polym. Adv. Technol. 2022, 33, 1100–1108. [Google Scholar] [CrossRef]
- Yuen, A.Y.; Lopez-Martinez, E.; Gomez-Bengoa, E.; Cortajarena, A.L.; Aguirresarobe, R.H.; Bossion, A.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Preparation of Biodegradable Cationic Polycarbonates and Hydrogels through the Direct Polymerization of Quaternized Cyclic Carbonates. ACS Biomater. Sci. Eng. 2017, 3, 1567–1575. [Google Scholar] [CrossRef]
- Dhingra, S.; Joshi, A.; Singh, N.; Saha, S. Infection Resistant Polymer Brush Coating on the Surface of Biodegradable Polyester. Mater. Sci. Eng. C 2021, 118, 111465. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zheng, L.; Hu, K.; Li, L.; Li, C.; Zhu, L.; Wang, H.; Xiao, Y.; Wu, S.; Liu, J.; et al. Cationic Polyesters with Antibacterial Properties: Facile and Controllable Synthesis and Antibacterial Study. Eur. Polym. J. 2019, 110, 41–48. [Google Scholar] [CrossRef]
- Krumm, C.; Trump, S.; Benski, L.; Wilken, J.; Oberhaus, F.; Köller, M.; Tiller, J.C. Fast-Acting Antibacterial, Self-Deactivating Polyionene Esters. ACS Appl. Mater. Interfaces 2020, 12, 21201–21209. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Geng, Z.; Finn, M.G.; Collard, D.M. Azide-Substituted Polylactide: A Biodegradable Substrate for Antimicrobial Materials via Click Chemistry Attachment of Quaternary Ammonium Groups. Biomacromolecules 2019, 20, 3366–3374. [Google Scholar] [CrossRef]
- Zhu, W.; Du, H.; Huang, Y.; Sun, S.; Xu, N.; Ni, H.; Cai, X.; Li, X.; Shen, Z. Cationic Poly(Ester-Phosphoester)s: Facile Synthesis and Antibacterial Properties. J. Polym. Sci. Part Polym. Chem. 2013, 51, 3667–3673. [Google Scholar] [CrossRef]
- Hamana, K.; Matsuzaki, S. Polyamines as a Chemotaxonomic Marker in Bacterial Systematics. Crit. Rev. Microbiol. 1992, 18, 261–283. [Google Scholar] [CrossRef]
- Recent Advances in the Development of Polyamine Analogues as Antitumor Agents. Available online: https://pubs.acs.org/doi/10.1021/jm900187v (accessed on 18 February 2023).
- Douglas, E.J.A.; Alkhzem, A.H.; Wonfor, T.; Li, S.; Woodman, T.J.; Blagbrough, I.S.; Laabei, M. Antibacterial Activity of Novel Linear Polyamines against Staphylococcus Aureus. Front. Microbiol. 2022, 13, 948343. [Google Scholar] [CrossRef]
- Karigiannis, G.; Papaioannou, D. Structure, Biological Activity and Synthesis of Polyamine Analogues and Conjugates. Eur. J. Org. Chem. 2000, 2000, 1841–1863. [Google Scholar] [CrossRef]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging Players in Bacteria–Host Interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Zhou, J.; Zhang, S.; Saltzman, W.M. Enzyme-Synthesized Poly(Amine-Co-Esters) as Nonviral Vectors for Gene Delivery. J. Biomed. Mater. Res. A 2011, 96A, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Cheng, C.J.; Patel, T.R.; Weller, C.E.; Piepmeier, J.M.; Jiang, Z.; Saltzman, W.M. Biodegradable Poly(Amine-Co-Ester) Terpolymers for Targeted Gene Delivery. Nat. Mater. 2011, 11, 82–90. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Li, W.; Pan, Y.; Fei, P.; Wang, H.; Zhang, W.; Zhi, L.; Meng, J. Preparation and Characterization of Antibacterial Polyamine-Based Cyclophosphazene Nanofiltration Membranes. J. Membr. Sci. 2019, 592, 117371. [Google Scholar] [CrossRef]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced Polymer-Based Composites and Structures for Biomedical Applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Q.; Gao, W.; Tang, X.; Yi, H.; Tang, X. The Mechanism of Metal-Based Antibacterial Materials and the Progress of Food Packaging Applications: A Review. Ceram. Int. 2022, 48, 34148–34168. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Simakin, A.V.; Sarimov, R.M.; Smirnova, V.V.; Astashev, M.E.; Serov, D.A.; Yanykin, D.V.; Chausov, D.N.; Shkirin, A.V.; Uvarov, O.V.; Rotanov, E.; et al. New Structural Nanocomposite Based on PLGA and Al2O3 NPs as a Balance between Antibacterial Activity and Biocompatibility with Eukaryotic Cells. J. Compos. Sci. 2022, 6, 298. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Stanić, V.; Tanasković, S.B. Antibacterial Activity of Metal Oxide Nanoparticles. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–274. ISBN 978-0-12-819943-5. [Google Scholar]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak Simunec, D.; Kyratzis, I.; Sola, A.; Wen, C. Biodegradable PLA-ZnO Nanocomposite Biomaterials with Antibacterial Properties, Tissue Engineering Viability, and Enhanced Biocompatibility. Smart Mater. Manuf. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Kurtycz, P.; Karwowska, E.; Ciach, T.; Olszyna, A.; Kunicki, A. Biodegradable Polylactide (PLA) Fiber Mats Containing Al2O3-Ag Nanopowder Prepared by Electrospinning Technique—Antibacterial Properties. Fibers Polym. 2013, 14, 1248–1253. [Google Scholar] [CrossRef]
- Alippilakkotte, S.; Kumar, S.; Sreejith, L. Fabrication of PLA/Ag Nanofibers by Green Synthesis Method Using Momordica Charantia Fruit Extract for Wound Dressing Applications. Colloids Surf. Physicochem. Eng. Asp. 2017, 529, 771–782. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of Biocompatibility and Bactericidal Activity of Hierarchically Porous PLA-TiO2 Nanocomposite Films Fabricated by Breath-Figure Method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Barczyńska-Felusiak, R.; Pastusiak, M.; Rychter, P.; Kaczmarczyk, B.; Sobota, M.; Wanic, A.; Kaps, A.; Jaworska-Kik, M.; Orchel, A.; Dobrzyński, P. Synthesis of the Bacteriostatic Poly(l-Lactide) by Using Zinc (II)[(Acac)(L)H2O] (L = Aminoacid-Based Chelate Ligands) as an Effective ROP Initiator. Int. J. Mol. Sci. 2021, 22, 6950. [Google Scholar] [CrossRef]
- Norouzi, M.A.; Montazer, M.; Harifi, T.; Karimi, P. Flower Buds like PVA/ZnO Composite Nanofibers Assembly: Antibacterial, in Vivo Wound Healing, Cytotoxicity and Histological Studies. Polym. Test. 2021, 93, 106914. [Google Scholar] [CrossRef]
- Wang, G.; Fakhri, A. Preparation of CuS/Polyvinyl Alcohol-Chitosan Nanocomposites with Photocatalysis Activity and Antibacterial Behavior against G+/G− Bacteria. Int. J. Biol. Macromol. 2020, 155, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Ismail, A.M.; Awwad, N.S.; Ibrahium, H.A. Physical Characterization and Antibacterial Activity of PVA/Chitosan Matrix Doped by Selenium Nanoparticles Prepared via One-Pot Laser Ablation Route. J. Mater. Res. Technol. 2020, 9, 9598–9606. [Google Scholar] [CrossRef]
- Al-hakimi, A.N.; Asnag, G.M.; Alminderej, F.; Alhagri, I.A.; Al-Hazmy, S.M.; Abdallah, E.M. Enhanced Structural, Optical, Electrical Properties and Antibacterial Activity of Selenium Nanoparticles Loaded PVA/CMC Blend for Electrochemical Batteries and Food Packaging Applications. Polym. Test. 2022, 116, 107794. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL Membranes Incorporated with Biosynthesized Silver Nanoparticles as Antibacterial Wound Dressings. Appl. Nanosci. Switz. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G.; et al. Controlled Degradability of PCL-ZnO Nanofibrous Scaffolds for Bone Tissue Engineering and Their Antibacterial Activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of Poly(Lactic-Co-Glycolic Acid)/ZnO Nanorods/Ag Nanoparticles Hybrid Coating on Ti Implants for Enhanced Antibacterial Activity and Biocompatibility. Mater. Sci. Eng. C 2017, 79, 629–637. [Google Scholar] [CrossRef]
- Mohammadi, G.; Valizadeh, H.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Milani, M.; Azhdarzadeh, M.; Kiafar, F.; Nokhodchi, A. Development of Azithromycin-PLGA Nanoparticles: Physicochemical Characterization and Antibacterial Effect against Salmonella Typhi. Colloids Surf. B Biointerfaces 2010, 80, 34–39. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Lednev, V.N.; Simakin, A.V.; Uvarov, O.V.; Kucherov, R.N.; Ivashkin, P.I.; Dorokhov, A.S.; Izmailov, A.Y. Biosafety Construction Composite Based on Iron Oxide Nanoparticles and PLGA. Inventions 2022, 7, 61. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Wojtyła, S.; Stochel, G.; Macyk, W. The Quenching Effect of Chitosan Crosslinking on ZnO Nanoparticles Photocatalytic Activity. RSC Adv. 2015, 5, 80089–80097. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of Chitosan Nano Dressing for Wound Healing: Characterization, in Vitro and in Vivo Studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Z.; Qi, C.; He, M.; Yu, T.; Shi, L. Construction of Chitosan/Ag Nanocomposite Sponges and Their Properties. Int. J. Biol. Macromol. 2021, 192, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–Silver Oxide Nanocomposite Film: Preparation and Antimicrobial Activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Vasantharaj, S.; Chandarshekar, B.; Bhuvaneshwari, V. Synthesis and Characterization of Chitosan/Iron Oxide Nanocomposite for Biomedical Applications. Int. J. Biol. Macromol. 2019, 132, 880–887. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.Y.; Liao, C.W.; Chen, B.H. Green Synthesis, Characterization and Evaluation of Catalytic and Antibacterial Activities of Chitosan, Glycol Chitosan and Poly(γ-Glutamic Acid) Capped Gold Nanoparticles. Int. J. Biol. Macromol. 2020, 161, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Al-nemrawi, N.K.; Alkhatib, R.Q.; Ayyad, H.; Alshraiedeh, N.A. Formulation and Characterization of Tobramycin-Chitosan Nanoparticles Coated with Zinc Oxide Nanoparticles. Saudi Pharm. J. 2022, 30, 454–461. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, H.; Wu, Y.; Ma, Z.; Tian, L.; Jiang, L. Facile Synthesis of Flower-like ZnO Loading Cellulose-Chitosan Nanocomposite Films by Biomimetic Approach with Enhanced Performance. Appl. Surf. Sci. 2023, 614, 156119. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Ye, Z.; Liu, Y.; Wang, Q.; Chen, Q.; Jiang, Y.; Qi, J.; Tian, D.; Xu, J.; et al. A Large-Nanosphere/Small-Nanosphere (Cellulose/Silver) Antibacterial Composite with Prominent Catalytic Properties for the Degradation of p-Nitrophenol. Appl. Surf. Sci. 2023, 608, 155192. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Cao, H.; Jia, L.; Liu, P. Cellulose Nanofibers Aerogels Functionalized with AgO: Preparation, Characterization and Antibacterial Activity. Int. J. Biol. Macromol. 2022, 194, 58–65. [Google Scholar] [CrossRef]
- Popescu, M.C.; Ungureanu, C.; Buse, E.; Nastase, F.; Tucureanu, V.; Suchea, M.; Draga, S.; Popescu, M.A. Antibacterial Efficiency of Cellulose-Based Fibers Covered with ZnO and Al2O3 by Atomic Layer Deposition. Appl. Surf. Sci. 2019, 481, 1287–1298. [Google Scholar] [CrossRef]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-Coated Silver Nanoparticles for Improved Barrier and Controlled Antimicrobial Properties of Nanocellulose Films Used in Food Packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and Antibacterial Properties of ZnO-Alginate Nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Pirsa, S.; Farzi, J. Biodegradable Nano Composite Film Based on Modified Starch-Albumin/MgO; Antibacterial, Antioxidant and Structural Properties. Polym. Test. 2021, 97, 107182. [Google Scholar] [CrossRef]
- Mahlambi, P.N.; Moloto, M.J. Starch-Capped Silver Oxide (Ag2O) Nanoparticles: Synthesis, Characterization and Antibacterial Activity. Dig. J. Nanomater. Biostructures 2022, 17, 921–930. [Google Scholar] [CrossRef]
- Ragab, E.; Shaban, M.; Khalek, A.A.; Mohamed, F. Design and Characterization of PANI/Starch/Fe2O3 Bio Composite for Wastewater Remediation. Int. J. Biol. Macromol. 2021, 181, 301–312. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring Mechanical and Antibacterial Properties of Chitosan/Gelatin Nanofiber Membranes with Fe3O4 Nanoparticles for Potential Wound Dressing Application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Hosseini, M.; Sarafbidabad, M.; Fakhri, A.; NoorMohammadi, Z.; Tahami, S. Preparation and Characterization of MnS2/Chitosan-sodium Alginate and Calcium Alginate Nanocomposites for Degradation of Analgesic Drug: Photocorrosion, Mechanical, Antimicrobial and Antioxidant Properties Studies. Int. J. Biol. Macromol. 2018, 118, 1494–1500. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Thangavelu, K.P. Application of Tetracycline Hydrochloride Loaded-Fungal Chitosan and Aloe Vera Extract Based Composite Sponges for Wound Dressing. J. Adv. Res. 2018, 14, 63–71. [Google Scholar] [CrossRef]
- Uysal, E.; Ates, S.; Safaltin, S.; Dikmetas, D.N.; Devecioglu, D.; Guler, F.K.; Gurmen, S. Synthesis of Calcium, Copper and Iron Alginate Hydrogels Doped with Ag Nanoparticles Produced by Chemical Reduction Method. Mater. Chem. Phys. 2022, 281, 125843. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Production of Poly-Hydroxyalkanoate as Secondary Metabolite with Main Focus on Sustainable Energy. Renew. Sustain. Energy Rev. 2017, 72, 95–104. [Google Scholar] [CrossRef]
- Smole, M.S.; Hribernik, S.; Kleinschek, K.S.; Kreže, T.; Smole, M.S.; Hribernik, S.; Kleinschek, K.S.; Kreže, T. Plant Fibres for Textile and Technical Applications; IntechOpen: London, UK, 2013; ISBN 978-953-51-1184-9. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agricultural Residues—Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Sedlarik, V. Antimicrobial Modifications of Polymers. In Biodegradation—Life of Science; IntechOpen: London, UK, 2013. [Google Scholar]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Vu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Abedi, B.; Bodaghi, H.; Davarynejad, G.; Haratizadeh, H.; Conte, A. Investigation of Developed Clay-Nanocomposite Packaging Film on Quality of Peach Fruit (Prunus persica Cv. Alberta) during Cold Storage. J. Food Process. Preserv. 2018, 42, e13466. [Google Scholar] [CrossRef]
- Caseri, W.R. Nanocomposites of Polymers and Inorganic Particles: Preparation, Structure and Properties. Mater. Sci. Technol. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Khalina, A.; Sapuan, S.M.; Rahmah, M. Development of Sugar Palm Yarn/Glass Fibre Reinforced Unsaturated Polyester Hybrid Composites. Mater. Res. Express 2018, 5, 045308. [Google Scholar] [CrossRef]
- Yorseng, K.; Rangappa, S.M.; Pulikkalparambil, H.; Siengchin, S.; Parameswaranpillai, J. Accelerated Weathering Studies of Kenaf/Sisal Fiber Fabric Reinforced Fully Biobased Hybrid Bioepoxy Composites for Semi-Structural Applications: Morphology, Thermo-Mechanical, Water Absorption Behavior and Surface Hydrophobicity. Constr. Build. Mater. 2020, 235, 117464. [Google Scholar] [CrossRef]
- Pappu, A.; Pickering, K.L.; Thakur, V.K. Manufacturing and Characterization of Sustainable Hybrid Composites Using Sisal and Hemp Fibres as Reinforcement of Poly (Lactic Acid) via Injection Moulding. Ind. Crops Prod. 2019, 137, 260–269. [Google Scholar] [CrossRef]
- Jawaid, M.; Abdul Khalil, H.P.S.; Abu Bakar, A. Woven Hybrid Composites: Tensile and Flexural Properties of Oil Palm-Woven Jute Fibres Based Epoxy Composites. Mater. Sci. Eng. A 2011, 528, 5190–5195. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Paridah, M.T.; Saba, N.; Nasir, M.; Shahroze, R.M. Dynamic and Thermo-Mechanical Properties of Hybridized Kenaf/PALF Reinforced Phenolic Composites. Polym. Compos. 2019, 40, 3814–3822. [Google Scholar] [CrossRef]
- Han, S.O.; Karevan, M.; Bhuiyan, M.A.; Park, J.H.; Kalaitzidou, K. Effect of Exfoliated Graphite Nanoplatelets on the Mechanical and Viscoelastic Properties of Poly(Lactic Acid) Biocomposites Reinforced with Kenaf Fibers. J. Mater. Sci. 2012, 47, 3535–3543. [Google Scholar] [CrossRef]
- Klekotka, U.; Rogacz, D.; Szymanek, I.; Malejko, J.; Rychter, P.; Kalska-Szostko, B. Ecotoxicological Assessment of Magnetite and Magnetite/Ag Nanoparticles on Terrestrial and Aquatic Biota from Different Trophic Levels. Chemosphere 2022, 308, 136207. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and Characterization of Intelligent Starch/PVA Films for Simultaneous Colorimetric Indication and Antimicrobial Activity for Food Packaging Applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.S.; Oliveira, M.; de Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial Nanostructured Starch Based Films for Packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Isotton, F.S.; Bernardo, G.L.; Baldasso, C.; Rosa, L.M.; Zeni, M. The Plasticizer Effect on Preparation and Properties of Etherified Corn Starchs Films. Ind. Crops Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food Packaging Bags Based on Thermoplastic Corn Starch Reinforced with Talc Nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Morphological Characteristics and Barrier Properties of Thermoplastic Starch/Chitosan Blown Film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef]
- Pyla, R.; Kim, T.-J.; Silva, J.L.; Jung, Y.-S. Enhanced Antimicrobial Activity of Starch-Based Film Impregnated with Thermally Processed Tannic Acid, a Strong Antioxidant. Int. J. Food Microbiol. 2010, 137, 154–160. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial Activity and Physical Properties of Chitosan–Tapioca Starch Based Edible Films and Coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Shen, X.; Wu, J.; Chen, Y.; Zhao, G. Antimicrobial and Physical Properties of Sweet Potato Starch Films Incorporated with Potassium Sorbate or Chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A Novel Active Packaging Material Based on Starch-Halloysite Nanocomposites Incorporating Antimicrobial Peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic Starch/Clay Nanocomposites Loaded with Essential Oil Constituents as Packaging for Strawberries—In Vivo Antimicrobial Synergy over Botrytis Cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Ziaee, Z.; Xiao, H.; Zheng, A.; Zhao, Y. Preparation and Properties of Nonleaching Antimicrobial Linear Low-Density Polyethylene Films. Ind. Eng. Chem. Res. 2015, 54, 1824–1831. [Google Scholar] [CrossRef]
- Barzegar, H.; Azizi, M.H.; Barzegar, M.; Hamidi-Esfahani, Z. Effect of Potassium Sorbate on Antimicrobial and Physical Properties of Starch-Clay Nanocomposite Films. Carbohydr. Polym. 2014, 110, 26–31. [Google Scholar] [CrossRef]
- Romainor, A.N.; Chin, S.F.; Lihan, S. Antimicrobial Starch-Based Film for Food Packaging Application. Starch-Stärke 2022, 74, 2100207. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Aider, M. Chitosan Application for Active Bio-Based Films Production and Potential in the Food Industry: Review. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Versatile Use of Chitosan and Hyaluronan in Medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.; Seydim, A.C. Development of Chitosan and Polycaprolactone Based Active Bilayer Films Enhanced with Nanocellulose and Grape Seed Extract. Carbohydr. Polym. 2018, 195, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The Effect of the Layer-by-Layer (LBL) Edible Coating on Strawberry Quality and Metabolites during Storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of Chitosan-TiO2 Composite Film with Efficient Antimicrobial Activities under Visible Light for Food Packaging Applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of Silver/Titanium Dioxide/Chitosan Adipate Nanocomposite as an Antibacterial Coating for Fruit Storage. LWT-Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Desoky, W.M.; Hussein, E.M.; El-Shaer, N.H.; Gomaa, M.; Gamal, A.A.; Esawy, M.A.; Guirguis, O.W. Biological Applications Study of Bio-Nanocomposites Based on Chitosan/TiO2 Nanoparticles Polymeric Films Modified by Oleic Acid. J. Biomed. Mater. Res. A 2021, 109, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of Red Apple Pomace Extract/Chitosan-Based Films Reinforced by TiO2 Nanoparticles as a Multifunctional Packaging Material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and Antibacterial Properties of Novel High Performance Chitosan/Nanocomposite Films. Int. J. Biol. Macromol. 2015, 76, 25–32. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-Zinc Oxide Nanoparticle Composite Coating for Active Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of Chitosan/Zinc Oxide/Melissa Officinalis Essential Oil Nano-Composite Film and Evaluation of Physical, Mechanical and Antimicrobial Properties by Response Surface Method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial Biodegradable Chitosan-Based Composite Nano-Layers for Food Packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, H.; Afraz, S.; Moradi, M.; Hassanpour, S. Antimicrobial Chitosan-Agarose Full Polysaccharide Silver Nanocomposite Films. Int. J. Biol. Macromol. 2021, 179, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and Characterization of Antioxidant, Antimicrobial and PH-Sensitive Films Based on Chitosan, Silver Nanoparticles and Purple Corn Extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of Chitosan/Essential Oils/Silver Nanoparticles Composite Films Packaging and Gamma Irradiation on Shelf Life of Strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer Blend of PLA/PHBV Based Bionanocomposites Reinforced with Nanocrystalline Cellulose for Potential Application as Packaging Material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Maresca, D.; Mauriello, G. Development of Antimicrobial Cellulose Nanofiber-Based Films Activated with Nisin for Food Packaging Applications. Foods 2022, 11, 3051. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.A.K.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–35. [Google Scholar] [CrossRef]
- Srikandace, Y.; Indrarti, L.; Indriyati; Sancoyorini, M.K. Antibacterial Activity of Bacterial Cellulose-Based Edible Film Incorporated with Citrus Spp Essential Oil. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012004. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated Cellulose Addition Improved the Physicochemical and Bioactive Properties of Biodegradable Films Based on Soy Protein and Clove Essential Oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wu, M.; Ma, J.; Lu, P. Bio-Based Antimicrobial Packaging from Sugarcane Bagasse Nanocellulose/Nisin Hybrid Films. Int. J. Biol. Macromol. 2020, 161, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.A.; Fadel, S.M.; Hassan, M.L. Influence of TEMPO-Oxidized NFC on the Mechanical, Barrier Properties and Nisin Release of Hydroxypropyl Methylcellulose Bioactive Films. Int. J. Biol. Macromol. 2018, 113, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, C.; Musatti, A.; Barbiroli, A.; Saini, S.; Bras, J.; Cavicchioli, D.; Rollini, M. Cellulose Nanofiber (CNF)–Sakacin-A Active Material: Production, Characterization and Application in Storage Trials of Smoked Salmon. J. Sci. Food Agric. 2019, 99, 4731–4738. [Google Scholar] [CrossRef]
- Kalajahi, S.E.M.; Alizadeh, A.; Hamishehkar, H.; Almasi, H.; Asefi, N. Orange Juice Processing Waste as a Biopolymer Base For Biodegradable Film Formation Reinforced With Cellulose Nanofiber and Activated With Nettle Essential Oil. Int. J. Polym. Sci. 2021, 30, 258–269. [Google Scholar]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of PH Indicator and Antimicrobial Cellulose Nanofibre Packaging Film Based on Purple Sweet Potato Anthocyanin and Oregano Essential Oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S. Preparation and Characterization of Whey Protein Isolate/Polydextrose-Based Nanocomposite Film Incorporated with Cellulose Nanofiber and L. Plantarum: A New Probiotic Active Packaging System. Lebensm.-Wiss. Ie Technol. 2020, 121, 108978. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and Characterization of Carboxymethyl Cellulose Based Probiotic Nanocomposite Film Containing Cellulose Nanofiber and Inulin for Chicken Fillet Shelf Life Extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.-W.; Kim, J. Cellulose Nanofiber-Based Nanocomposite Films Reinforced with Zinc Oxide Nanorods and Grapefruit Seed Extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Jude, S.; Thomas, S.; Guo, Q. Bionanocomposite Films Based on Potato, Tapioca Starch and Chitosan Reinforced with Cellulose Nanofiber Isolated from Turmeric Spent. J. Taiwan Inst. Chem. Eng. 2019, 96, 664–671. [Google Scholar] [CrossRef]
- Mohammadi sadati, S.M.; Shahgholian-Ghahfarrokhi, N.; Shahrousvand, E.; Mohammadi-Rovshandeh, J.; Shahrousvand, M. Edible Chitosan/Cellulose Nanofiber Nanocomposite Films for Potential Use as Food Packaging. Mater. Technol. 2022, 37, 1276–1288. [Google Scholar] [CrossRef]
- Gharoy Ahangar, E.; Abbaspour-Fard, M.H.; Shahtahmassebi, N.; Khojastehpour, M.; Maddahi, P. Preparation and Characterization of PVA/ZnO Nanocomposite. J. Food Process. Preserv. 2015, 39, 1442–1451. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Antimicrobial and Physical-Mechanical Properties of Agar-Based Films Incorporated with Grapefruit Seed Extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A Bioadsorbent for Chemical Contaminant Remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.-M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial Cellulose Films with ZnO Nanoparticles and Propolis Extracts: Synergistic Antimicrobial Effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef] [PubMed]
- Tarabiah, A.; Alhadlaq, H.A.; Alaizeri, Z.M.; Ahmed, A.A.; Asnag, G.M.; Ahamed, M. Enhanced Structural, Optical, Electrical Properties and Antibacterial Activity of PEO/CMC Doped ZnO Nanorods for Energy Storage and Food Packaging Application. Int. J. Polym. Sci. 2022, 29, 167. [Google Scholar] [CrossRef]
- Su, C.H.; Velusamy, P.; Kumar, G.V.; Adhikary, S.; Pandian, K.; Anbu, P. Studies of Antibacterial Efficacy of Different Biopolymer Protected Silver Nanoparticles Synthesized under Reflux Condition. J. Mol. Struct. 2017, 1128, 718–723. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial Activity of Alginate/Clay Nanocomposite Films Enriched with Essential Oils against Three Common Foodborne Pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Hosseini, S.M.H.; Sepidname, M.; Negahdarifar, M.; Li, P. Development of a Novel Colorimetric Sensor Based on Alginate Beads for Monitoring Rainbow Trout Spoilage. J. Food Sci. Technol. 2018, 55, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of Edible Films and Coatings from Alginates and Carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Galus, S. Development of Edible Coatings in the Preservation of Fruits and Vegetables. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 377–390. ISBN 978-3-030-19416-1. [Google Scholar]
- Sanchez-García, M.D. Carrageenan Polysaccharides for Food Packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 594–609. ISBN 978-1-84569-738-9. [Google Scholar]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined Effects of Chemical Dip and/or Carrageenan Coating and/or Controlled Atmosphere on Quality of Fresh-Cut Banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending Shelf-Life of Minimally Processed Apples with Edible Coatings and Antibrowning Agents. LWT-Food Sci. Technol. 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef]
- Sogut, E.; Ili Balqis, A.M.; Nur Hanani, Z.A.; Seydim, A.C. The Properties of κ-Carrageenan and Whey Protein Isolate Blended Films Containing Pomegranate Seed Oil. Polym. Test. 2019, 77, 105886. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Lajevardi, A.; Rahimi, T.; Tanzifi, M.; Ghorbanpour, M. An Investigation of the Role of Fabrication Process in the Physicochemical Properties of κ-Carrageenan-Based Films Incorporated with Zataria Multiflora Extract and Nanoclay. Food Packag. Shelf Life 2020, 23, 100435. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-Carrageenan Films Incorporated Plant Essential Oils with Improved Antimicrobial Activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA Films from Environment Friendly Additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Bayraktar, I.; Doganay, D.; Coskun, S.; Kaynak, C.; Akca, G.; Unalan, H.E. 3D Printed Antibacterial Silver Nanowire/Polylactide Nanocomposites. Compos. Part B Eng. 2019, 172, 671–678. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Saharudin, N.I.; Olaiya, N.G.; Khalil, H.P.S.A.; Haafiz, M.K.M.; Ikramullah, I.; Muksin, U.; Olaiya, F.G.; Abdullah, C.K.; Yahya, E.B. Functional Properties and Molecular Degradation of Schizostachyum Brachycladum Bamboo Cellulose Nanofibre in PLA-Chitosan Bionanocomposites. Molecules 2021, 26, 2008. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; P, H.; Saha, S. Efficient and Prolonged Antibacterial Activity from Porous PLGA Microparticles and Their Application in Food Preservation. Mater. Sci. Eng. C 2020, 108, 110496. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, Characterization and Antimicrobial Properties of Electrospun Polylactide Films Containing Allium ursinum L. Extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Granata, G.; Riccobene, C.; Napoli, E.; Geraci, C. Polymeric Nanocapsules Containing Fennel Essential Oil: Their Preparation, Physicochemical Characterization, Stability over Time and in Simulated Gastrointestinal Conditions. Pharmaceutics 2022, 14, 873. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Mosnáčková, K.; Musioł, M.; Opálek, A.; Bučková, M.; Rychter, P.; Eckstein Andicsová, A. Electrospun Nisin-Loaded Poly(ε-Caprolactone)-Based Active Food Packaging. Materials 2022, 15, 4540. [Google Scholar] [CrossRef]
- Delamarche, E.; Mattlet, A.; Livi, S.; Gérard, J.-F.; Bayard, R.; Massardier, V. Tailoring Biodegradability of Poly(Butylene Succinate)/Poly(Lactic Acid) Blends With a Deep Eutectic Solvent. Front. Mater. 2020, 7, 7. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Lanč, M.; Kludský, M.; Velas, J.; Hrdlička, Z.; Michalcová, A.; Dendisová, M.; Friess, K. Poly(Butylene Succinate)-Cellulose Triacetate Blends: Permeation, Pervaporation, Sorption and Physical Structure. Polym. Test. 2018, 65, 468–479. [Google Scholar] [CrossRef]
- Lai, S.-M.; Kao, Y.-H.; Liu, Y.-K.; Chiu, F.-C. Preparation and Properties of Luffa Fiber- and Kenaf Fiber-Filled Poly(Butylene Succinate-Co-Lactate)/Starch Blend-Based Biocomposites. Polym. Test. 2016, 50, 191–199. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial Food Packaging Prepared from Poly(Butylene Succinate) and Zinc Oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Veranitisagul, C.; Wattanathana, W.; Wannapaiboon, S.; Hanlumyuang, Y.; Sukthavorn, K.; Nootsuwan, N.; Chotiwan, S.; Phuthong, W.; Jongrungruangchok, S.; Laobuthee, A. Antimicrobial, Conductive, and Mechanical Properties of AgCB/PBS Composite System. J. Chem. 2019, 2019, e3487529. [Google Scholar] [CrossRef]
- de Souza, A.G.; Komatsu, L.G.H.; Barbosa, R.F.S.; Parra, D.F.; Rosa, D.S. The Effect of ZnO Nanoparticles as Ag-Carrier in PBAT for Antimicrobial Films. Polym. Bull. 2022, 79, 4031–4048. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable Polymers with a Range of Applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chun Chen, J.; Chen, G.Q. Polyhydroxyalkanoate (PHA) Scaffolds with Good Mechanical Properties and Biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, Y.; Wang, X.-L.; Wang, Y.-Z. Biodegradation Behavior of PHBV Films in a Pilot-Scale Composting Condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Ha, C.-S.; Cho, W.-J. Miscibility, Properties, and Biodegradability of Microbial Polyester Containing Blends. Prog. Polym. Sci. 2002, 4, 759–809. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The Impact of Zinc Oxide Particle Morphology as an Antimicrobial and When Incorporated in Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Films for Food Packaging and Food Contact Surfaces Applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kržan, A.; Žagar, E. Degradation of PLA/ZnO and PHBV/ZnO Composites Prepared by Melt Processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Oves, M.; Alosaimi, A.M.; Hussein, M.A. A Promising Antimicrobial Bionanocomposite Based Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Reinforced Silver Doped Zinc Oxide Nanoparticles. Sci. Rep. 2022, 12, 14299. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) with Zinc Oxide Nanoparticles for Food Packaging. J. Food Process Eng. 2022, 45, e13814. [Google Scholar] [CrossRef]
- Chen, J.; Wei, D.; Gong, W.; Zheng, A.; Guan, Y. Hydrogen-Bond Assembly of Poly(Vinyl Alcohol) and Polyhexamethylene Guanidine for Nonleaching and Transparent Antimicrobial Films. ACS Appl. Mater. Interfaces 2018, 10, 37535–37543. [Google Scholar] [CrossRef] [PubMed]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of Miscible PVA/PEG Blends and Effect of Graphene Concentration on Thermal, Crystallization, Morphological, and Mechanical Properties of PVA/PEG (10 wt%) Blend. Int. J. Polym. Sci. 2018, 2018, e8527693. [Google Scholar] [CrossRef]
- Galya, T.; Sedlařík, V.; Kuřitka, I.; Novotný, R.; Sedlaříková, J.; Sáha, P. Antibacterial Poly(Vinyl Alcohol) Film Containing Silver Nanoparticles: Preparation and Characterization. J. Appl. Polym. Sci. 2008, 110, 3178–3185. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L. Physical and Antibacterial Properties of Polyvinyl Alcohol Films Reinforced with Quaternized Cellulose. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial Films Based on PVA and PVA–Chitosan Modified with Poly(Hexamethylene Guanidine). Polymers 2019, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Morales, J.; Amariei, G.; Letón, P.; Rosal, R. Antimicrobial Activity of Poly(Vinyl Alcohol)-Poly(Acrylic Acid) Electrospun Nanofibers. Colloids Surf. B Biointerfaces 2016, 146, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.; Ullah, S.; Kim, I.S. Electrospun Momordica Charantia Incorporated Polyvinyl Alcohol (PVA) Nanofibers for Antibacterial Applications. Mater. Today Commun. 2020, 24, 101161. [Google Scholar] [CrossRef]
- da Silva, F.T.; da Cunha, K.F.; Fonseca, L.M.; Antunes, M.D.; Halal, S.L.M.E.; Fiorentini, Â.M.; Zavareze, E.d.R.; Dias, A.R.G. Action of Ginger Essential Oil (Zingiber Officinale) Encapsulated in Proteins Ultrafine Fibers on the Antimicrobial Control in Situ. Int. J. Biol. Macromol. 2018, 118, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.G.-G.; Almasi, H. Physical Characteristics, Release Properties, and Antioxidant and Antimicrobial Activities of Whey Protein Isolate Films Incorporated with Thyme (Thymus Vulgaris L.) Extract-Loaded Nanoliposomes. Food Bioprocess Technol. 2018, 11, 1552–1565. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol Loaded Electrospun Fibrous Films from Zein and Poly(Lactic Acid) for Active Food Packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef]
- Kim, H.; Beak, S.-E.; Song, K.B. Development of a Hagfish Skin Gelatin Film Containing Cinnamon Bark Essential Oil. LWT 2018, 96, 583–588. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Ojagh, S.M.; Fooladi, A.A.I.; Shabanpour, B.; Gharahei, M. Effects of Probiotic Cells on the Mechanical and Antibacterial Properties of Sodium-Caseinate Films. Appl. Food Biotechnol. 2018, 5, 155–162. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yılmaz, B.A.; Sargin, I. Effect of Different Animal Fat and Plant Oil Additives on Physicochemical, Mechanical, Antimicrobial and Antioxidant Properties of Chitosan Films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Tonyali, B.; McDaniel, A.; Amamcharla, J.; Trinetta, V.; Yucel, U. Release Kinetics of Cinnamaldehyde, Eugenol, and Thymol from Sustainable and Biodegradable Active Packaging Films. Food Packag. Shelf Life 2020, 24, 100484. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, G.; Long, Z.; Xiao, H.; Qian, L. Bio-Wax Latex-Modified Paper as Antimicrobial and Water-Vapor-Resistant Packaging Material. J. Wood Chem. Technol. 2016, 36, 182–191. [Google Scholar] [CrossRef]
- Strategies to Mitigate Cross Contamination of Non-Critical Medical Devices; APIC: Arlington, TX, USA, 2021; Available online: https://apic.org/noncritical-is-critical (accessed on 8 March 2023).
- Ishihama, H.; Ishii, K.; Nagai, S.; Kakinuma, H.; Sasaki, A.; Yoshioka, K.; Kuramoto, T.; Shiono, Y.; Funao, H.; Isogai, N.; et al. An Antibacterial Coated Polymer Prevents Biofilm Formation and Implant-Associated Infection. Sci. Rep. 2021, 11, 3602. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef]
- Perrault, D.P.; Sharma, A.; Kim, J.F.; Gurtner, G.C.; Wan, D.C. Surgical Applications of Materials Engineered with Antimicrobial Properties. Bioengineering 2022, 9, 138. [Google Scholar] [CrossRef]
- Antimicrobial Polymeric Composites for High-Touch Surfaces in Healthcare Applications|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2468451122000289?token=7E030662074CF75BBDC45393F6136A73E16A068DCA42ABAEF805B03BF8D4565CE0E451657AD39912EC426327D6EFE08F&originRegion=eu-west-1&originCreation=20230329140545 (accessed on 29 March 2023).
- Eltorai, A.E.; Haglin, J.; Perera, S.; Brea, B.A.; Ruttiman, R.; Garcia, D.R.; Born, C.T.; Daniels, A.H. Antimicrobial Technology in Orthopedic and Spinal Implants. World J. Orthop. 2016, 7, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Recent Advances on Antimicrobial Wound Dressing—A Review|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0939641117315023?token=4066831EE25B0FBA600937AF35D45D4E78ADC350A1682622E5774E49F18A3BD4C97BA25E0E5F8CA2D0207D789C57509E&originRegion=eu-west-1&originCreation=20230329145140 (accessed on 29 March 2023).
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; You, L.; Tarafder, S.; Zou, L.; Fang, Z.; Chen, J.; Lee, C.H.; Zhang, Q. Curcumin-Releasing Chitosan/Aloe Membrane for Skin Regeneration. Chem. Eng. J. 2019, 359, 1111–1119. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Wang, E.; Li, T.; Chang, J.; Wu, C. Bioinspired Multifunctional Biomaterials with Hierarchical Microstructure for Wound Dressing. Acta Biomater. 2019, 100, 270–279. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In Vivo Evaluation of Curcumin Nanoformulation Loaded Methoxy Poly(Ethylene Glycol)-Graft-Chitosan Composite Film for Wound Healing Application. Carbohydr. Polym. 2012, 1, 84–90. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-I. Characterization and Antibacterial Properties of Genipin-Crosslinked Chitosan/Poly(Ethylene Glycol)/ZnO/Ag Nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric Oxide-Releasing Chitosan Film for Enhanced Antibacterial and in Vivo Wound-Healing Efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-Inspired Adhesive Antioxidant Antibacterial Hemostatic Composite Hydrogel Wound Dressing via Photo-Polymerization for Infected Skin Wound Healing. Bioact. Mater. 2021, 8, 341–354. [Google Scholar] [CrossRef]
- Yin, M.; Wan, S.; Ren, X.; Chu, C.-C. Development of Inherently Antibacterial, Biodegradable, and Biologically Active Chitosan/Pseudo-Protein Hybrid Hydrogels as Biofunctional Wound Dressings. ACS Appl. Mater. Interfaces 2021, 13, 14688–14699. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via PH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Qiu, L.; Cai, T.; Jiang, C.; Sun, Y.; Li, Y.; Peng, H.; Xiong, H. Bacteria-Triggered Hyaluronan/AgNPs/Gentamicin Nanocarrier for Synergistic Bacteria Disinfection and Wound Healing Application. Chem. Eng. J. 2020, 380, 122582. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small Weinh. Bergstr. Ger. 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Noninvasive Rapid Bacteria-Killing and Acceleration of Wound Healing through Photothermal/Photodynamic/Copper Ion Synergistic Action of a Hybrid Hydrogel. Biomater. Sci. 2018, 6, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Zhu, J.; Han, H.; Li, F.; Wang, X.; Yu, J.; Qin, X.; Wu, D. Peptide-Functionalized Amino Acid-Derived Pseudoprotein-Based Hydrogel with Hemorrhage Control and Antibacterial Activity for Wound Healing. Chem. Mater. 2019, 31, 4436–4450. [Google Scholar] [CrossRef]
- Deng, H.; Yu, Z.; Chen, S.; Fei, L.; Sha, Q.; Zhou, N.; Chen, Z.; Xu, C. Facile and Eco-Friendly Fabrication of Polysaccharides-Based Nanocomposite Hydrogel for Photothermal Treatment of Wound Infection. Carbohydr. Polym. 2020, 230, 115565. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-Inspired, Antibacterial, Conductive, Antioxidant, Injectable Composite Hydrogel Wound Dressing to Promote the Regeneration of Infected Skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Synthesis of a Novel Anti-Freezing, Non-Drying Antibacterial Hydrogel Dressing by One-Pot Method. Chem. Eng. J. 2019, 372, 216–225. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Chen, W.; Xu, T.; Yun, S.; Xu, Z.; Xu, Z.; Sato, T.; Chi, B.; Xu, H. A Biomimetic Mussel-Inspired ε-Poly-l-Lysine Hydrogel with Robust Tissue-Anchor and Anti-Infection Capacity. Adv. Funct. Mater. 2017, 27, 1604894. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced Liposome-Loaded Scaffolds for Therapeutic and Tissue Engineering Applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Q.; He, M.; Zhang, X.; Liu, X.; Zhao, C. Antibiofouling Zwitterionic Gradational Membranes with Moisture Retention Capability and Sustained Antimicrobial Property for Chronic Wound Infection and Skin Regeneration. Biomacromolecules 2019, 20, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-Gelatin Based Nanofibers Loaded with Ciprofloxacin Hydrochloride and Quercetin: A Potential Antibacterial and Anti-Oxidant Dressing Material for Accelerated Healing of a Full Thickness Wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core-Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, e1900328. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Golzar, H.; Hashemi, M.; Mansouri, V.; Omidi, M.; Yazdian, F.; Yadegari, A.; Tayebi, L. Optimizing the Nanostructure of Graphene Oxide/Silver/Arginine for Effective Wound Healing. Nanotechnology 2018, 29, 475101. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Appl. Mater. Interfaces 2020, 12, 3371–3381. [Google Scholar] [CrossRef]
- Nudelman, R.; Alhmoud, H.; Delalat, B.; Fleicher, S.; Fine, E.; Guliakhmedova, T.; Elnathan, R.; Nyska, A.; Voelcker, N.H.; Gozin, M.; et al. Jellyfish-Based Smart Wound Dressing Devices Containing In Situ Synthesized Antibacterial Nanoparticles. Adv. Funct. Mater. 2019, 29, 1902783. [Google Scholar] [CrossRef]
- Liu, B.; Yao, T.; Ren, L.; Zhao, Y.; Yuan, X. Antibacterial PCL Electrospun Membranes Containing Synthetic Polypeptides for Biomedical Purposes. Colloids Surf. B Biointerfaces 2018, 172, 330–337. [Google Scholar] [CrossRef]
- Li, W.; Yu, Q.; Yao, H.; Zhu, Y.; Topham, P.D.; Yue, K.; Ren, L.; Wang, L. Superhydrophobic Hierarchical Fiber/Bead Composite Membranes for Efficient Treatment of Burns. Acta Biomater. 2019, 92, 60–70. [Google Scholar] [CrossRef]
- Wang, X.; Lv, F.; Li, T.; Han, Y.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Electrospun Micropatterned Nanocomposites Incorporated with Cu2S Nanoflowers for Skin Tumor Therapy and Wound Healing. ACS Nano 2017, 11, 11337–11349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable Antibacterial Conductive Nanocomposite Cryogels with Rapid Shape Recovery for Noncompressible Hemorrhage and Wound Healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef] [PubMed]
- Cho Lee, A.-R.; Leem, H.; Lee, J.; Park, K.C. Reversal of Silver Sulfadiazine-Impaired Wound Healing by Epidermal Growth Factor. Biomaterials 2005, 26, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Ma, W.; Li, L.; Lin, X.; Wang, Y.; Ren, X.; Huang, T.-S. Novel ZnO/N-Halamine-Mediated Multifunctional Dressings as Quick Antibacterial Agent for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 31411–31420. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, H.; Ma, X.; Hong, H.; Yuan, Y.; Liu, C. Bioinspired, Injectable, Quaternized Hydroxyethyl Cellulose Composite Hydrogel Coordinated by Mesocellular Silica Foam for Rapid, Noncompressible Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 34595–34608. [Google Scholar] [CrossRef]
- Gao, H.; Zhong, Z.; Xia, H.; Hu, Q.; Ye, Q.; Wang, Y.; Chen, L.; Du, Y.; Shi, X.; Zhang, L. Construction of Cellulose Nanofibers/Quaternized Chitin/Organic Rectorite Composites and Their Application as Wound Dressing Materials. Biomater. Sci. 2019, 7, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Sanad, R.A.-B.; Abdel-Bar, H.M. Chitosan-Hyaluronic Acid Composite Sponge Scaffold Enriched with Andrographolide-Loaded Lipid Nanoparticles for Enhanced Wound Healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.; Guo, B.; Yin, Z.; Zhu, D.; Han, Y. Injectable Dry Cryogels with Excellent Blood-Sucking Expansion and Blood Clotting to Cease Hemorrhage for Lethal Deep-Wounds, Coagulopathy and Tissue Regeneration. Chem. Eng. J. 2021, 403, 126329. [Google Scholar] [CrossRef]
- Ding, X.; Wang, A.; Tong, W.; Xu, F.-J. Biodegradable Antibacterial Polymeric Nanosystems: A New Hope to Cope with Multidrug-Resistant Bacteria. Small 2019, 15, 1900999. [Google Scholar] [CrossRef]
- Zong, T.-X.; Silveira, A.P.; Morais, J.A.V.; Sampaio, M.C.; Muehlmann, L.A.; Zhang, J.; Jiang, C.-S.; Liu, S.-K. Recent Advances in Antimicrobial Nano-Drug Delivery Systems. Nanomaterials 2022, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Nanosystems-1st Edition. Available online: https://www.elsevier.com/books/antimicrobial-nanosystems/978-0-323-91156-6 (accessed on 30 March 2023).
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Mukherjee, S.; Das, G.; Ramesh, A. Micellar Chemotherapeutic Platform Based on a Bifunctional Salicaldehyde Amphiphile Delivers a “Combo-Effect” for Heightened Killing of MRSA. J. Mater. Chem. B 2018, 6, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial Micelles with Vancomycin-Mediated Targeting and PH/Lipase-Triggered Release of Antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 36814–36823. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management. Adv. Mater. 2018, 30, 1803618. [Google Scholar] [CrossRef]
- ICAM-1-Targeted and Antibacterial Peptide Modified Polymeric Nanoparticles for Specific Combating Sepsis|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0264127522006293?token=F48384B4F354D000144C209E8B848137D20FDB4021368A97F0E8B6B4F97D91E3AF71014CF706ACC285391A47159582E9&originRegion=eu-west-1&originCreation=20230331162510 (accessed on 31 March 2023).
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019, 29, 1806883. [Google Scholar] [CrossRef]
- Casanova, M.; Olleik, H.; Hdiouech, S.; Roblin, C.; Cavalier, J.-F.; Point, V.; Jeannot, K.; Caron, B.; Perrier, J.; Charriau, S.; et al. Evaluation of the Efficiency of Random and Diblock Methacrylate-Based Amphiphilic Cationic Polymers against Major Bacterial Pathogens Associated with Cystic Fibrosis. Antibiotics 2023, 12, 120. [Google Scholar] [CrossRef]
- Sebastian, J.; Mary Samuel, J. Anticancer Potential of Poly(2-Aminobenzoic Acid)-Blend-Aloe Vera against the Human Breast Cancer Cell Line MDA-MB-231. J. Bioact. Compat. Polym. 2023, 38, 58–73. [Google Scholar] [CrossRef]
- Biswas, S.; Datta, L.P.; Das, T.K. A Bioinspired Stimuli-Responsive Amino Acid-Based Antibacterial Drug Delivery System in Cancer Therapy. New J. Chem. 2022, 46, 7024–7031. [Google Scholar] [CrossRef]
- Antimicrobial Plastics Market. Available online: https://www.reportsanddata.com/report-detail/antimicrobial-plastics-market (accessed on 19 February 2023).
- Research and Markets Ltd. Bioresorbable Polymers Market Forecast to 2028-COVID-19 Impact and Global Analysis by Type and Application. Available online: https://www.researchandmarkets.com/reports/5548297/bioresorbable-polymers-market-forecast-to-2028 (accessed on 19 February 2023).
- Present and Future Trends in Biodegradable Polymers. Available online: https://www.plasticstoday.com/biopolymers/present-and-future-trends-biodegradable-polymers (accessed on 19 February 2023).
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]



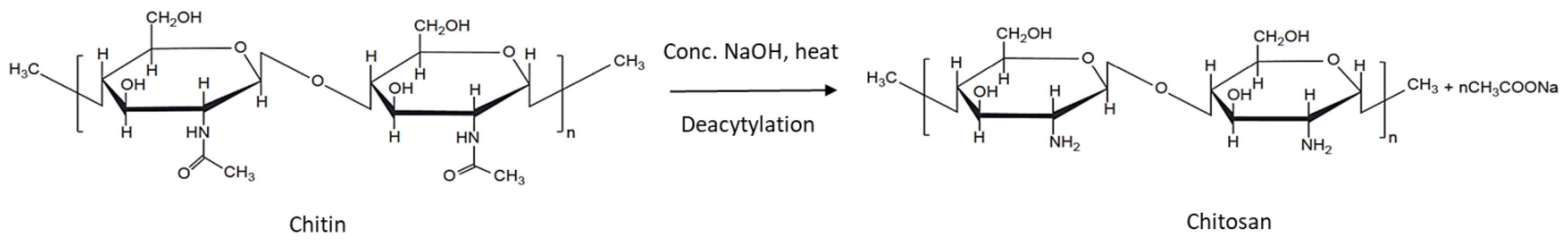

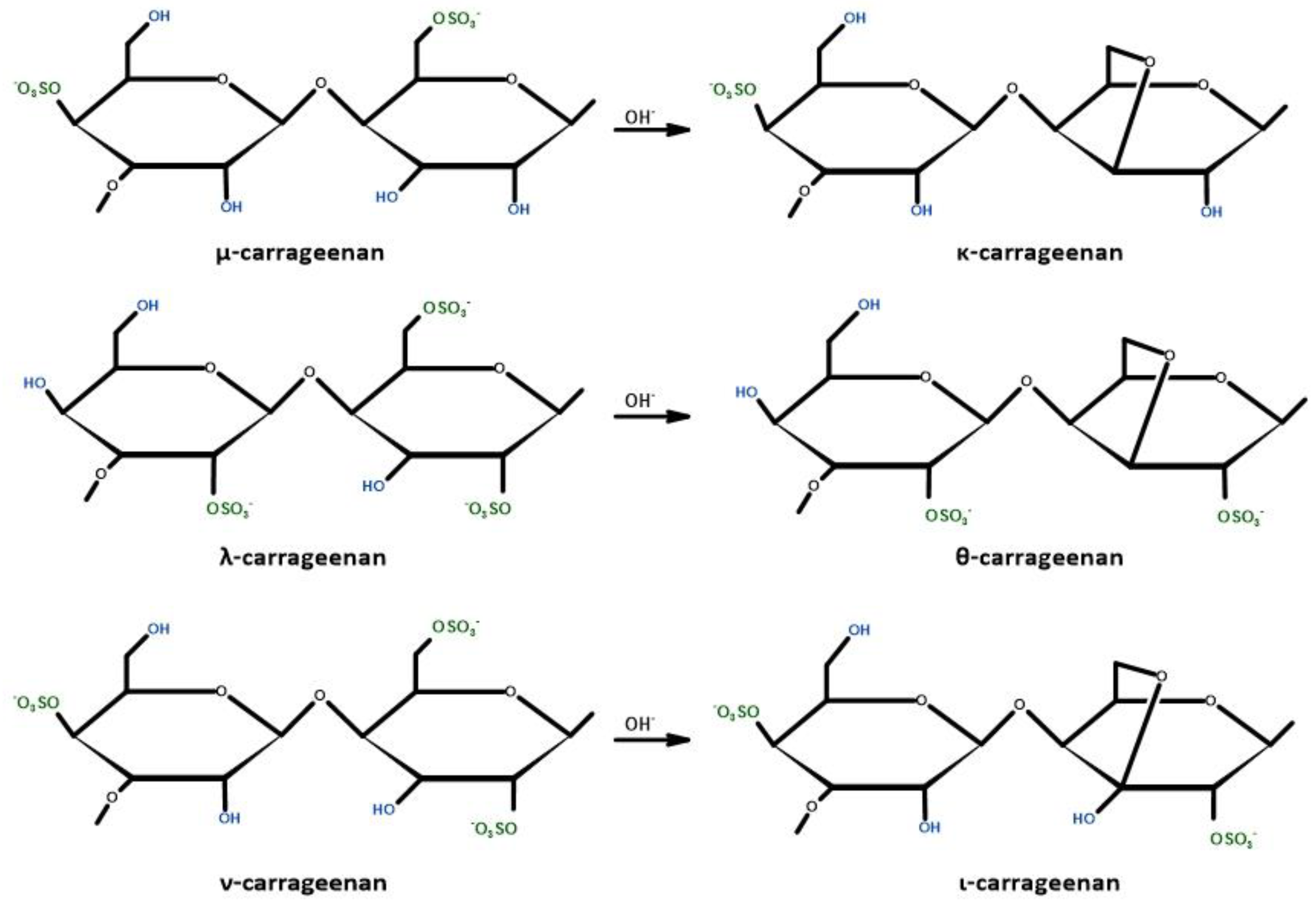

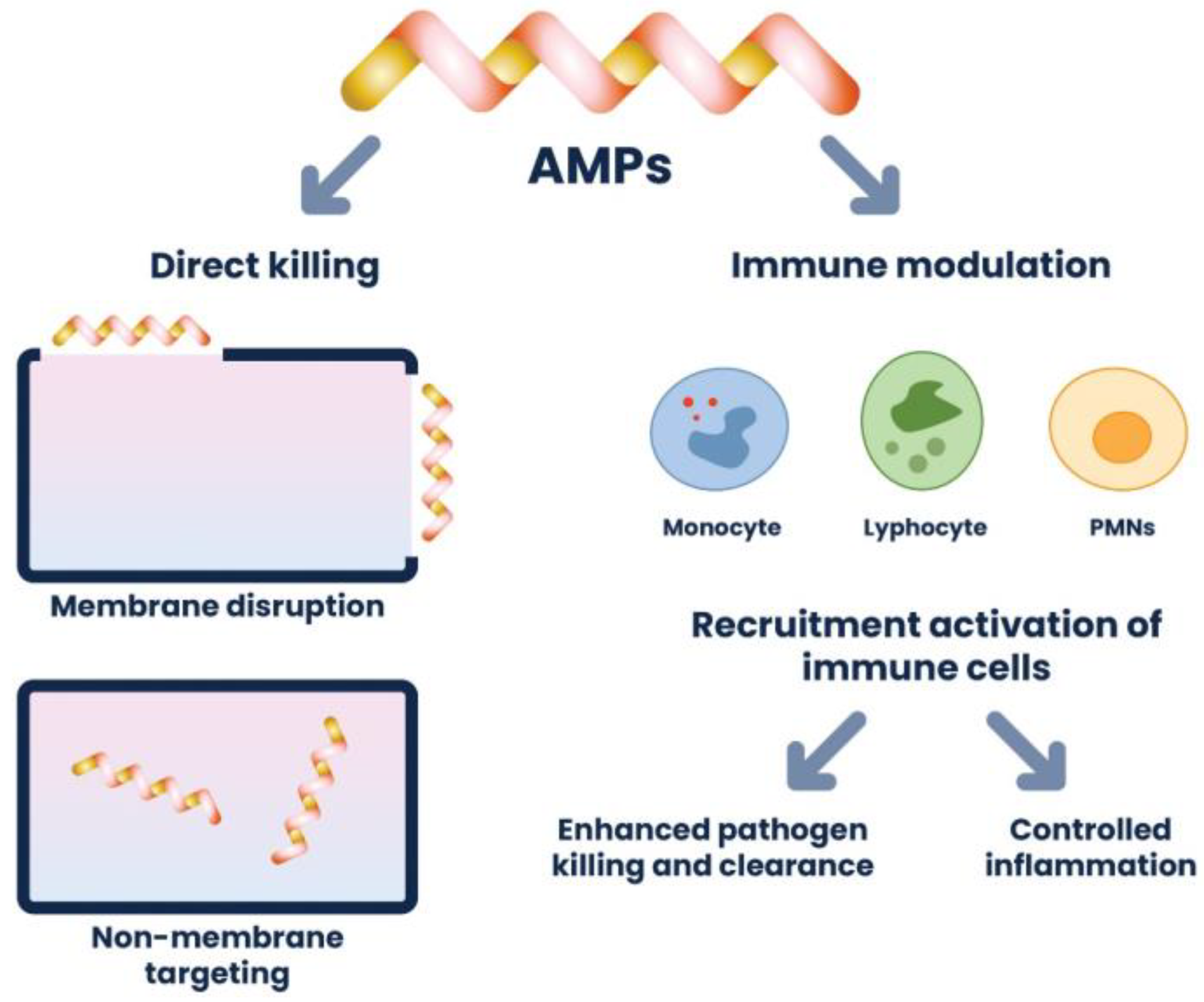
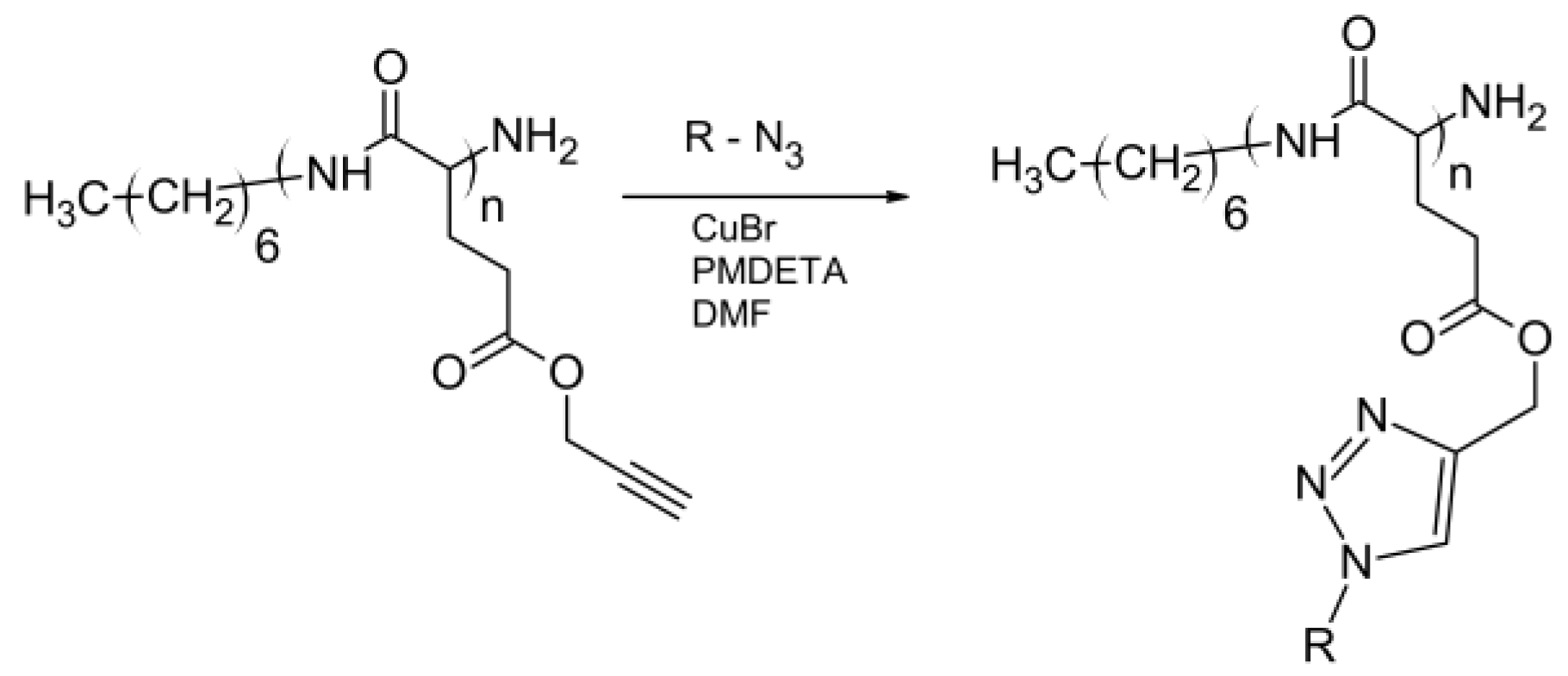


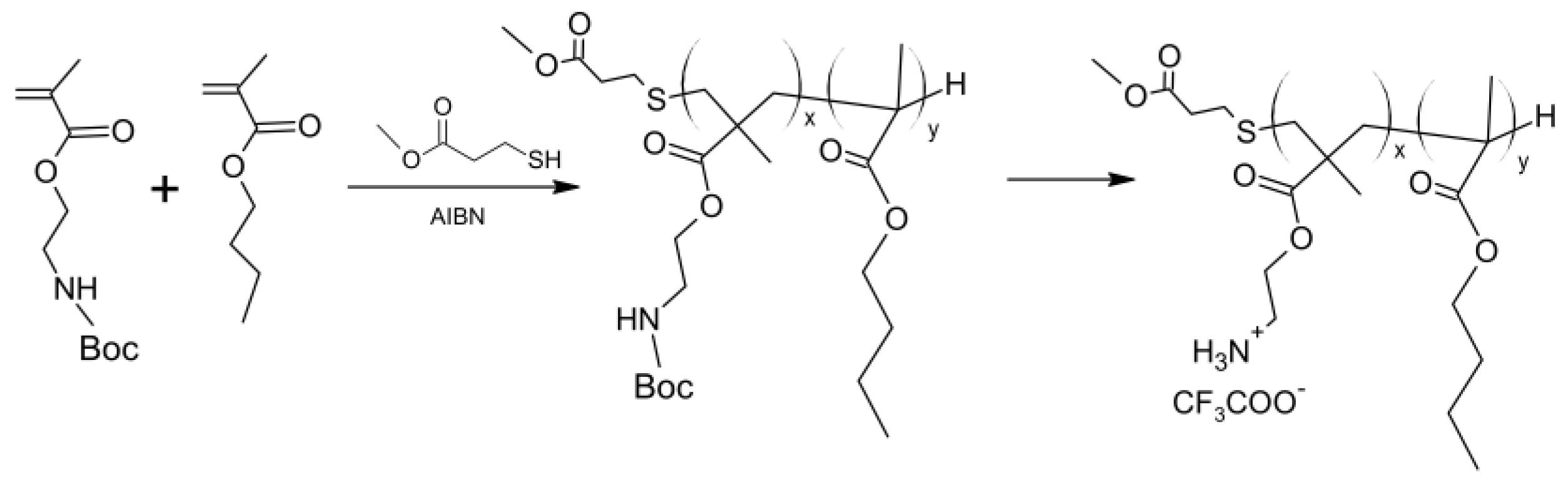
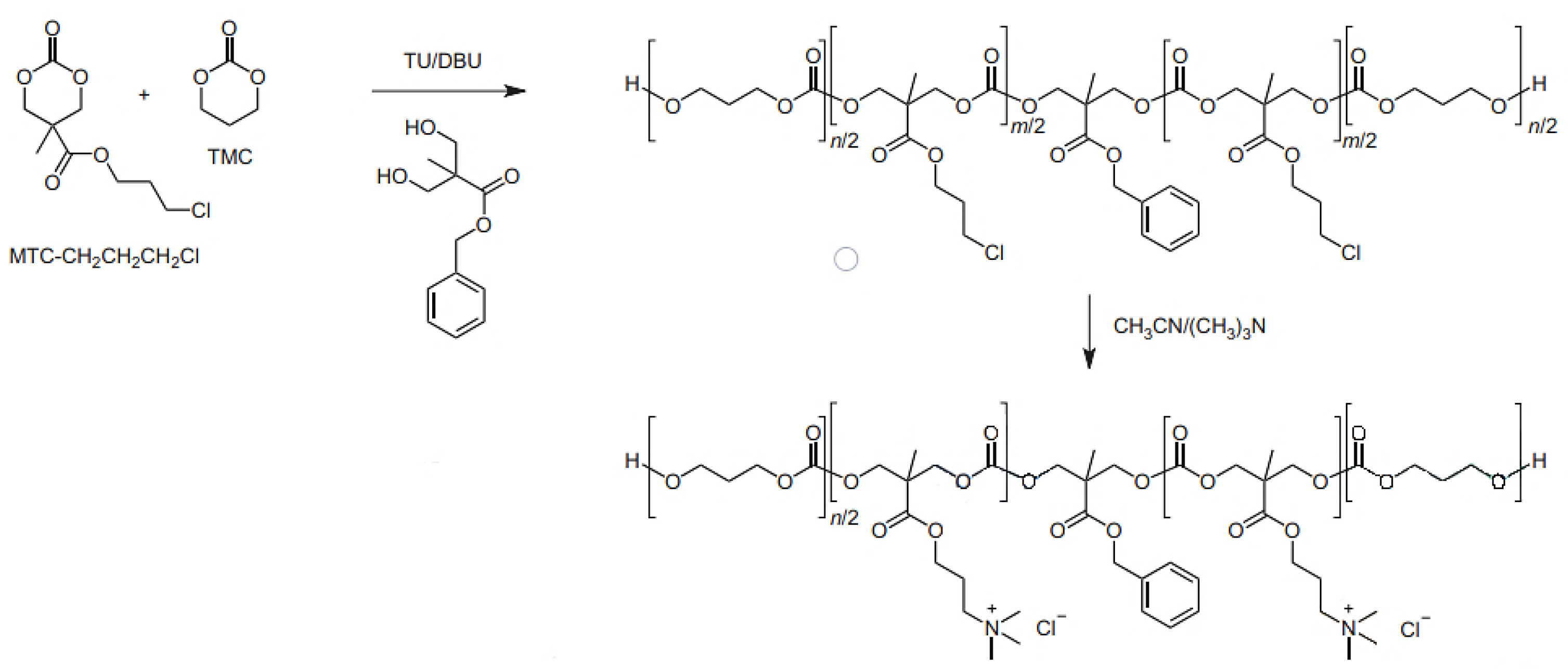
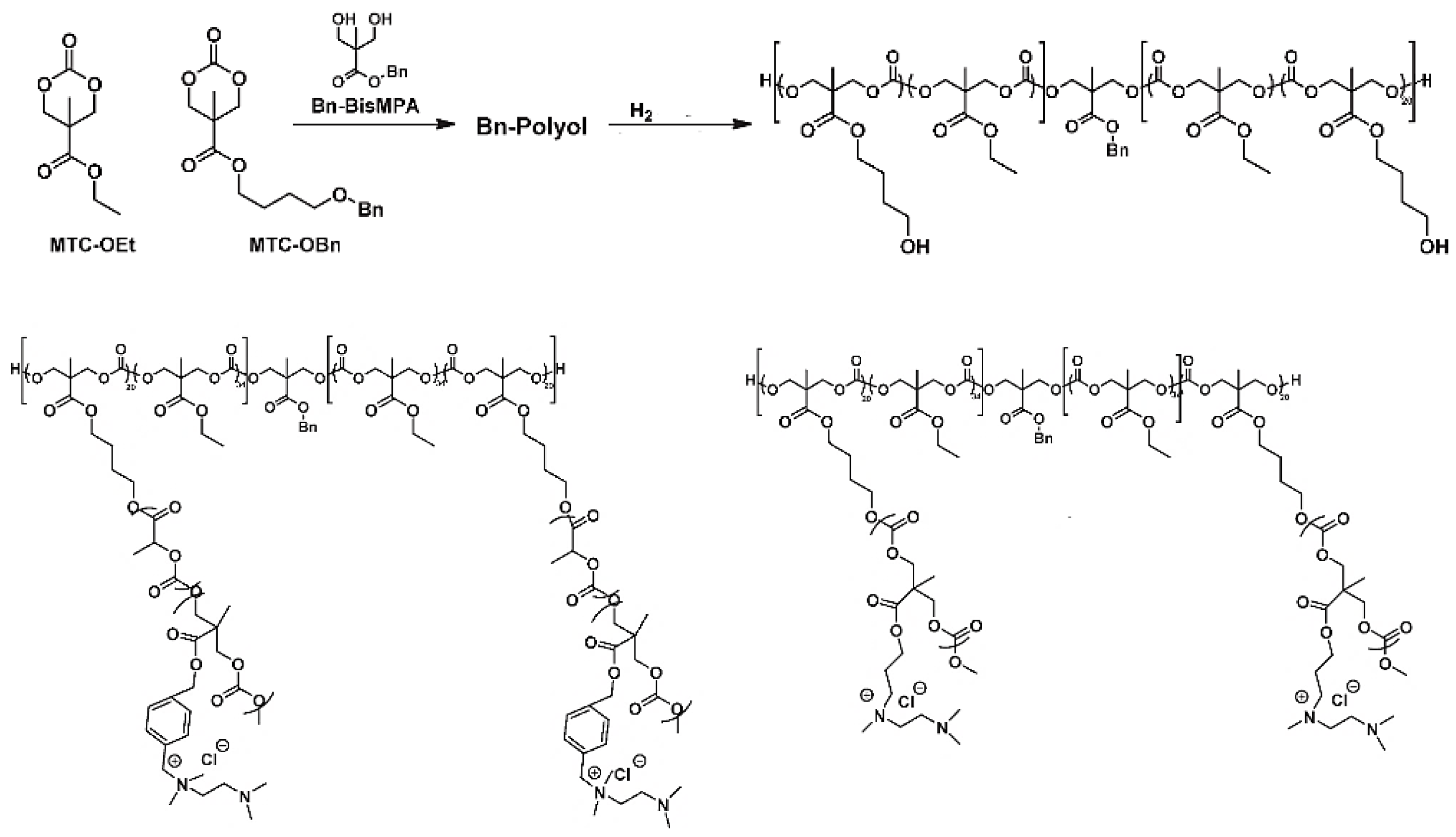

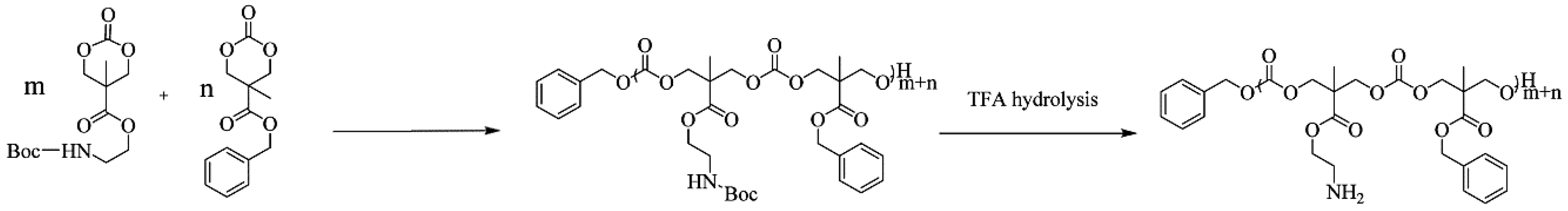
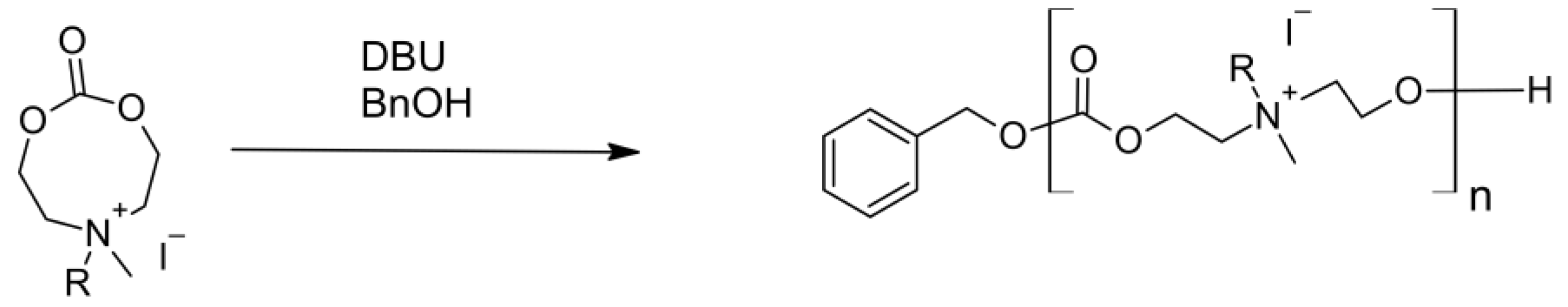
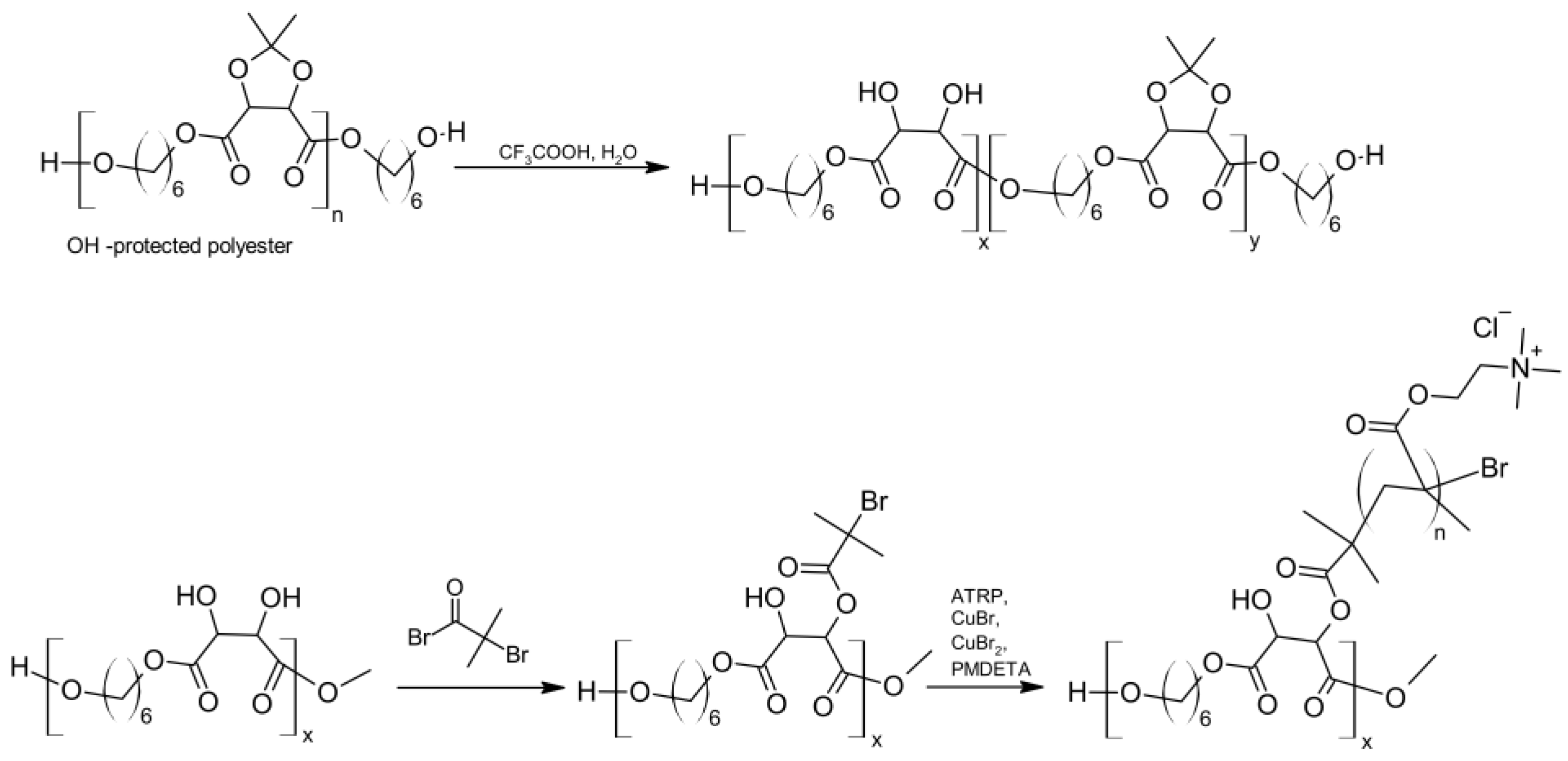



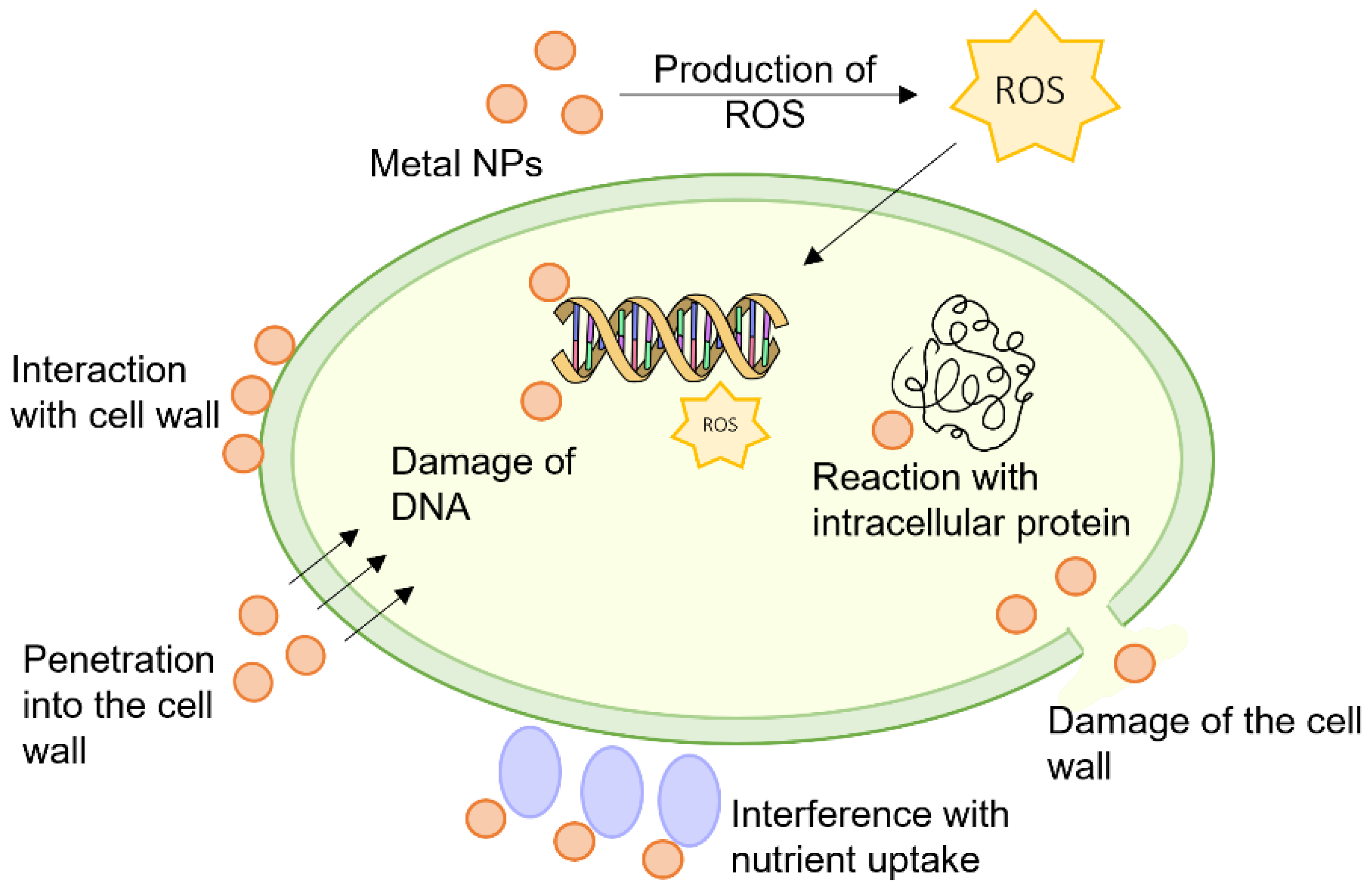
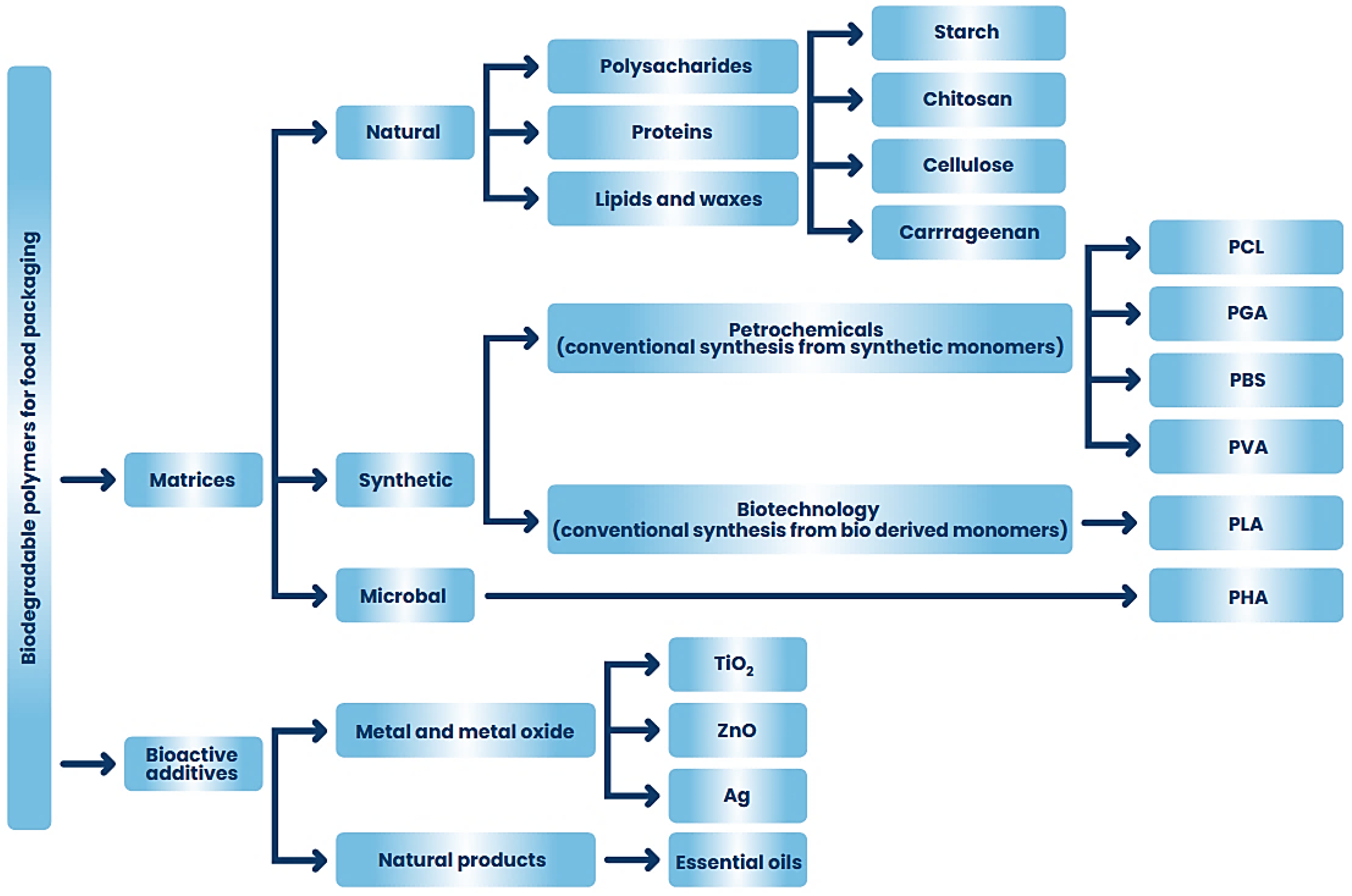
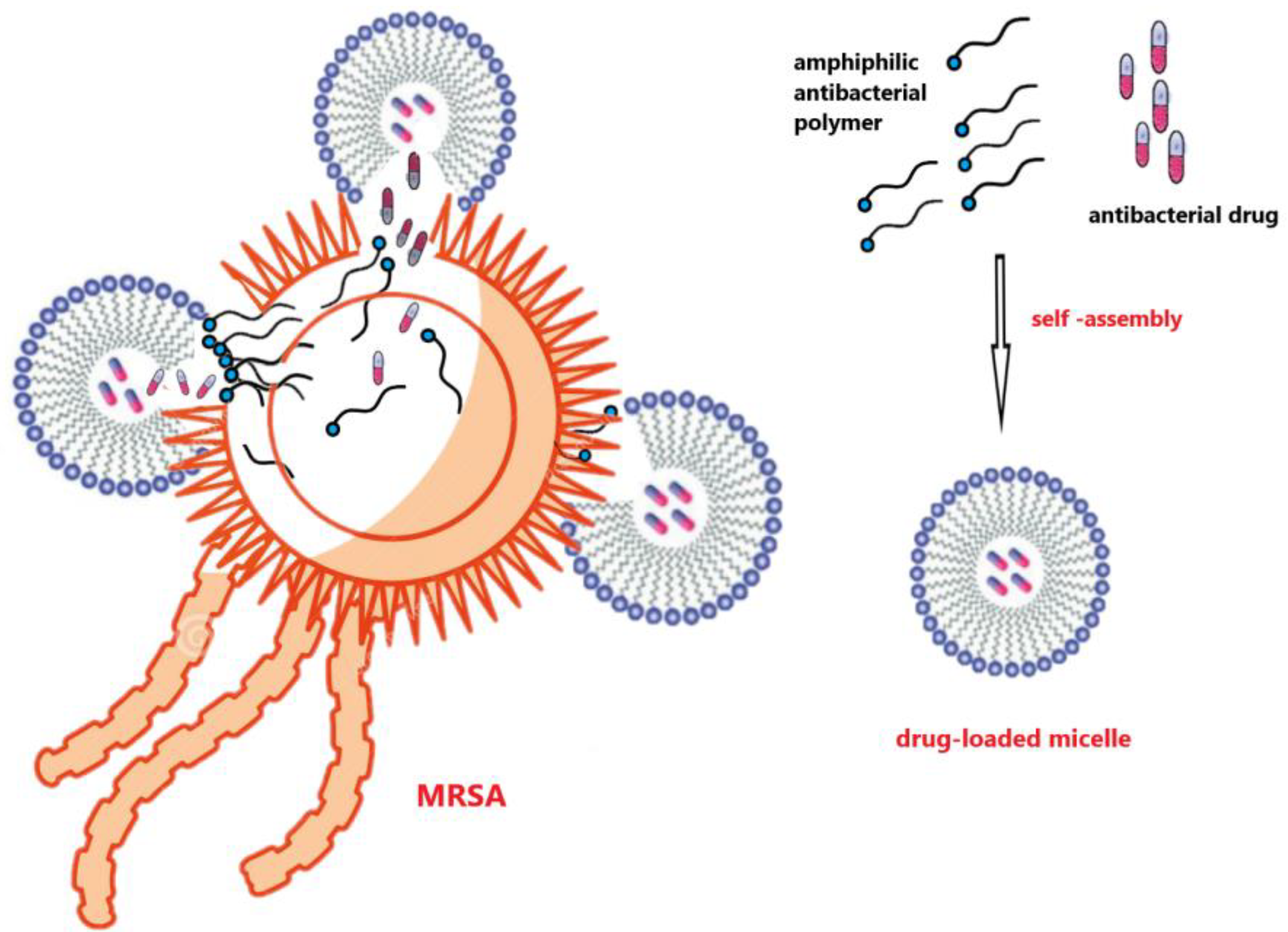
| Materials | Typ of Microorganism | Antimicrobial Activity | Ref. |
|---|---|---|---|
| CS (chitosan) (0.2% w/v) | B. cereus | ZOI = 4 mm | [81] |
| P. aeruginosa | ZOI = 2 mm | ||
| CS (1.5% w/v) | B. cereus | ZOI = 12 mm | |
| P. aeruginosa | ZOI = 10 mm | ||
| CS (Mw = 322.04 kDa) | S. aureus | MIC = 80 μg/mL | [82] |
| E. coli | MIC = 80 μg/mL | ||
| CS (Mw = 41.1 kDa) | S. aureus | MIC = 32 μg/mL | |
| E. coli | MIC = 64 μg/mL | ||
| CS (Mw = 14.3 kDa) | S. aureus | MIC = 32 μg/mL | |
| E. coli | MIC = 32 μg/mL | ||
| CS (Mw = 5.06 kDa) | S. aureus | MIC = 32 μg/mL | |
| E. coli | MIC = 16 μg/mL | ||
| CS (Mw = 110 kDa; DA = 2) | E. coli | ZOI = 12.5 mm; MIC = 0.01% | [74] |
| K. pneumoniae | ZOI = 12.0 mm; MIC = 0.002% | ||
| S. typhi | ZOI = 10.5 mm; MIC = 0.005% | ||
| S. aureus | ZOI = 9.5 mm; MIC = 0.025% | ||
| B. cereus | ZOI = 10.0 mm; MIC = 0.025% | ||
| CS (Mw = 42.5 kDa; DA = 2) | E. coli | ZOI = 12.5 mm; MIC = 0.005% | [74] |
| K. pneumoniae | ZOI = 11.5 mm; MIC = 0.001% | ||
| S. typhi | ZOI = 10.5 mm; MIC = 0.002% | ||
| S. aureus | ZOI = 8.5 mm; MIC = 0.025% | ||
| B. cereus | ZOI = 9.0 mm; MIC = 0.025% | ||
| CS (67 kDa; 5% w/v) | B. cereus | ZOI = 51.5 ± 0.42 mm; MIC = 0.625% | [83] |
| S. aureus | ZOI = 33.4 ± 0.53 mm; MIC = 1.25% | ||
| E. coli | ZOI = 49.7 ± 0.31 mm; MIC = 0.156% | ||
| S. typhi | ZOI = 41.7 ± 0.60 mm; MIC = 0.312% | ||
| P. aeruginosa | ZOI = 50.4 ± 0.71 mm; MIC = 0.312% | ||
| A. niger | ZOI = 53 ± 0.94 mm; MIC = 0.07% | ||
| C. albicans | ZOI = 30.8 ± 0.88 mm; MIC = 1.25% | ||
| CS (0.5% w/v) | E. coli | MIC = 256 μg/mL | [84] |
| S. enteritidis | MIC = 128 μg/mL | ||
| L. monocytogenes | MIC = 128 μg/mL | ||
| S. aureus | MIC = 256 μg/mL | ||
| B. cereus | MIC = 256 μg/mL | ||
| C. albicans | MIC = 64 μg/mL | ||
| QC (quaternized chitosan) | E. coli | ZOI = 12.3 ± 0.1 mm | [85] |
| S. aureus | ZOI = 13.7 ± 0.1 mm | ||
| P. aeruginosa | ZOI = 14.3 ± 0.1 mm | ||
| C. albicans | ZOI = 14.6 ± 0.1 mm | ||
| QC + Ag NPs (0.125% wt%) | E. coli | ZOI = 15.6 ± 0.1 mm | [85] |
| S. aureus | ZOI = 16.3 ± 0.1 mm | ||
| P. aeruginosa | ZOI = 17.7 ± 0.1 mm | ||
| C. albicans | ZOI = 19.6 ± 0.1 mm | ||
| 2,6 DAC (2,6-diamino chitosan) | E. coli | MIC = 16–32 μg/mL | [86] |
| S. aureus | MIC = 16 μg/mL | ||
| P. aeruginosa | MIC = 8 μg/mL | ||
| CMCs (Carboxymethyl chitosan) | B. subtilis | ZOI = 5 mm | [87] |
| S. aureus | ZOI = 7 mm | ||
| S. faecalis | ZOI = 6 mm | ||
| E. coli | ZOI = 8 mm | ||
| P. aeruginosa | ZOI = 7 mm | ||
| TMC(N,N,N-trimethyl chitosan) | E. coli | MIC = 0.125 μg/mL | [88] |
| S. aureus | MIC = 0.0625 μg/mL | ||
| E. facialis | MIC = 128 μg/mL | ||
| P. aeruginosa | MIC = 256 μg/mL | ||
| SCS (Sulfonated chitosan) | E. coli | MIC = 0.13 μg/mL | [89] |
| S. aureus | MIC = 2.00 μg/mL | ||
| A. sacchari | MIC = 64 μg/mL | ||
| B. cinerea | MIC = 0.25 μg/mL | ||
| Phosphorylated chitosan (concentration 100%) | V. cholerae | good activity (11–15 mm dia) | [90] |
| K. pneumoniae | weak activity (7–10 mm dia) | ||
| Salmonella sp. | weak activity (7–10 mm dia) | ||
| S. aureus | very good activity (above 16 mm dia) | ||
| V. alginolyticus | weak activity (7–10 mm dia) | ||
| V. parahemolyticus | good activity (11–15 mm dia) | ||
| P. vugaris | weak activity (7–10 mm dia) | ||
| CS | E. coli | ZOI = 24 ± 0.63 mm | [91] |
| K. pneumoniae | ZOI = 26 ± 0.73 mm | ||
| S. aureus | means not detected | ||
| S. mutans | means not detected | ||
| C. albicans | ZOI = 26 ± 0.79 mm | ||
| A. fumigatus | ZOI = 16 ± 0.83 mm | ||
| CSSBs (Chitosan Schiff bases) with 2-chloroquinoline-3-carbaldehyde | E. coli | ZOI = 22 ± 0.73 mm | [91] |
| K. pneumoniae | ZOI = 22 ± 0.73 mm | ||
| S. aureus | ZOI = 22 ± 0.30 mm | ||
| S. mutans | ZOI = 15 ± 0.89 mm | ||
| C. albicans | ZOI = 34 ± 0.99 mm | ||
| A. fumigatus | ZOI = 26 ± 0.91 mm | ||
| CSSBs with quinazoline-6-carbaldehyde | E. coli | ZOI = 27 ± 0.83 mm | [91] |
| K. pneumoniae | ZOI = 27 ± 0.72 mm | ||
| S. aureus | ZOI = 20 ± 1.20 mm | ||
| S. mutans | ZOI = 17 ± 0.50 mm | ||
| C. albicans | ZOI = 31 ± 1.29 mm | ||
| A. fumigatus | ZOI = 25 ± 0.72 mm | ||
| CSSBs with oxazole-4-carbaldehyde | E. coli | ZOI = 22 ± 0.98 mm | [91] |
| K. pneumoniae | ZOI = 26 ± 0.65 mm | ||
| S. aureus | ZOI = 19 ± 0.62 mm | ||
| S. mutans | ZOI = 18 ± 1.20 mm | ||
| C. albicans | ZOI = 26 ± 0.49 mm | ||
| A. fumigatus | ZOI = 21 ± 0.65 mm | ||
| CS-NPs | E. coli | MIC = 117 μg/mL | [92] |
| S. choleraesuis | MIC = 117 μg/mL | ||
| S. aureus | MIC = 234 μg/mL | ||
| CS-Ag+ NPs | E. coli | MIC = 3 μg/mL | [92] |
| S. choleraesuis | MIC = 3 μg/mL | ||
| S. aureus | MIC = 6 μg/mL | ||
| CS-Cu2+ NPs | E. coli | MIC = 9 μg/mL | [92] |
| S. choleraesuis | MIC = 9 μg/mL | ||
| S. aureus | MIC = 21 μg/mL | ||
| CS-Zn2+ NPs | E. coli | MIC = 18 μg/mL | [92] |
| S. choleraesuis | MIC = 18 μg/mL | ||
| S. aureus | MIC = 36 μg/mL | ||
| CS-Mn2+ NPs | E.coli | MIC = 73 μg/mL | [92] |
| S.choleraesuis | MIC = 73 μg/mL | ||
| S. aureus | MIC = 85 μg/mL | ||
| CS-Fe2+ NPs | E. coli | MIC = 121 μg/mL | [92] |
| S. choleraesuis | MIC = 121 μg/mL | ||
| S. aureus | MIC = 146 μg/mL |
| Materials | Bacteria | Antimicrobial Activity | Ref. |
|---|---|---|---|
| Carr/AgNP/Clay | Listeria monocytogenes | DI = 6.34 mm | [156] |
| Escherichia coli | DI = 7.43 mm | ||
| Carr/AgNP (melanin-mediated synthesis) | Listeria monocytogenes | MIC = 64 μM | [157] |
| Escherichia coli | MIC = 16 μM | ||
| Carr/HNT-AgNP(SDS) | Listeria monocytogenes | reduced the bacterial count within 12 h | [158] |
| Escherichia coli | reduced the bacterial count within 12 h | ||
| Carr/AgNP (from pine needle) | Staphylococcus aureus | 3.5 Log CFU/mL reduction | [159] |
| Escherichia coli | 2 Log CFU/mL reduction | ||
| SSPS/AgNPs/Carr | Staphylococcus aureus | ZOI = 1.83 ± 0.13 mm | [154] |
| Escherichia coli | ZOI = 1.92 ± 0.25 mm | ||
| CA–AgNPs (10 mM) | Staphylococcus aureus | ZOI = 17.67 + 1.15 mm | [160] |
| Escherichia coli | ZOI = 12.67 + 0.41 mm | ||
| Carr/AgNP (AgNO3 0.6%) | Staphylococcus aureus | ZOI = 7.59 | [161] |
| Carr/AgNP (AgNO3 1.2%) | Staphylococcus aureus | ZOI = 9.12 | |
| Ag/Fe/g-C3N4-Carr | Klebsiella pneumoniae | excellent antimicrobial | [162] |
| Enterococcus faecalis | excellent antimicrobial | ||
| Carr/Fe3O4@NH2-Ag | Listeria monocytogenes | 10.09 vs. 3.93 log reduction | [163] |
| Escherichia coli | 8.82 vs. 5.02 log reduction | ||
| Carr/Fe3O4@SNP | Listeria monocytogenes | decrease growth by about 4 log cycles after 12 h of incubation compared to neat Carr | [164] |
| Escherichia coli | decrease growth by about 3.5 log cycles after 12 h of incubation compared to neat Carr | ||
| Pul/Carr/DL/CuS | Listeria monocytogenes | decrease growth by about 2 log cycles after 12 h of culturing compared to the control | [165] |
| Escherichia coli | decrease growth by about 7 log cycles after 12 h of culturing compared to the control | ||
| Carr/CuS | Staphylococcus aureus | reduced the bacterial counts 69.8 ± 1.8% | [166] |
| Escherichia coli | reduced the bacterial counts52.6 ± 5.4% | ||
| Carr/ZnONPs | Staphylococcus aureus | reduced the bacterial count (3 to 4 log reductions) within 24 h | [167] |
| Escherichia coli | reduced the bacterial count (4 log reductions) within 24 h | ||
| Carr/CuONPs | Staphylococcus aureus | reduced the bacterial count (3 to 4 log reductions) within 24 h | |
| Escherichia coli | reduced the bacterial count (4 log reductions) within 24 h | ||
| Carr/SiO2NPs | Staphylococcus aureus | no antibacterial activity | |
| Escherichia coli | reduced the bacterial count (3 log reductions) within 24 h | ||
| Kappaphycus alvarezii/ZnONPs | Staphylococcus aureus | reduced the bacterial count (2 to 4 log reductions) within 24 h | |
| Escherichia coli | |||
| Kappaphycus alvarezii/CuONPs | Staphylococcus aureus | ||
| Escherichia coli | |||
| Kappaphycus alvarezii/SiO2NPs | Staphylococcus aureus | ||
| Escherichia coli | |||
| Carr/Lig/AgNPs/CaCl2 hydrogel | Staphylococcus aureus | completely killed within 6 h of incubation | [168] |
| Escherichia coli | completely killed within 3 h of incubation | ||
| Carr/Lig/AgNPs/CuCl2 hydrogel | Staphylococcus aureus | completely killed within 3 h of incubation | |
| Escherichia coli | completely killed within 3 h of incubation | ||
| Carr/Lig/AgNPs/MgCl2 hydrogel | Staphylococcus aureus | completely killed within 6 h of incubation | |
| Escherichia coli | completely killed within 6 h of incubation | ||
| Carr/CNC/AgNPs cryogel | Staphylococcus aureus | R = 100% | [169] |
| Escherichia coli | R = 100% | ||
| Ag/Carr/Gelatin hydrogel | Streptococcus agalactiae | zone of clearance = 21 mm | [170] |
| Streptococcus pyogenes | zone of clearance = 18 mm | ||
| Escherichia coli | zone of clearance = 19 mm | ||
| Carr/KCl/ZnO/CuO | Listeria monocytogenes | increased slightly till 3 h of incubation then decreased linearly as the time increased | [171] |
| Escherichia coli | completely killed within 6–9 h of incubation | ||
| gelatin/carr/bacterial cellulose hydrogel scaffolds | Staphylococcus aureus | 5.4 ± 0.43 mm | [172] |
| Escherichia coli | 3.1 ± 0.88 mm | ||
| Klebsiella pneumonia | resistance against the bacteria | ||
| KaMA-ZnO/PD hydrogel | Staphylococcus aureus | ZOI = ~2.05 mm | [173] |
| Escherichia coli | ZOI = 2.8 ± 0.4 mm | ||
| H-OCA-Dop-Zn hydrogel | Staphylococcus aureus | 96.3% bacterial reduction | [174] |
| Escherichia coli | 99.6% bacterial reduction | ||
| CO-CNF 400 mg SHκ-carrageenan oligosaccharides linked cellulose nanofibers hydrogel loaded 400 mg surfactin and Herbmedotcin | Streptococcus mutans | ZOI = 26.33 ± 1.52 mmMIC = 60% | [175] |
| Porphyromonas gingivalis | ZOI = 18.33 ± 0.57 mmMIC = 50% | ||
| Fusobacterium nucleatum | ZOI = 20.33 ± 0.63 mmMIC = 70% | ||
| Pseudomonas aeruginosa | ZOI = 20.66 ± 1.25 mmMIC = 40% | ||
| ampicillin sodium salt-loaded PVA/HA-ĸ-Carr hydrogel | Staphylococcus aureus | ZOI = 12 mm | [176] |
| Escherichia coli | ZOI = 13 mm | ||
| ciprofloxacin-loaded PVA/Carr/HA hydrogel | Staphylococcus aureus | MIC and/or ZOI not given. Only photos are show | [178] |
| Escherichia coli | |||
| Berberine-loaded /Carr/KGM hydrogel | Staphylococcus aureus | ZOI = 16.1 ± 0.2 mm | [179] |
| Candida albicans | ZOI = 12.4 ± 0.1 mm | ||
| Carr/GSE/SNP hydrogel | Staphylococcus epidermis | destroy the bacteria within 3 h | [180] |
| Escherichia coli | destroy the bacteria within 3 h | [181] | |
| AR/rGO/Carr hydrogel | Staphylococcus aureus | ZOI ≈ 33 mm | [182] |
| Pseudomonas aeruginosa | ZOI ≈ 31 mm | ||
| Escherichia coli | ZOI ≈ 29 mm | ||
| Carr/Agar/MMT/CLPCarr/Agar/MMT/LDC/CLP | Staphylococcus aureus | ZOI = 25.7 ± 1.2 mm | [183] |
| Escherichia coli | ZOI = 31.0 ± 1.0 mm | ||
| Staphylococcus aureus | ZOI = 29.3 ± 1.2 mm | ||
| Escherichia coli | ZOI = 29.7 ± 0.6 mm |
| Peptide Name/Analog | Application | Clinical Phase |
|---|---|---|
| Medicine application | ||
| Human lactoferrin | Bacterial infections and mycoses | Approved |
| Vancomycin | Staphylococcal infections | Approved |
| Gramicidin/Cationic polycyclic peptide | Purulent skin disease | Approved |
| Colistin | Multidrug-resistance Gram-negative infections | Phase IV |
| SGX942(Dusquetide)/IDR-1 | Oral mucositis | Phase III |
| Pexiganan/Magainin | Topical application for diabetic foot ulcers | Phase III |
| PXL01/Lactoferrin | Postsurgical adhesions | Phase II |
| Omiganan/Indolicidin | Acne, atopic dermatitis | Phase II |
| PAC 113/Histatin | Oral candidiasis (mouth wash) Treatment of inflammation and ulceration | Phase II |
| PMX-30063 Brilacidin/Defensin mimetic | Acute bacterial skin infection | Phase II |
| OP145/LL-37 | Bacterial ear infection Topical cream for prevention of catheter | Phase I/II |
| LL-37/Leucine | Melanoma | Phase I/II |
| Food industry | ||
| Nisin | Dairy (L. monocytogenes and S. aureus) | Approved |
| Polylysine/Natural cationic antibacterial agent | Sushi, boiled rice, noodles, meat, and drinks | Approved |
| Polymer | Nanoparticle | Form of Composite | Application | Bacteria | Antibacterial Activity | References |
|---|---|---|---|---|---|---|
| PLA-polylactide | ZnO | Nanofibers, nanofibrous mats, | wound dressings, tissue regenerative applications | E. coli, S. aureus | MIC = 6.5 ± 10−4 mg/L | [361] |
| Carbon doped TiO2 | Nanocomposite films (H-PLA/3GST) | Wound dressing | S. aureus | ZOI = 28 mm | [364] | |
| Al2O3 + Ag | PLA-Al2O3/Ag fiber mats (25% of Al2O3/Ag powder concentration) | Antibacterial applications | E. coli | ZOI = 10.55 mm | [362] | |
| Sarcina lutea | ZOI = 13.88 mm | |||||
| Ag | Nanofiber membrane (PLA—2Ag) | wound dressing | E. coli | ZOI = 7.57 mm | [363] | |
| S. aureus | ZOI = 6.75 mm | |||||
| Zn[(acac)(L)H2O], where L represents N-(2-pyridin-4-ylethylidene) phenylalanine | Initiator of polymerization, M/I ratio as 1:400 | drug delivery, wound healing | S. aureus S. epidermidis, P. aeruginosa | MIC = 100 µg/mL | [365] | |
| PVA-poly vinyl alcohol | ZnO | nanocomposite fibers | wound healing and tissue reconstruction | E. coli | MIC = 62.5 μg/ mL | [366] |
| S. aureus | MIC = 250 μg/ mL | |||||
| Se | PVA/Chitosan/SeNPs nanocomposite films (at 30 min laser ablation) | antimicrobial applications | E. coli | Activ. Index = 69% | [368] | |
| S. aureus | Activ. Index = 54% | |||||
| P. aeruginosa | Activ. Index = 64% | |||||
| B. subtilis | Activ. Index = 44% | |||||
| PVA/carboxymethyl cellulose blend with selenium nanoparticles | Candidate for food packaging | E. coli | D(inhibition zone diameter) = 33 mm2 | [369] | ||
| S. aureus | D = 49 mm2 | |||||
| P. aeruginosa | D = 6 mm2 | |||||
| B. cereus | D = 39 mm2 | |||||
| CuS + chitosan | CuS/PVACS nanocomposite | Antibacterial aplications | E. coli | DI = 13.51 ± 0.33 mm | [367] | |
| S. aureus | DI = 23.81 ± 0.09 mm | |||||
| P. syringae | DI = 18.23 ± 0.41 mm | |||||
| S. pneumoniae | DI = 27.11 ± 0.31 mm | |||||
| PCL-polycaprolactone | ZnO | scaffolds of PCL compounded with hydroxyapatite and ZnO (6% ZnO) | bone tissue engineering | S. aureus | R = 96% | [371] |
| Ag | nanocomposite membranes PCL/Ag (1%) | Wound dressing | E. coli | DI = 7.9 ± 0.6 mm | [370] | |
| S. aureus | DI = 11.6 ± 0.5 mm | |||||
| PLGA-poly(lactide-co-glycolide) | Ag + ZnO | PLGA/Ag/ZnO nanorods composite coating | - | E. coli | R = 60.8% | [372] |
| S. aureus | R = 70.3% | |||||
| azithromycin | poly(lactide-co-glycolide) nanoparticles loaded with azithromycin | Drug delivery | Salmonella typhi | MIC = 3.12 µg/mL | [373] | |
| Al2O3 | PLGA/Al2O3 (0.1%) nanocomposite | candidate for packaging materials, biomedicine | E. coli | 74% reduction in bacteria growth | [358] | |
| Fe2O3 | PLGA/Fe2O3 (0.01%) nanocomposite | Candidate for packaging material in agriculture | E. coli | over 5-fold reduction in the number of cells | [374] | |
| Chitosan | ZnO | Nanocomposite films | Food packaging | E. coli | Plate count values: 2.5 ± 0.421× 107 cfu/g | [141] |
| S. aureus | 9 ± 0.367× 107 cfu/g | |||||
| ZnO | ZnO nanoparticles embedded in TPP—crosslinked chitosan | Sunscreen agent | E. coli | 5 log reduction | [375] | |
| S. aureus | Total reduction | |||||
| TiO2 + pectin | TiO2 nanotubes loaded with a drug on chitosan coating | Drug carrier | E. coli | ZOI = 45 mm | [376] | |
| S. aureus | ZOI = 45 mm | |||||
| P. aeruginosa | ZOI = 47 mm | |||||
| B. subtilis | ZOI = 49 mm | |||||
| Ag | Ag nanoparticles in the porous chitosan matrix—sponges (15 mM Ag) | wound dressing | E. coli | 6 Log reductions of viable cell numbers after 2 h of exposure | [377] | |
| S. aureus | ||||||
| Ag2O | The solution of the chitosan– Ag2O encapsulated nanocomposite film | Food packaging | E. coli | ZOI = 16 mm | [378] | |
| S. aureus | ZOI = 23 mm | |||||
| B. subtilis | ZOI = 20 mm | |||||
| P. aeruginosa | ZOI = 24 mm | |||||
| Fe3O4 + gelatin | Fe3O4/CS/GE nanofiber membrane (1% Fe3O4) | Wound dressing | E. coli | ZOI = 20 mm | [392] | |
| S. aureus | ZOI = 19 mm | |||||
| FeO | CS/FeO nanocomposite (40 μg/mL FeO) | Biological applications | B. subtilis | ZOI = 13.0 ± 0.5 mm | [379] | |
| S. aureus | ZOI = 12.0 ± 0.5 mm | |||||
| E. coli | ZOI = 15.0 ± 0.5 mm | |||||
| Au | chitosan-capped gold nanoparticles | Antibacterial applications | E. coli | MIC = 64 μg/mL | [380] | |
| S. aureus | MIC = 128 μg/mL | |||||
| Salmonella enterica | MIC = 32 μg/mL | |||||
| L. monocytogenes | MIC = 4 μg/mL | |||||
| MnS + CaAlg | MnS2/CS-CaAlg | Antibacterial applications | E. coli, S. aureus | reduced the bacterial count (6 log to 7 log reductions in 60 min) | [393] | |
| Tobramycin | Tobramycin-chitosan nanoparticles (TOB-CS NPs) | Drug delivery | E. coli | MIC = 11.30 ± 0.12 mg/mL | [381] | |
| S. aureus | MIC = 15.60 ± 0.09 mg/mL | |||||
| Tobramycin + ZnO | Tobramycin-chitosan nanoparticles (TOB-CS NPs) coated with zinc oxide nanoparticles (ZnO NPs) | Drug delivery | E. coli | MIC = 8.40 ± 0.11 mg/mL | [381] | |
| S. aureus | MIC = 10.70 ± 0.08 mg/mL | |||||
| tetracycline | tetracycline hydrochloride loaded into 1% fungal chitosan | Drug delivery | E. coli | ZOI = 26.17 ± 1.53 mm | [394] | |
| B. sbtilis | ZOI = 20.0 ± 1.4 mm | |||||
| S. aureus | ZOI = 22.0 ± 1.4 mm | |||||
| cellulose | ZnO + chitosan | cellulose/chitosan composite films loaded with ZnO nanoplates (3% ZnO) | wastewater treatment, antibacterial materials | E. coli | ZOI = 13.6 ± 0.2 mm | [382] |
| S. aureus | ZOI = 20.6 ± 0.2 mm | |||||
| Ag | spherical-nano cellulose (SNC)/silver-nanoparticle (AgNP) | Antibacterial and catalytic applications | E. coli | DI = 12.1 ± 0.91 mm | [383] | |
| S. aureus | DI = 11.3 ± 0.01 mm | |||||
| AgO | Cellulose nanofibers /PVA/SA-AgO (CPS-AgO) aerogel | Antibacterial aerogel | E. coli | ZOI = 23 mm | [384] | |
| S. aureus | ZOI = 20 mm | |||||
| Al2O3 | Conformal coatings with Al2O3 on viscose fabrics | Antibacterial applications | E. coli | Inhibition rate of bacterial growth = 71% | [385] | |
| S. aureus | Inhibition rate of bacterial growth = 65% | |||||
| dextran | Ag | Dextran-coated silver nanoparticles | Food packaging | E. coli | R = 99.9% (after 24 h of exposure) | [386] |
| alginate | ZnO | ZnO nanoparticles on alginate (~11 wt.% ZnO) | Antibacterial applications | E. coli | R = 100% | [388] |
| S. aureus | R = 99.91% (after 2 h of exposure) | |||||
| Ag | copper alginate hydrogel doped with Ag nanoparticles (5% Ag) | Antibacterial applications | E. coli | ZOI ≈ 23 mm | [395] | |
| S. aureus | ZOI ≈ 35 mm | |||||
| starch | MgO + albumin | starch-Albumin-magnesium oxide (S-A-MgO) film | Antibacterial applications | E. coli | DI = 5 ± 0.13 mm | [389] |
| S. aureus | DI = 6 ± 0.22 mm | |||||
| Ag2O | Starch-capped Ag2O nanoparticles (2:1 Ag2O ratio) | Antibacterial applications | E. coli | ZOI = 14 ± 0.11 mm | [390] | |
| S. aureus | ZOI = 15 ± 0.19 mm | |||||
| Fe2O3 | polyaniline/starch/hematite biocomposite | water purifier | S. typhimurium | IZ = 18.02 mm | [391] | |
| S. aureus | IZ = 13.98 mm | |||||
| B. subtilis | IZ = 14.12 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. https://doi.org/10.3390/ijms24087473
Smola-Dmochowska A, Lewicka K, Macyk A, Rychter P, Pamuła E, Dobrzyński P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. International Journal of Molecular Sciences. 2023; 24(8):7473. https://doi.org/10.3390/ijms24087473
Chicago/Turabian StyleSmola-Dmochowska, Anna, Kamila Lewicka, Alicja Macyk, Piotr Rychter, Elżbieta Pamuła, and Piotr Dobrzyński. 2023. "Biodegradable Polymers and Polymer Composites with Antibacterial Properties" International Journal of Molecular Sciences 24, no. 8: 7473. https://doi.org/10.3390/ijms24087473





